Post-Bloom CPPU Application Is Effective at Improving Fruit Set and Suppressing Coloration but Ineffective at Increasing Fruit Size in Litchi
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Treatments
2.2. Methods
2.3. CPPU Residue Analysis
2.4. Statistics
3. Results and Analysis
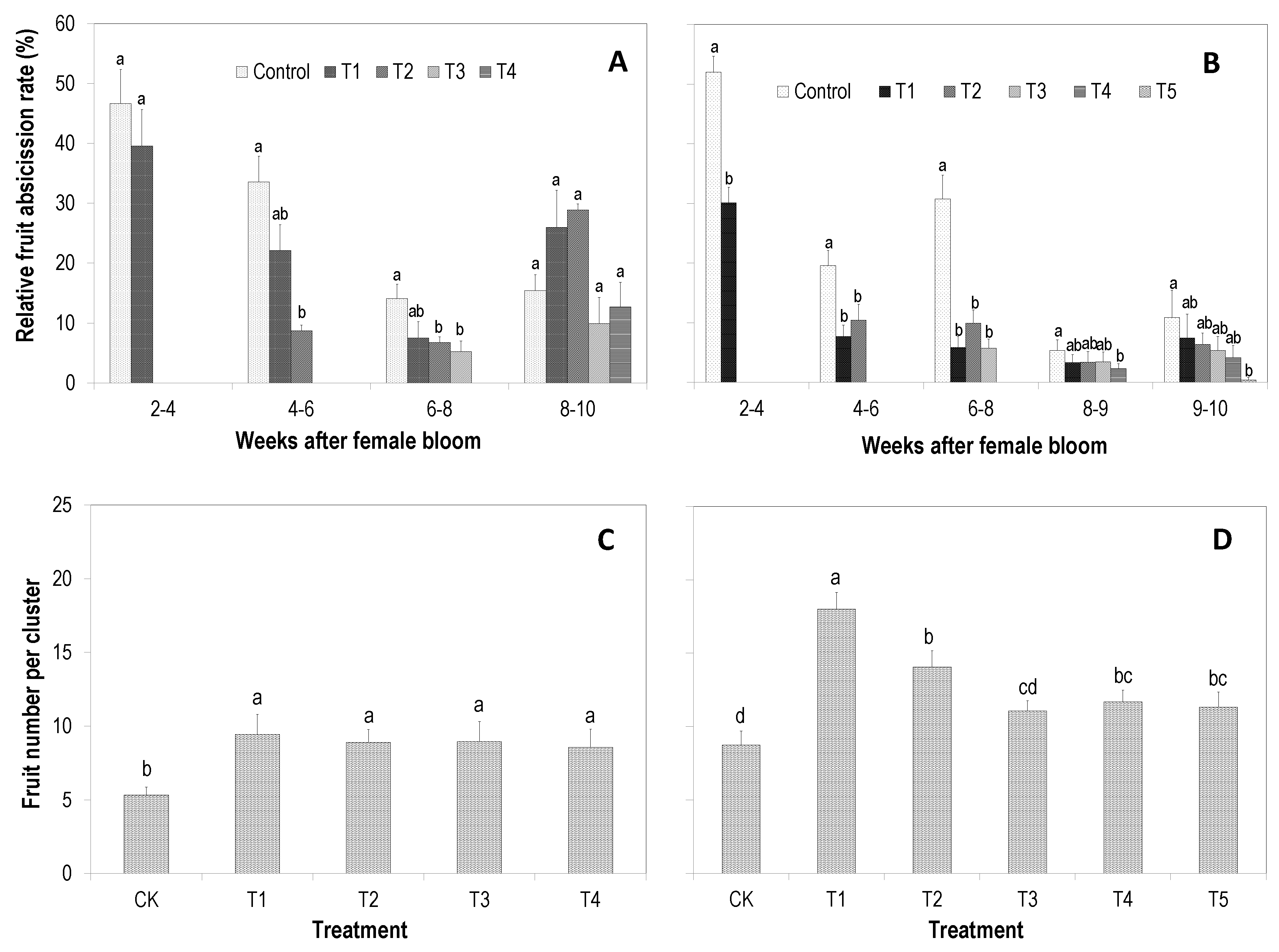
3.1. Effect on Relative Fruit Drop in Different Periods
3.2. Effect on Appearance and Quality at Harvest
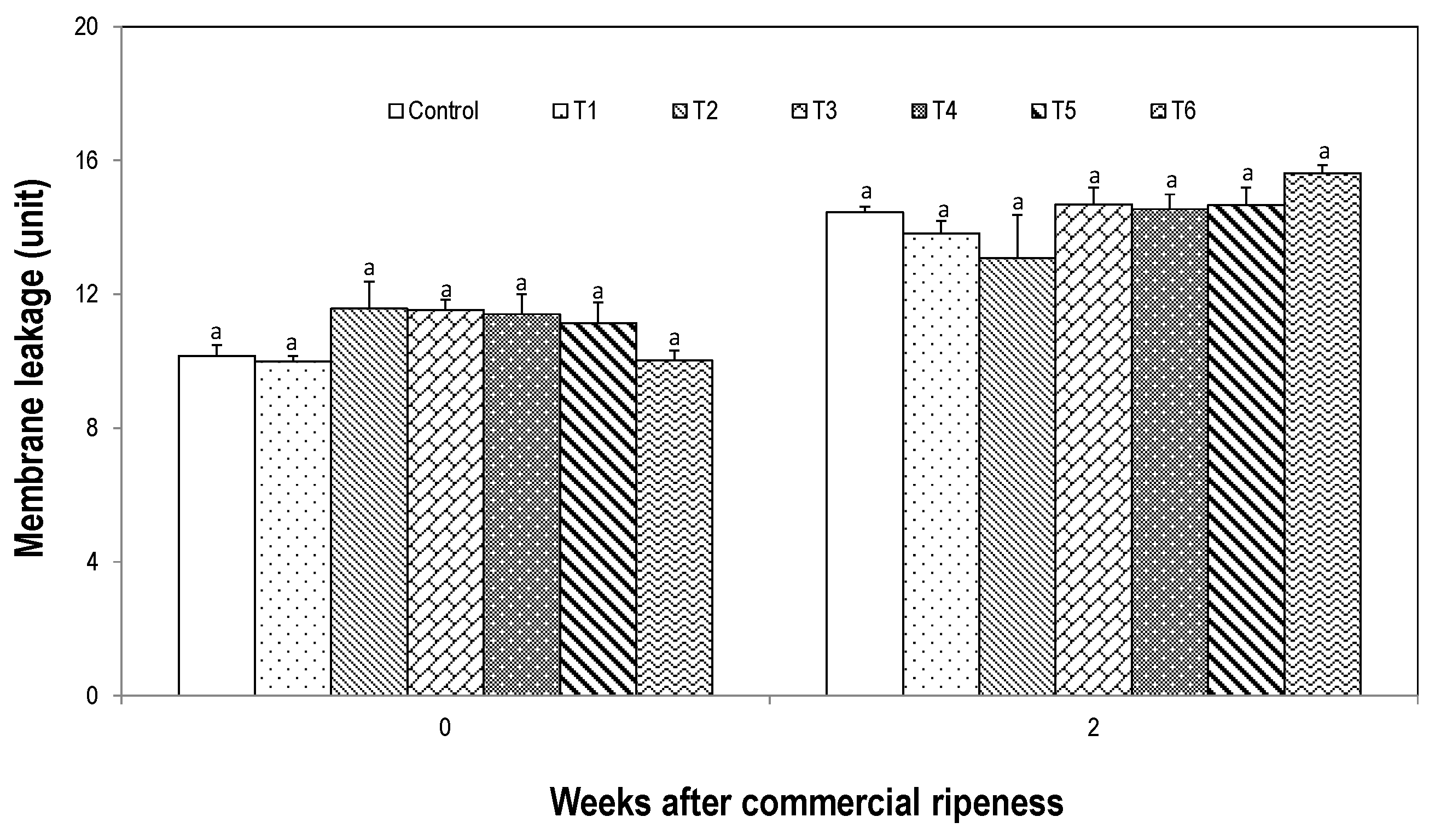
3.3. Effect on Quality and Membrane Leakage in Overripe Fruit
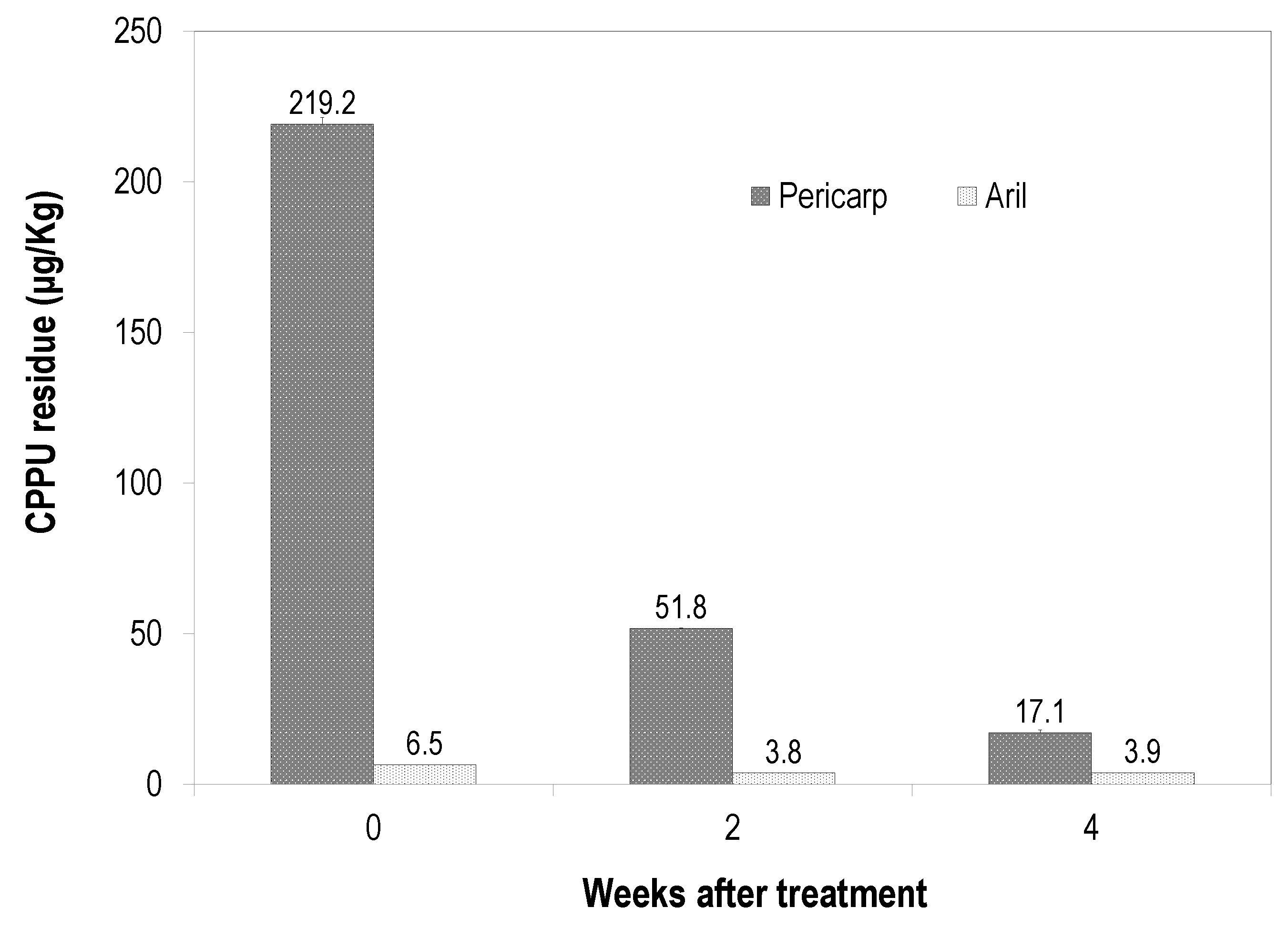
3.4. Dynamics of CPPU Residue in the Pericarp and Aril
4. Discussion
4.1. CPPU Increases Fruit Set but Not Fruit Size in Litchi
4.2. CPPU Treatments Strongly Suppressed Fruit Coloration but Had No Effect on TSS Accumulation and Maintenance
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Gillaspy, G.; Ben-David, H.; Gruissem, W. Fruits: A developmental perspective. Plant Cell 1993, 5, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, Y. A matter of size: Developmental control of organ size in plants. Curr. Opin. Plant Biol. 2001, 4, 533–539. [Google Scholar] [CrossRef]
- Li, J.; Huang, X.; Huang, H. An overview of factors related to fruit size in litchi chinensis Sonn. Acta Hortic. 2010, 863, 477–482. [Google Scholar] [CrossRef]
- Li, Y.-H.; Zhang, Z.; Sun, G.-M. Changes in cell number and cell size during pineapple (Ananas comosus L.) fruit development and their relationship with fruit size. Aust. J. Bot. 2010, 58, 673–678. [Google Scholar] [CrossRef]
- Antognozzi, E.; Battistelli, A.; Famiani, F.; Moscatello, S.; Stănică, F.; Tombesi, A. Influence of CPPU on carbohydrate accumulation and metabolism in fruits of Actinidia deliciosa (A. Chev.). Sci. Hortic. 1996, 65, 37–47. [Google Scholar] [CrossRef]
