Establishment of Direct Organogenesis Protocol for Arachis hypogaea cv. Virginia in Liquid Medium by Temporary Immersion System (TIS)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Decontamination of the Seeds and Explant Preparation
2.3. Determination of Seed Viability and Germination Rate
2.4. In Vitro Propagation via Temporary Immersion System (TIS)
2.5. Data Collection and Statistical Analysis
3. Results
3.1. Decontamination of the Seeds
3.2. Evaluation of Seed Viability via TTC Test and Its Confirmation by Germination Trials
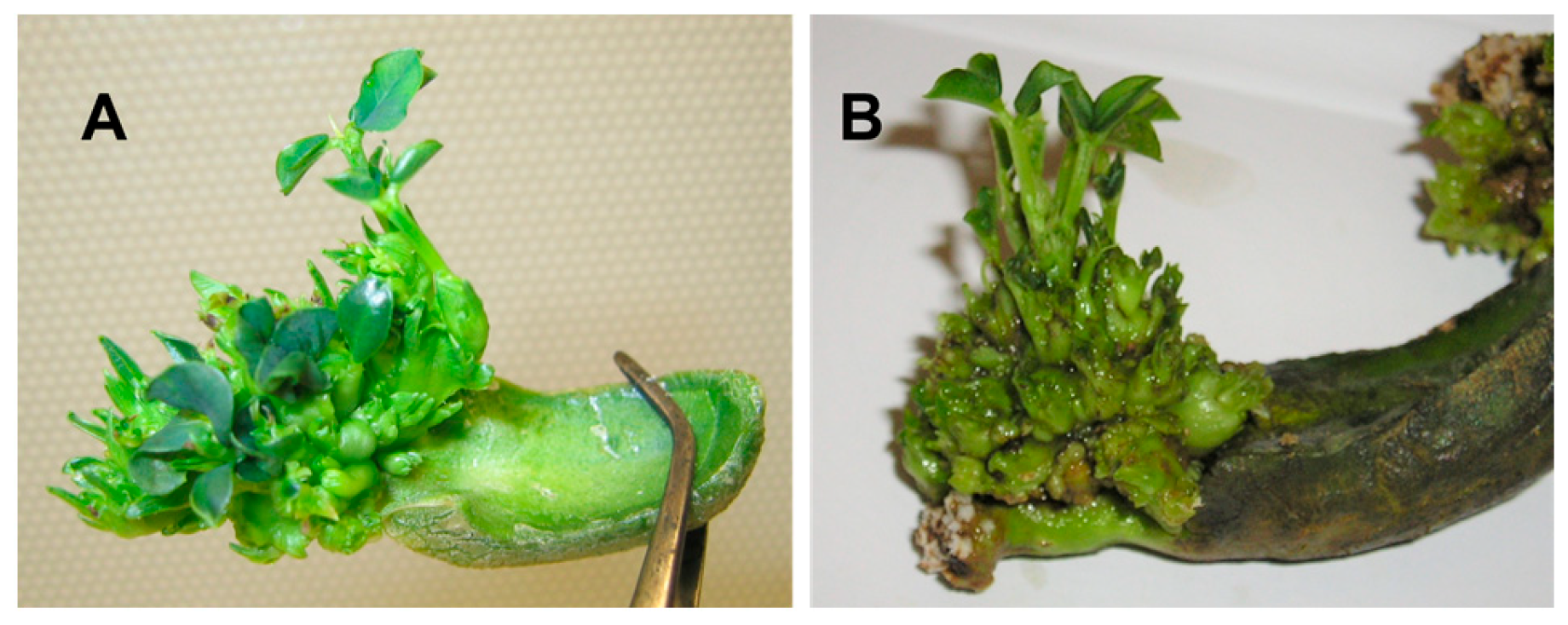
3.3. Direct Organogenesis of the De-Embryonated Seeds
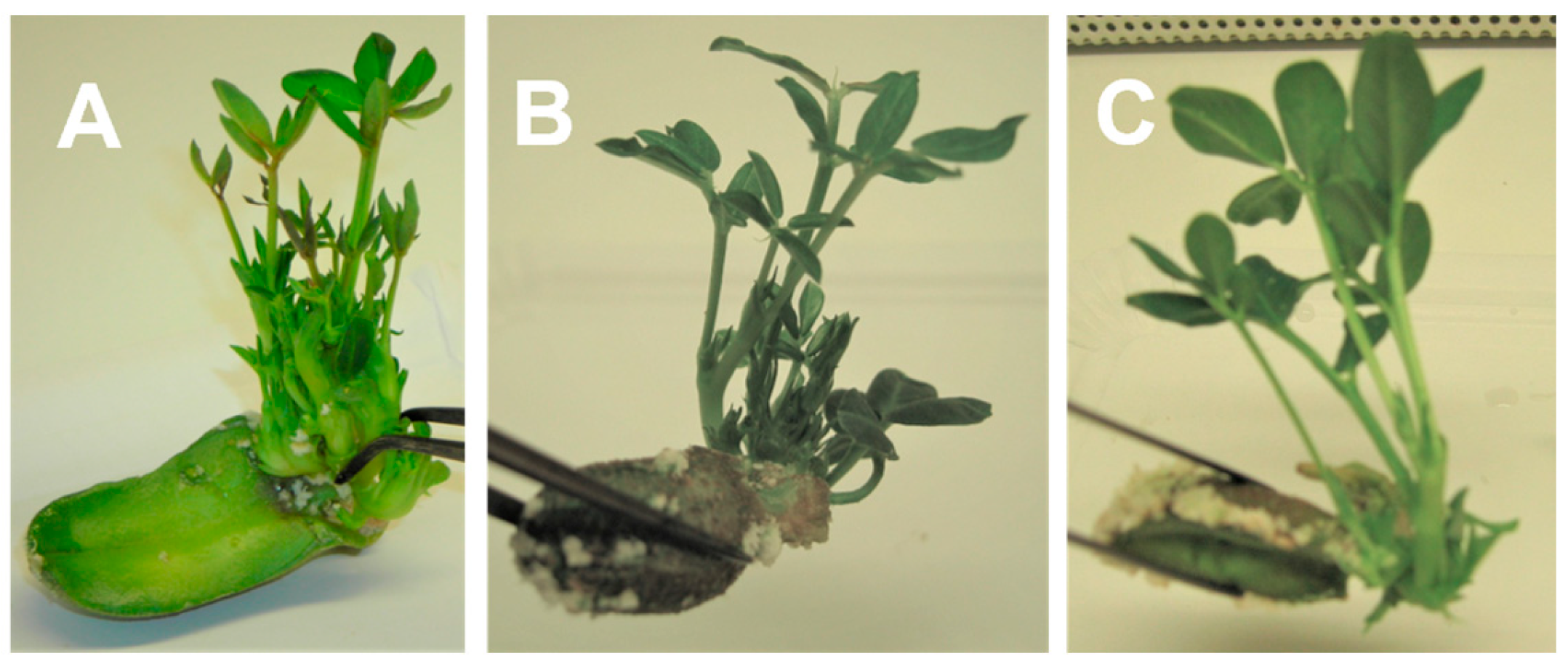
3.4. Rooting of the Shoots and Acclimatization of the Plantlets
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pal, A.; Pal, A.K. Physiological Basis of Salt Tolerance in Groundnut (Arachis hypogaea L.). Int. J. Curr. Microb. Appl. Sci. 2017, 6, 2157–2171. [Google Scholar] [CrossRef]
- FAO Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/3/CA0239EN/ca0239en.pdf (accessed on 10 August 2022).