- Yu, J.Q.; Li, Y.; Qian, Y.R.; Zhu, Z.J. Changes of endogenous hormone level in pollinated and N-(2-chloropyridyl)-N′-phenylurea (CPPU)-induced parthenocarpic fruits of Lagenaria leucantha. J. Hortic. Sci. Biotechnol. 2001, 76, 231–234. [Google Scholar]
- Flaishman, M.A.; Shargal, A.; Stern, R.A. The synthetic cytokinin CPPU increases fruit size and yield of ‘Spadona’ and ‘Costia’ pear (Pyrus communis L.). J. Hortic. Sci. Biotechnol. 2001, 76, 145–149. [Google Scholar] [CrossRef]
- Zabadal, T.J.; Bukovac, M.J. Effect of CPPU on Fruit Development of Selected Seedless and Seeded Grape Cultivars. HortScience 2006, 41, 154–157. [Google Scholar] [CrossRef]
- Cai, L.; Huang, Y.; Xiao, P.; Zhang, M. Effects of CPPU on fruit size, quality and maturation time in ‘Fujiminori’ grape. Hubei Agric. Sci. 1996, 3, 38–41. [Google Scholar]
- Greene, D.W. CPPU thins and influence fruit quality of Mcintosh apples. Hortscience 1991, 26, 686. [Google Scholar]
- Curry, E.A.; Greene, D.W. CPPU influences fruit quality, fruit set, return bloom and preharvest drop of apples. HortScience 1993, 28, 115–119. [Google Scholar] [CrossRef]
- Stern, R.A.; Ben-Arie, R.; Neria, O.; Flaishman, M. CPPU and BA increase fruit size of ‘Royal Gala’ (Malus domestica) apple in a warm climate. J. Hortic. Sci. Biotechnol. 2003, 78, 297–302. [Google Scholar] [CrossRef]
- Kano, Y. Effects of CPPU treatment on fruit and rind development of watermelons (Citrullus lanatus Matsum. et Nakai). J. Hortic. Sci. Biotechnol. 2000, 75, 651–654. [Google Scholar] [CrossRef]
- Kano, Y. Effects of GA and CPPU treatments on cell size and types of sugars accumulated in Japanese pear fruit. J. Hortic. Sci. Biotechnol. 2003, 78, 331–334. [Google Scholar] [CrossRef]
- Hamada, K.; Hasegawa, K.; Ogata, T. Effects of CPPU and strapping on fruit size and maturity in ‘Hiratanenashi’ Japanese persimmon. J. Hortic. Sci. Biotechnol. 2008, 83, 477–480. [Google Scholar] [CrossRef]
- Peppi, M.C.; Fidelibus, M.W. Effects of forchlorfenuron and abscisic acid on the quality of ‘Flame Seedless’ grapes. HortScience 2008, 43, 173–176. [Google Scholar] [CrossRef]
- Maoz, I.; Bahar, A.; Kaplunov, T.; Zutchi, Y.; Daus, A.; Lurie, S.; Lichter, A. Effect of the Cytokinin Forchlorfenuron on Tannin Content of Thompson Seedless Table Grapes. Am. J. Enol. Vitic. 2014, 65, 230–237. [Google Scholar] [CrossRef]
- Nesmith, D.S. Response of Rabbiteye Blueberry (Vaccinium ashei Reade) to the Growth Regulators CPPU and Gibberellic Acid. HortScience 2002, 37, 666–668. [Google Scholar] [CrossRef]
- Zhuang, L.J.; Qiu, Z.H. Status of litchi industry in China in 2019 and policy suggestions. South China Fruits 2021, 50, 184–188. (In Chinese) [Google Scholar]
- Stern, R.A.; Gazit, S. The Reproductive Biology of the Lychee. Hortic. Rev. 2002, 28, 393–453. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, Q.; Duan, Y.; Zhang, X.; Zhang, L. Usage and residues of plant growth regulators on litchi. Chin. J. Trop. Agric. 2019, 39, 67–75. [Google Scholar]
- Stern, R.A.; Nerya, O.; Ben-Arie, R. The cytokinin CPPU delays maturity in litchi cv. ‘Mauritius’ and extends storage-life. J. Hortic. Sci. Biotechnol. 2006, 81, 158–162. [Google Scholar] [CrossRef]
- Fahima, A.; Levinkron, S.; Maytal, Y.; Hugger, A.; Lax, I.; Huang, X.; Eyal, Y.; Lichter, A.; Goren, M.; Stern, R.A.; et al. Cytokinin treatment modifies litchi fruit pericarp anatomy leading to reduced susceptibility to post-harvest pericarp browning. Plant Sci. 2019, 283, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.S.; Luo, Y.C.; Wang, S.W.; Wang, H.C.; Harpaz-Saad, S.; Huang, X.M. Residue analysis and the Effect of preharvest forchlorfenuron (CPPU) application on on-tree quality maintenance of ripe fruit in ‘Feizixiao’ litchi (Litchi chinensis Sonn.). Front. Plant Sci. 2022, 13, 829635. [Google Scholar] [CrossRef]