- Bakoye, O.; Baoua, I.; Sitou, L.; Moctar, M.R.; Amadou, L.; Njoroge, A.W.; Murdock, L.L.; Baributsa, D. Groundnut Production and Storage in the Sahel: Challenges and Opportunities in the Maradi and Zinder Regions of Niger. J. Agric. Sci. 2019, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Savage, G.P.; Keenan, J.I. The Composition and Nutritive Value of Groundnut Kernels. In The Groundnut Crop: Scientific basis for improvement; Smart, J., Ed.; Chapman and Hall: London, UK, 1994; pp. 173–213. ISBN 9789401043151. [Google Scholar]
- Shokunbi, O.S.; Fayomi, E.T.; Sonuga, O.S.; Tayo, G.O. Nutrient Composition of Five Varieties of Commonly Consumed Nigerian Groundnut (Arachis hypogaea L.). Grasas Y Aceites 2012, 63, 14–18. [Google Scholar] [CrossRef]
- Janila, P.; Nigam, S.N.; Abhishek, R.; Anil Kumar, V.; Manohar, S.S.; Venuprasad, R. Iron and Zinc Concentrations in Peanut (Arachis hypogaea L.) Seeds and Their Relationship with Other Nutritional and Yield Parameters. J. Agric. Sci. 2014, 153, 975–994. [Google Scholar] [CrossRef]
- Arya, S.S.; Salve, A.R.; Chauhan, S. Peanuts as Functional Food: A Review. J. Food Sci. Technol. 2015, 53, 31–41. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M.; Goktepe, I.; Dai, J. Peanut Skin Procyanidins: Composition and Antioxidant Activities as Affected by Processing. J. Food Compos. Anal. 2006, 19, 364–371. [Google Scholar] [CrossRef]
- Coates, A.M.; Howe, P.R. Edible Nuts and Metabolic Health. Curr. Opin. Lipidol. 2007, 18, 25–30. [Google Scholar] [CrossRef]
- Kumar, V.; Thirumalaisamy, P.P. Diseases of Groundnut. In Diseases of Field Crops and Their Management; Dubey, S.C., Aggarwal, R., Patro, T.S.S.K., Sharma, P., Eds.; Today and Tomorrows Printers and Publishers: New Delhi, India, 2016; pp. 445–494. ISBN 9788170354178. [Google Scholar]
- Palanivel, S.; Jayabalan, N. Direct Multiple Shoot Induction from Different Mature Seed Explants of Groundnut (Arachis hypogaea L.). Philippines J. Sci. 2002, 131, 127–135. [Google Scholar]
- Iqbal, M.M.; Nazir, F.; Iqbal, J.; Tehrim, S.; Zafar, Y. İn vitro Micropropagation of Peanut (Arachis hypogaea) through Direct Somatic Embryogenesis and Callus Culture. Int. J. Agric. Biol. 2011, 13, 811–814. [Google Scholar]
- Venkatachalam, P.; Kavipriya, V. Efficient Method for in vitro Plant Regeneration from Cotyledonary Node Explants of Peanut (Arachis hypogaea L.). In Proceedings of the International Conference on Nuclear Energy, Environmental and Biological Sciences (ICNEEBS’2012), Bangkok, Thailand, 8–9 September 2012; pp. 8–9. [Google Scholar]
- Ozudogru, E.A.; Kaya, E.; Lambardi, M. In vitro Propagation of Peanut (Arachis hypogaea L.) by Shoot Tip Culture. Methods Mol. Biol. 2012, 11013, 77–87. [Google Scholar] [CrossRef]
- Das, A.; Goyali, J.C.; Ferdausi, A. Tissue Culture Approaches to Improve Nutritional Quality and Stress Response in Peanut. Eur. J. Biol. 2021, 11, 332–347. [Google Scholar]
- Ramírez-Mosqueda, M.A.; Iglesias-Andreu, L.G.; Ramírez-Madero, G.; Hernández-Rincón, E.U. Micropropagation of Stevia Rebaudiana Bert. In Temporary Immersion Systems and Evaluation of Genetic Fidelity. S. Afr. J. Bot. 2016, 106, 238–243. [Google Scholar] [CrossRef]