- Greene, D.W. CPPU Influences Fruit Quality and Fruit Abscission of ‘McIntosh’ Apples. HortScience 2001, 36, 1292–1295. [Google Scholar] [CrossRef]
- Huang, X.-M.; Wang, H.-C.; Yuan, W.-Q.; Lu, J.-M.; Yin, J.-H.; Luo, S.; Huang, H.-B. A study of rapid senescence of detached litchi: Roles of water loss and calcium. Postharvest Biol. Technol. 2005, 36, 177–189. [Google Scholar] [CrossRef]
- Maroto, J.V.; Miguel, A.; Lopez-Galarza, S.; San Bautista, A.; Pascual, B.; Alagarda, J.; Guardiola, J.L. Parthenocarpic fruit set in triploid watermelon induced by CPPU and 2,4-D application. Plant Growth Regul. 2005, 45, 209–213. [Google Scholar] [CrossRef]
- Hayata, Y.; Niimi, Y.; Inoue, K.; Kondo, S. CPPU and BA, with and without pollination affect set, growth and quality of muskmelon (Cucumis melo L.). HortScience 2002, 35, 868–870. [Google Scholar] [CrossRef]
- Sabir, I.A.; Liu, X.; Jiu, S.; Whiting, M.; Zhang, C. Plant growth regulators modify fruit set, fruit quality, and return bloom in sweet cherry. HortScience 2021, 56, 922–931. [Google Scholar] [CrossRef]
- Cong, L.; Wu, T.; Liu, H.; Wang, H.; Zhang, H.; Zhao, G.; Wen, Y.; Shi, Q.; Xu, L.; Wang, Z. CPPU may induce gibberellin-independent parthenocarpy associated with PbRR9 in ‘Dangshansu’ pear. Hortic. Res. 2020, 7, 1993–2005. [Google Scholar] [CrossRef]
- Qian, C.; Ren, N.; Wang, J.; Xu, Q.; Chen, X.; Qi, X. Effects of exogenous application of CPPU, NAA and GA4+7 on parthenocarpy and fruit quality in cucumber (Cucumis sativus L.). Food Chem. 2018, 243, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, L.; Mostafa, Y.; El-Berry, I. Effect of NAA and CPPU on fruit drop, yield and quality of avocado trees. Egypt. J. Hortic. 2020, 47, 137–147. [Google Scholar] [CrossRef]
- Fujisawa, H.; Kawai, Y.; Ishikawa, K. Effect of GA and CPPU application on fruit set and fruit characteristics of northern highbush blueberries. J. Agric. Sci. Tokyo Nogyo Daigaku 2011, 56, 118–124. [Google Scholar]