- Szopa, A.; Kokotkiewicz, A.; Bednarz, M.; Jafernik, K.; Luczkiewicz, M.; Ekiert, H. Bioreactor Type Affects the Accumulation of Phenolic Acids and Flavonoids in Microshoot Cultures of Schisandra chinensis (Turcz.) Baill. PCTOC 2019, 139, 199–206. [Google Scholar] [CrossRef]
- Ptak, A.; Morańska, E.; Skrzypek, E.; Warchoł, M.; Spina, R.; Laurain-Mattar, D.; Simlat, M. Carbohydrates Stimulated Amaryllidaceae Alkaloids Biosynthesis in Leucojum aestivum L. Plants Cultured in RITA®Bioreactor. PeerJ 2020, 8, e8688. [Google Scholar] [CrossRef]
- Welander, M.; Sayegh, A.; Hagwall, F.; Kuznetsova, T.; Holefors, A. Technical Improvement of a New Bioreactor for Large Scale Micropropagation of Several Vaccinium Cultivars. Acta Hortic. 2017, 1180, 387–392. [Google Scholar] [CrossRef]
- Othmani, A.; Bayoudh, C.; Sellemi, A.; Drira, N. Temporary Immersion System for Date Palm Micropropagation. In Date Palm Biotechnology Protocols Volume 1: Tissue Culture Applications, Methods in Molecular Biology; Al-Khayri, J., Jain, S., Johnson, D., Eds.; Humana Press: New York, NY, USA, 2017; Volume 1637, pp. 239–249. ISBN 9781493971558. [Google Scholar]
- Rico, S.; Garrido, J.; Sánchez, C.; Ferreiro-Vera, C.; Codesido, V.; Vidal, N. A Temporary Immersion System to Improve Cannabis Sativa Micropropagation. Front. Plant. Sci. 2022, 13, 895971. [Google Scholar] [CrossRef]
- Uma, S.; Karthic, R.; Kalpana, S.; Backiyarani, S.; Saraswathi, M.S. A Novel Temporary Immersion Bioreactor System for Large Scale Multiplication of Banana (Rasthali AAB—Silk). Sci. Rep. 2021, 11, 20371. [Google Scholar] [CrossRef]
- Balota, M.; Phipps, P. Comparison of Virginia and Runner-Type Peanut Cultivars for Development, Disease, Yield Potential, and Grade Factors in Eastern Virginia. Peanut Sci. 2013, 40, 15–23. [Google Scholar] [CrossRef][Green Version]
- Balota, M.; Tillman, B.L.; Paula-Moraes, S.V.; Anco, D. “Walton”, a New Virginia-Type Peanut Suitable for Virginia and Northern U.S. Growing Regions. J. Plant. Regist. 2021, 15, 422–434. [Google Scholar] [CrossRef]
- Pallas, J.E.; Stansell, J.R.; Bruce, R.R. Peanut Seed Germination as Related to Soil Water Regime during Pod Development. Agron. J. 1977, 69, 381–383. [Google Scholar] [CrossRef]
- Gaines, T.P.; Parker, M.B.; Walker, M.E. Limestone and Gypsum Effects on Calcium Nutrition of “Florunner” and “NC-7” Peanuts. Commun. Soil Sci. Plant. Anal. 1991, 22, 117–135. [Google Scholar] [CrossRef]
- ISTA. I.S.T. International Rules for Seed Testing. Available online: https://www.seedtest.org/en/publications/international-rules-seed-testing-1168.html (accessed on 30 June 2022).
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lambardi, M.; Sharma, K.K.; Thorpe, T.A. Optimization of in vitro Bud Induction and Plantlet Formation from Mature Embryos of Aleppo Pine (Pinus halepensis Mill.). In Vitro Cell Dev. Biol.-Plant 1993, 29, 189–199. [Google Scholar] [CrossRef]