- Huang, H.; Xu, J. The developmental patterns of fruit tissues and their correlative relationships in Litchi chinensis Sonn. Sci. Hortic. 1983, 19, 335–342. [Google Scholar] [CrossRef]
- Lötter, J.D.V. A study of the preharvest ripening of Hayward kiwifruit and how it is altered by N-(2-Chloro-4-pyridyl)-N-phenylurea (CPPU). Acta Hortic. 1992, 297, 357–366. [Google Scholar] [CrossRef]
- Wei, Y.-Z.; Hu, F.-C.; Hu, G.-B.; Li, X.-J.; Huang, X.-M.; Wang, H.-C. Differential expression of anthocyanin biosynthetic gGenes in relation to anthocyanin accumulation in the pericarp of Litchi chinensis Sonn. PLoS ONE 2011, 6, e19455. [Google Scholar] [CrossRef]
- Hu, B.; Li, J.; Wang, D.; Wang, H.; Qin, Y.; Hu, G.; Zhao, J. Transcriptome profiling of Litchi chinensis pericarp in response to exogenous cytokinins and abscisic acid. Plant Growth Regul. 2017, 84, 437–450. [Google Scholar] [CrossRef]
- Lai, B.; Li, X.-J.; Hu, B.; Qin, Y.-H.; Huang, X.-M.; Wang, H.-C.; Hu, G.-B. LcMYB1 Is a key determinant of differential anthocyanin accumulation among genotypes, tissues, developmental phases and ABA and light stimuli in Litchi chinensis. PLoS ONE 2014, 9, e86293. [Google Scholar] [CrossRef]
- Wang, H.; Huang, H.; Huang, X. Differential effects of abscisic acid and ethylene on the fruit maturation of Litchi chinensis Sonn. Plant Growth Regul. 2007, 52, 189–198. [Google Scholar] [CrossRef]
- Pesis, E.; Dvir, O.; Feygenberg, O.; Ben Arie, R.; Ackerman, M.; Lichter, A. Production of acetaldehyde and ethanol during maturation and modified atmosphere storage of litchi fruit. Postharvest Biol. Technol. 2002, 26, 157–165. [Google Scholar] [CrossRef]


| Treatment | Fruit Weight (g) | Pericarp Weight (g) | Flesh Weight (g) | Flesh Recovery (%) | TSS (%) | Color Parameters | ||
|---|---|---|---|---|---|---|---|---|
| a | b | L | ||||||