- Marascuilo, L.A.; McSweeney, M. Post Hoc Multiple Comparisons in Sample Preparations for Test of Homogenesity. In Nonparametric and Distribution-Free Methods for the Social Sciences; McSweeney, M., Marascuilo, L.A., Eds.; Brooks/Cole Publishing Company: Monterey, CA, USA, 1977; pp. 141–147. [Google Scholar]
- Ozudogru, E.A.; Ozden-Tokatli, Y.; Akcin, A. Effect of Silver Nitrate on Multiple Shoot Formation of Virginia-Type Peanut through Shoot Tip Culture. Vitr. Cell. Dev. Biol.-Plant 2005, 41, 151–156. [Google Scholar] [CrossRef]
- Canavar, Ö. The Influence of Storage Time on Fatty Acid, Tocopherol and Seed Quality of Peanut. Qual. Assur. Saf. Crop. Foods 2015, 7, 165–174. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Chen, F. Effect of Storage Temperature on Lipid Oxidation and Changes in Nutrient Contents in Peanuts. Food Sci. Nutr. 2019, 7, 2280–2290. [Google Scholar] [CrossRef]
- Matand, K.; Wu, N.; Wu, H.; Tucker, E.; Love, K. More Improved Peanut (Arachis hypogaea L.) Protocol for Direct Shoot Organogenesis in Mature Dry-Cotyledonary and Root Tissues. J. Biotech. Res. 2013, 5, 24–34. [Google Scholar]
- Mehta, U.; Mohan Ram, H. Regeneration of Plantlets from the Cotyledons of Cajanus Cajan. Indian J. Exp. Bot. 1980, 18, 800–802. [Google Scholar]
- Mathews, V.H.; Rao, P.S. İn vitro Production of Multiple Seedlings from Single Seeds of Mung Bean (Vigna radiata L. Wilczek) Z. Pflanzenphysiol. 1984, 113, 325–329. [Google Scholar] [CrossRef]
- Dunstan, D.; Thorpe, A. Regeneration in Forest Trees. In Cell Culture and Somatic Cell Genetics of Plants; Vasil, E., Ed.; Academic Press: Orlando, FL, USA, 1986; pp. 223–243. [Google Scholar]
- McKently, A.H.; Moore, G.A.; Gardner, F.P. In vitro Plant Regeneration of Peanut from Seed Explants. Crop. Sci. 1990, 30, 192–196. [Google Scholar] [CrossRef]
- Pestana, M.C.; Lacorte, C.; de Freitas, V.G.; de Oliveira, D.E.; Mansur, E. İn vitro Regeneration of Peanut (Arachis hypogaea L.) through Organogenesis: Effect of Culture Temperature and Silver Nitrate. Vitr. Cell Dev. Biol.-Plant 1999, 35, 214–216. [Google Scholar] [CrossRef]
- Radhakrishnan, T.; Murthy, T.G.K.; Chandran, K.; Bandyopadhyay, A. Micropropagation in Peanut (Arachis hypogaea L.). Biol. Plant. 2000, 43, 447–450. [Google Scholar] [CrossRef]
- Tiwari, S.; Tuli, R. Factors Promoting Efficient in vitro Regeneration from De-Embryonated Cotyledon Explants of Arachis hypogaea L. PCTOC 2007, 92, 15–24. [Google Scholar] [CrossRef]
- Maina, S.; Emongor, Q.; Sharma, K.; Gichuki, S.; Gathaara, M.; de Villiers, S. Surface Sterilant Effect on the Regeneration Efficiency from Cotyledon Explants of Groundnut (Arachis hypogea L.) Varieties Adapted to Eastern and Southern Africa. Afr. J. Biotechnol. 2010, 9, 2866–2871. [Google Scholar]
- Burns, S.P.; Gallo, M.; Tillman, B.L. Expansion of a Direct Shoot Organogenesis System in Peanut (Arachis hypogaea L.) to Include US Cultivars. Vitr. Cell Dev. Biol.-Plant 2011, 48, 58–66. [Google Scholar] [CrossRef]
- Ozkan, H.; Aasim, M. Potential of Pretreated Explants of Peanut (Arachis hypogaea Linn.) to Micropropagation under in vitro Conditions. Pak. J. Agric. Sci. 2019, 56, 775–780. [Google Scholar]
- Hoa, P.T.B.; Tue, N.H.; Trang, P.T.Q.; Hang, L.T.; Tien, N.Q.D.; Loc, N.H. An Efficient Protocol for in vitro Regeneration of Peanut (Arachis hypogaea L.) Cultivar L14. Biosci. J. 2021, 37, e37019. [Google Scholar] [CrossRef]
- Hokanson, K.E.; Pooler, M.R. Regeneration of Ornamental Cherry (Prunus) Taxa from Mature Stored Seed. HortScience 2000, 35, 745–748. [Google Scholar] [CrossRef]
- Ainsley, P.J.; Hammerschlag, F.A.; Bertozzi, T.; Collins, G.G.; Sedgley, M. Regeneration of Almond from Immature Seed Cotyledons. PCTOC 2001, 67, 221–226. [Google Scholar] [CrossRef]
- Singh, A.K.; Chand, S.; Pattnaik, S.; Chand, P.K. Adventitious Shoot Organogenesis and Plant Regeneration from Cotyledons of Dalbergia sissoo Roxb., a Timber Yielding Tree Legume. PCTOC 2002, 68, 203–209. [Google Scholar] [CrossRef]


| T1 * (%) | T2 (%) | T3 (%) | T4 (%) | |
|---|---|---|---|---|
| Upon seed arrival | 40 | 28 | 20 | 12 |
| After conservation at 4 °C in darkness | 36 | 16 | 32 | 16 |
| Variety | Upon Seed Arrival | After Conservation at 4 °C in Darkness | ||||
|---|---|---|---|---|---|---|
| Viability According to TTC Test (%) | Germination of the Entire Seed (%) | Germination of the Excised Embryo (%) | Viability According to TTC Test (%) | Germination of the Entire Seed (%) | Germination of the Excised Embryo (%) | |
| Virginia | 100 a * | 100 a | 100 a | 100 a | 100 a | 100 a |
| NC7 | 100 a | 100 a | 100 a | 100 a | 100 a | 100 a |
| 7 × 77 | 85 b * | 75 b | 80 b | 80 b | 70 b | 65 b |
| Com74 | 85 b | 85 b | 85 b | 85 b | 90 ab | 80 b |
| Medium Composition | % of Regenerating Explants * | Number of Shoots per Regenerating Explant ** | SFC *** | Average Shoot Length (cm) ** |
|---|---|---|---|---|
| MS + 27.5 µM BA | 26.6 a | 3.2 ± 0.9 b | 0.85 | 0.56 ± 0.04 b |
| MS + 55 µM BA | 6.0 c | 4.3 ± 2.4 b | 0.26 | 0.72 ± 0.14 b |
| MS + 110 µM BA | 8.5 c | 4.2 ± 2.3 b | 0.36 | 0.97 ± 0.25 a |
| MS + 5 µM TDZ | 10.0 bc | 5.8 ± 2.3 a | 0.58 | 1.21 ± 0.33 a |
| MS + 7.5 µM TDZ | 13.3 b | 4.2 ± 0.8 b | 0.56 | 1.00 ± 0.01 a |
| MS + 10 µM TDZ | 14.0 b | 7.8 ± 4.8 a | 1.09 | 0.96 ± 0.18 a |
| Medium Composition | Medium Immersion Regime | % of Regenerating Explants * | Number of Shoots per Regenerating Explant ** | SFC *** | Average Shoot Length (cm) ** |
|---|---|---|---|---|---|
| MS + 27.5 µM BA | 8 min/16 h, 12 min daily immersion | 8.7 e | 4.1 ± 0.7 bc | 0.36 | N.R. **** |
| MS + 55 µM BA | 8 min/16 h, 12 min daily immersion | 13.5 d | 3.2 ± 0.5 c | 0.43 | N.R. |
| MS + 110 µM BA | 8 min/16 h, 12 min daily immersion | 11.1 e | 2.2 ± 0.4 d | 0.24 | N.R. |
| MS + 5 µM TDZ | 8 min/16 h, 12 min daily immersion | 15.7 d | 2.7 ± 0.9 d | 0.42 | N.R. |
| MS + 7.5 µM TDZ | 8 min/16 h, 12 min daily immersion | 18.7 cd | 3.1 ± 0.7 c | 0.58 | N.R. |
| MS + 10 µM TDZ | 8 min/16 h, 12 min daily immersion | 23.3 cd | 3.8 ± 0.9 c | 0.88 | N.R. |
| MS + 27.5 µM BA | 8 min/24 h, 8 min daily immersion | - | - | - | - |
| MS + 55 µM BA | 8 min/24 h, 8 min daily immersion | - | - | - | - |
| MS + 110 µM BA | 8 min/24 h, 8 min daily immersion | 8.7 e | 4.9 ± 0.5 bc | 0.43 | N.R. |
| MS + 5 µM TDZ | 8 min/24 h, 8 min daily immersion | 12.7 d | 3.2 ± 0.8 c | 0.41 | N.R. |