| Control | 26.4 ± 1.45 ab | 4.82 ± 0.30 ab | 20.7 ± 1.07 ab | 78.7 ± 0.50 a | 17.1 ± 0.31 a | 4.4 ± 0.91 a | 18.3 ± 0.53 b | 40.8 ± 0.90 b |
| T1 | 24.5 ± 1.08 b | 4.71 ± 0.17 b | 19.0 ± 0.81 b | 77.5 ± 0.26 a | 16.8 ± 0.22 a | 2.5 ± 1.35 a | 18.8 ± 0.58 b | 41.2 ± 0.87 b |
| T2 | 27.2 ± 1.71 ab | 5.52 ± 0.38 a | 20.9 ± 1.33 ab | 76.9 ± 0.51 a | 17.0 ± 0.25 a | 3.9 ± 0.89 b | 21.4 ± 0.40 a | 44.3 ± 0.71 a |
| T3 | 25.6 ± 1.22 ab | 5.18 ± 0.20 a | 19.7 ± 1.11 ab | 76.8 ± 1.02 a | 16.9 ± 0.20 a | −8.0 ± 0.38 c | 22.0 ± 0.29 a | 44.2 ± 0.63 a |
| T4 | 28.1 ± 1.20 a | 5.53 ± 0.29 a | 21.6 ± 0.89 a | 76.8 ± 0.31 a | 17.4 ± 0.33 a | −5.5 ± 0.54 b | 21.7 ± 0.17 a | 44.2 ± 0.41 a |
| Treatment | Fruit Weight (g) | Pericarp Weight (g) | Flesh Weight (g) | Flesh Recovery (%) | TSS (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 11WAFB | 13 WAFB | 11WAFB | 13WAFB | 11WAFB | 13WAFB | 11WAFB | 13WAFB | 11WAFB | 13WAFB | |
| Control | 25.1 ± 0.56 a | 29.3 ± 0.25 a | 5.00 ± 0.20 b | 5.97 ± 0.04 a | 19.0 ± 0.41 a | 22.4 ± 0.27 a | 75.7 ± 0.84 a | 76.4 ± 0.34 a | 18.3 ± 0.30 a | 17.2 ± 0.35 a |
| T1 | 22.7 ± 0.60 b | 27.8 ± 0.33 b | 5.26 ± 0.29 ab | 6.40 ± 0.46 a | 16.4 ± 0.54 b | 20.3 ± 0.18 b | 72.2 ± 1.12 a | 73.2 ± 0.37 b | 18.5 ± 0.22 a | 16.7 ± 0.37 a |
| T2 | 24.2 ± 0.81 ab | 28.6 ± 0.80 ab | 5.52 ± 0.13 a | 6.07 ± 0.27 a | 17.9 ± 0.57 ab | 21.7 ± 0.62 ab | 73.8 ± 0.29 a | 76.1 ± 0.64 a | 18.1 ± 0.29 a | 16.7 ± 0.18 a |
| T3 | 25.6 ± 0.50 a | 30.6 ± 1.10 a | 5.08 ± 0.18 b | 6.38 ± 0.23 a | 19.3 ± 0.28 a | 23.3 ± 0.98 a | 75.4 ± 0.68 a | 76.1 ± 0.73 a | 18.1 ± 0.45 a | 16.5 ± 0.29 a |
| T4 | 24.8 ± 0.36 ab | 29.2 ± 0.75 ab | 5.27 ± 0.17 ab | 6.16 ± 0.21 a | 18.8 ± 0.25 a | 22.0 ± 0.60 a | 75.9 ± 0.12 a | 75.5 ± 0.25 a | 18.3 ± 0.39 a | 16.8 ± 0.23 a |
| T5 | 25.3 ± 0.91 a | 30.0 ± 0.72 a | 5.27 ± 0.22 ab | 5.93 ± 0.21 a | 18.8 ± 0.91 a | 21.3 ± 0.52 ab | 76.2 ± 0.40 a | 76.1 ± 0.79 a | 18.0 ± 0.20 a | 16.6 ± 0.34 a |