| MS + 7.5 µM TDZ | 8 min/24 h, 8 min daily immersion | 10.7 e | 3.7 ± 0.2 c | 0.40 | N.R. |
| MS + 10 µM TDZ | 8 min/24 h, 8 min daily immersion | 15.3 d | 5.1 ± 0.7 b | 0.78 | N.R. |
| MS + 27.5 µM BA | 16 min/8 h, 48 min daily immersion | 15.5 d | 4.1 ± 0.9 bc | 0.64 | 0.52 ± 0.11 d |
| MS + 55 µM BA | 16 min/8 h, 48 min daily immersion | 19.8 d | 4.2 ± 0.7 bc | 0.83 | 1.07 ± 0.43 c |
| MS + 110 µM BA | 16 min/8 h, 48 min daily immersion | 29.8 cd | 4.8 ± 0.9 bc | 1.43 | 0.44 ± 0.01 d |
| MS + 5 µM TDZ | 16 min/8 h, 48 min daily immersion | 38.6 c | 4.6 ± 0.5 bc | 1.78 | 0.84 ± 0.51 d |
| MS + 7.5 µM TDZ | 16 min/8 h, 48 min daily immersion | 21.3 cd | 5.2 ± 0.8 b | 1.11 | 1.51 ± 0.18 c |
| MS + 10 µM TDZ | 16 min/8 h, 48 min daily immersion | 17.0 d | 4.4 ± 1.3 bc | 0.75 | 0.67 ± 0.01 d |
| MS + 27.5 µM BA | 16 min/16 h, 24 min daily immersion | 37.3 c | 4.2 ± 0.6 bc | 1.57 | 2.95 ± 0.46 b |
| MS + 55 µM BA | 16 min/16 h, 24 min daily immersion | 43.7 bc | 5.6 ± 0.5 b | 2.45 | 2.20 ± 0.16 b |
| MS + 110 µM BA | 16 min/16 h, 24 min daily immersion | 71.7 a | 5.4 ± 0.8 b | 3.87 | 0.97 ± 0.25 c |
| MS + 5 µM TDZ | 16 min/16 h, 24 min daily immersion | 52.5 b | 4.8 ± 0.7 bc | 2.52 | 1.63 ± 0.15 c |
| MS + 7.5 µM TDZ | 16 min/16 h, 24 min daily immersion | 42.8 bc | 9.2 ± 1.8 a | 3.94 | 1.84 ± 0.11 c |
| MS + 10 µM TDZ | 16 min/16 h, 24 min daily immersion | 43.2 bc | 10.2 ± 1.5 a | 4.41 | 1.49 ± 0.08 c |
| MS + 27.5 µM BA | 16 min/24 h, 16 min daily immersion | 33.7 c | 7.5 ± 0.9 ab | 2.53 | 0.97 ± 0.07 c |
| MS + 55 µM BA | 16 min/24 h, 16 min daily immersion | 40.2 bc | 6.2 ± 0.6 b | 2.49 | 1.46 ± 0.13 c |
| MS + 110 µM BA | 16 min/24 h, 16 min daily immersion | 43.3 bc | 6.7 ± 0.9 b | 2.90 | 1.07 ± 0.07 c |
| MS + 5 µM TDZ | 16 min/24 h, 16 min daily immersion | 41.2 bc | 5.9 ± 0.9 b | 2.43 | 1.13 ± 0.11 c |
| MS + 7.5 µM TDZ | 16 min/24 h, 16 min daily immersion | 38.3 c | 6.4 ± 0.5 b | 2.45 | 1.21 ± 0.31 c |
| MS + 10 µM TDZ | 16 min/24 h, 16 min daily immersion | 35.5 c | 8.9 ± 2.9 a | 3.16 | 1.01 ± 0.04 c |
| MS + 27.5 µM BA | 24 min/8 h, 72 min daily immersion | 18.7 cd | 3.8 ± 0.4 c | 0.71 | 3.02 ± 1.07 ab |
| MS + 55 µM BA | 24 min/8 h, 72 min daily immersion | 31.3 c | 4.2 ± 0.4 bc | 1.31 | 2.51 ± 0.37 b |
| MS + 110 µM BA | 24 min/8 h, 72 min daily immersion | 38.3 c | 2.8 ± 0.7 d | 1.07 | 2.07 ± 0.18 b |
| MS + 5 µM TDZ | 24 min/8 h, 72 min daily immersion | 44.7 bc | 3.6 ± 0.5 c | 1.61 | 4.03 ± 1.11 a |
| MS + 7.5 µM TDZ | 24 min/8 h, 72 min daily immersion | 40.3 bc | 5.2 ± 0.8 b | 2.10 | 4.41 ± 0.91 a |
| MS + 10 µM TDZ | 24 min/8 h, 72 min daily immersion | 40.7 bc | 5.4 ± 0.7 b | 2.20 | 3.71 ± 1.06 a |
| MS + 27.5 µM BA | 24 min/16 h, 36 min daily immersion | 31.2 c | 3.5 ± 0.9 c | 1.09 | 1.82 ± 0.27 c |
| MS 55 µM BA | 24 min/16 h, 36 min daily immersion | 38.7 c | 3.2 ± 0.6 c | 1.24 | 1.71 ± 0.17 c |
| MS + 110 µM BA | 24 min/16 h, 36 min daily immersion | 59.7 b | 4.7 ± 0.9 bc | 2.81 | 3.01 ± 0.09 ab |
| MS + 5 µM TDZ | 24 min/16 h, 36 min daily immersion | 52.3 b | 4.9 ± 0.9 bc | 2.56 | 3.14 ± 0.07 a |
| MS + 7.5 µM TDZ | 24 min/16 h, 36 min daily immersion | 35.8 c | 4.4 ± 0.5 bc | 1.58 | 2.03 ± 0.41 b |
| MS + 10 µM TDZ | 24 min/16 h, 36 min daily immersion | 41.7 bc | 5.9 ± 2.9 b | 2.46 | 3.10 ± 0.61 a |
| Variety | Medium Immersion Regime | % of Regenerating Explants * | Number of Shoots per Regenerating Explant ** | SFC *** | Average Shoot Length (cm) ** |
|---|---|---|---|---|---|
| Regeneration on semi-solid MS medium | |||||
| NC7 | Semi-solid medium | 13.3 c | 2.5 ± 0.7 d | 0.33 | 0.96 ± 0.12 a |
| 7 × 77 | Semi-solid medium | 10.0 c | 2.2 ± 0.9 d | 0.22 | 0.73 ± 0.25 a |
| Com74 | Semi-solid medium | 16.6 c | 3.1 ± 0.3 c | 0.51 | 0.62 ± 0.09 ab |
| Regeneration in liquid medium (TIS; 16 min/16 h, corresponding to 24 min daily immersion) * | |||||
| NC7 | 16 min/16 h, 24 min daily immersion | 33.3 b | 1.3 ± 0.16 e | 0.43 | 0.78 ± 0.06 a |
| 7 × 77 | 16 min/16 h, 24 min daily immersion | 76.6 a | 5.9 ± 0.44 b | 4.52 | 0.72 ± 0.06 a |
| Com74 | 16 min/16 h, 24 min daily immersion | 46.6 b | 4.9 ± 0.68 b | 2.28 | 0.35 ± 0.03 b |
| Regeneration in liquid medium (TIS; 16 min/24 h, corresponding to 16 min daily immersion) * | |||||
| NC7 | 16 min/24 h, 16 min daily immersion | 40.0 b | 7.0 ± 1.1 a | 2.80 | 0.71 ± 0.12 a |
| 7 × 77 | 16 min/24 h, 16 min daily immersion | 23.3 bc | 3.7 ± 0.3 c | 0.86 | 0.40 ± 0.02 b |
| Com74 | 16 min/24 h, 16 min daily immersion | 30.0 b | 3.3 ± 0.2 c | 0.99 | 0.43 ± 0.03 b |
| Origin of Multiplication | Variety | % of Rooting Shoots * | Average Root Number ** | Acclimatization (%) * | |
|---|---|---|---|---|---|
| TIS | Virginia | 82 a | 10.7± 0.37 a | Virginia | 87 a |
| NC7 | 84 a | 8.5 ± 0.41 a | |||
| 7 × 77 | 75 ab | 9.2 ± 0.37 a | NC7 | 84 a | |
| Com74 | 71 ab | 6.0 ± 0.21 b | |||
| Semi-solid media | Virginia | 78 a | 9.5 ± 0.67 a | 7 × 77 | 78 a |
| NC7 | 67 ab | 6.4 ± 0.34 b | |||
| 7 × 77 | 58 b | 9.1 ± 0.67 a | Com74 | 81 a | |
| Com74 | 61 b | 6.5 ± 0,37 b | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozudogru, E.A.; Karlik, E.; Elazab, D.; Lambardi, M. Establishment of Direct Organogenesis Protocol for Arachis hypogaea cv. Virginia in Liquid Medium by Temporary Immersion System (TIS). Horticulturae 2022, 8, 1129. https://doi.org/10.3390/horticulturae8121129
Ozudogru EA, Karlik E, Elazab D, Lambardi M. Establishment of Direct Organogenesis Protocol for Arachis hypogaea cv. Virginia in Liquid Medium by Temporary Immersion System (TIS). Horticulturae. 2022; 8(12):1129. https://doi.org/10.3390/horticulturae8121129
Chicago/Turabian StyleOzudogru, Elif Aylin, Elif Karlik, Doaa Elazab, and Maurizio Lambardi. 2022. "Establishment of Direct Organogenesis Protocol for Arachis hypogaea cv. Virginia in Liquid Medium by Temporary Immersion System (TIS)" Horticulturae 8, no. 12: 1129. https://doi.org/10.3390/horticulturae8121129
APA StyleOzudogru, E. A., Karlik, E., Elazab, D., & Lambardi, M. (2022). Establishment of Direct Organogenesis Protocol for Arachis hypogaea cv. Virginia in Liquid Medium by Temporary Immersion System (TIS). Horticulturae, 8(12), 1129. https://doi.org/10.3390/horticulturae8121129