| T6 | 26.2 ± 0.24 a | 29.4 ± 0.60 a | 5.03 ± 0.14 b | 6.11 ± 0.16 a | 19.5 ± 0.20 a | 22.4 ± 0.41 a | 74.5 ± 0.41 a | 76.1 ± 0.26 a | 18.0 ± 0.24 a | 16.5 ± 0.26 a |
| Control | T1 | T2 | T3 | T4 | T5 | T6 | |
|---|---|---|---|---|---|---|---|
| Fruit weight (g) | 4.23 ± 0.60 a | 5.09 ± 0.97 a | 4.18 ± 0.84 a | 4.90 ± 0.96 a | 5.53 ± 1.07 a | 3.63 ± 0.37 a | 3.56 ± 0.66 a |
| TSS (%) | −1.16 ± 0.46 a | −1.80 ± 0.31 a | −1.35 ± 0.19 a | −1.56 ± 0.25 a | −1.24 ± 0.20 a | −1.54 ± 0.26 a | −1.42 ± 0.23 a |
| a value | 16.6 ± 1.75 b | 23.5 ± 2.19 a | 25.2 ± 1.85 a | 25.9 ± 2.72 a | 18.0 ± 2.58 ab | 12.2 ± 1.70 b | 3.29 ± 0.80 c |
| Membrane leakage (unit) | 4.29 ± 0.18 b | 3.83 ± 0.20 bc | 1.51 ± 0.20 d | 3.15 ± 0.24 c | 3.13 ± 0.24 c | 3.53 ± 0.19 bc | 5.6 ± 0.08 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Luo, Y.; Wang, H.; Huang, X. Post-Bloom CPPU Application Is Effective at Improving Fruit Set and Suppressing Coloration but Ineffective at Increasing Fruit Size in Litchi. Horticulturae 2022, 8, 1096. https://doi.org/10.3390/horticulturae8121096
Liu X, Luo Y, Wang H, Huang X. Post-Bloom CPPU Application Is Effective at Improving Fruit Set and Suppressing Coloration but Ineffective at Increasing Fruit Size in Litchi. Horticulturae. 2022; 8(12):1096. https://doi.org/10.3390/horticulturae8121096
Chicago/Turabian StyleLiu, Xinsheng, Yecheng Luo, Huicong Wang, and Xuming Huang. 2022. "Post-Bloom CPPU Application Is Effective at Improving Fruit Set and Suppressing Coloration but Ineffective at Increasing Fruit Size in Litchi" Horticulturae 8, no. 12: 1096. https://doi.org/10.3390/horticulturae8121096
APA StyleLiu, X., Luo, Y., Wang, H., & Huang, X. (2022). Post-Bloom CPPU Application Is Effective at Improving Fruit Set and Suppressing Coloration but Ineffective at Increasing Fruit Size in Litchi. Horticulturae, 8(12), 1096. https://doi.org/10.3390/horticulturae8121096







