Prevention and Control of Fusarium spp., the Causal Agents of Onion (Allium cepa) Basal Rot
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungicides Culture Plates Assay
2.2. Examination of Selected Pesticides in Pots under Field Conditions throughout a Full Growing Season
2.2.1. Growth Protocol and Conditions
2.2.2. Important Dates and Meteorological Data
2.2.3. Complementary Inoculation
2.2.4. Pesticide Treatments
2.2.5. Growth and Disease Estimation
2.3. Statistical Analysis
3. Results
3.1. Fungicides Culture Plates Assay
3.2. Examination of Selected Pesticides in Pots under Field Conditions throughout a Full Growing Season
3.2.1. Impact of the Treatments on the Plants’ Soil Surface Germination and Survival
3.2.2. Impact of the Treatments at the Mid-Season Sampling (Day 65)
3.2.3. Impact of the Treatments at the Season End (Day 115)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lebiush-Mordechai, S.; Erlich, O.; Maymon, M.; Freeman, S.; Ben-David, T.; Ofek, T.; Palevsky, E.; Tsror, L. Bulb and Root Rot in Lily (Lilium longiflorum) and Onion (Allium cepa) in Israel. J. Phytopathol. 2014, 162, 466–471. [Google Scholar] [CrossRef]
- Kalman, B.; Abraham, D.; Graph, S.; Perl-Treves, R.; Harel, Y.M.; Degani, O. Isolation and Identification of Fusarium spp., the Causal Agents of Onion (Allium cepa) Basal Rot in Northeastern Israel. Biology 2020, 9, 69. [Google Scholar] [CrossRef]
- Rabinowitch, H.D. Breeding alliaceous crops for pest resistance. Acta Hortic. 1994, 433, 223–246. [Google Scholar] [CrossRef]
- Esfahani, M.N. Genetic and virulence variation in Fusarium oxysporum f. sp. cepae causing root and basal rot of common onion in Iran. J. Phytopathol. 2018, 166, 572–580. [Google Scholar] [CrossRef]
- Lacy, M.; Roberts, D. Yields of onion cultivars in Midwestern organic soils infested with Fusarium oxysporum f. sp. cepae and Pyrenochaeta terrestris. Plant Dis. 1982, 66, 1003–1006. [Google Scholar] [CrossRef]
- Burgess, L.W.; Liddell, C.M.; Summerell, B.A. Laboratory Manual for Fusarium Research: Incorporating a Key and Descriptions of Common Species Found in Australasia; Dept. of Plant Pathology and Agricultural Entomology, University of Sydney: Sydney, Australasia, 1988. [Google Scholar]
- Summerell, B.A.; Leslie, J.F.; Liew, E.C.; Laurence, M.H.; Bullock, S.; Petrovic, T.; Bentley, A.R.; Howard, C.G.; Peterson, S.A.; Walsh, J.L. Fusarium species associated with plants in Australia. Fungal Divers. 2011, 46, 1–27. [Google Scholar] [CrossRef]
- Le, D.; Audenaert, K.; Haesaert, G. Fusarium basal rot: Profile of an increasingly important disease in Allium spp. Trop. Plant Pathol. 2021, 46, 241–253. [Google Scholar] [CrossRef]
- Sumner, D.R.; Gitaitis, R.D.; Gay, J.D.; Smittle, D.A.; Maw, B.W.; Tollner, E.W.; Hung, Y.C. Control of soilborne pathogenic fungi in fields of sweet onion. Plant Dis. 1997, 81, 885–891. [Google Scholar] [CrossRef]
- Stankovic, S.; Levic, J.; Petrovic, T.; Logrieco, A.; Moretti, A. Pathogenicity and mycotoxin production by Fusarium proliferatum isolated from onion and garlic in Serbia. Eur. J. Plant Pathol. 2007, 118, 165–172. [Google Scholar] [CrossRef]
- Gamliel, A.; Gillett, D.; Minkovsky, N.; Benikhis, M.; Dobrynin, S. Fusarium Proliferatum Disease Outburst in White Onions from Different Fields in the Southern Israel Arava Area. 2013. Available online: http://en.agri.arava.co.il/about (accessed on 20 September 2022).
- Sintayehu, A.; Sakhuja, P.; Fininsa, C.; Ahmed, S. Management of Fusarium basal rot (Fusarium oxysporum f. sp. cepae) on shallot through fungicidal bulb treatment. Crop Prot. 2011, 30, 560–565. [Google Scholar] [CrossRef]
- Leadbeater, A. Plant Health Management: Fungicides and Antibiotics; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Özer, N.; Köycü, N.; Mirik, M.; Soran, H.; Boyraz, D. Effect of some organic amendments on onion bulb rot. Phytoparasitica 2002, 30, 429–433. [Google Scholar] [CrossRef]
- Degani, O.; Kalman, B. Assessment of Commercial Fungicides against Onion (Allium cepa) Basal Rot Disease Caused by Fusarium oxysporum f. sp. cepae and Fusarium acutatum. J. Fungi 2021, 7, 235. [Google Scholar] [CrossRef]
- Behrani, G.; Syed, R.; Abro, M.; Jiskani, M.; Khanzada, M. Pathogenicity and chemical control of basal rot of onion caused by Fusarium oxysporum f. sp. cepae. Pak. J. Agric. Agric. Eng. Vet. Sci. 2015, 31, 60–70. [Google Scholar]
- Rajamohan, K.; Udhayakumar, R.; Sanjaygandhi, S.; Vengadesh Kumar, L.; Thamarai Selvi, M.; Sudhasha, S.; Yuvarani, R.J. Management of basal rot of onion caused by Fusarium oxysporum f. sp. cepae using bioregulators. J. Biopest. 2019, 12, 239–247. [Google Scholar]
- Degani, O.; Cernica, G. Diagnosis and Control of Harpophora maydis, the Cause of Late Wilt in Maize. Adv. Microbiol. 2014, 4, 94–105. [Google Scholar] [CrossRef]
- Broders, K.; Lipps, P.; Paul, P.; Dorrance, A. Evaluation of Fusarium graminearum associated with corn and soybean seed and seedling disease in Ohio. Plant Dis. 2007, 91, 1155–1160. [Google Scholar] [CrossRef]
- Degani, O.; Gordani, A.; Becher, P.; Chen, A.; Rabinovitz, O. Crop rotation and minimal tillage selectively affect maize growth promotion under late wilt disease stress. J. Fungi 2022, 8, 586. [Google Scholar] [CrossRef]
- Degani, O.; Gordani, A. New Antifungal Compound, 6-Pentyl-alpha;-Pyrone, against the Maize Late Wilt Pathogen, Magnaporthiopsis maydis. Agronomy 2022, 12, 2339. [Google Scholar] [CrossRef]
- Pandit, M.A.; Kumar, J.; Gulati, S.; Bhandari, N.; Mehta, P.; Katyal, R.; Rawat, C.D.; Mishra, V.; Kaur, J. Major Biological Control Strategies for Plant Pathogens. Pathogens 2022, 11, 273. [Google Scholar] [CrossRef]
- Elnahal, A.S.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.-S.M.; El-Tahan, A.M.; Rady, M.M.; AbuQamar, S.F.; El-Tarabily, K.A. The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: A review. Eur. J. Plant Pathol. 2022, 162, 759–792. [Google Scholar] [CrossRef]
- Ab Rahman, S.F.S.; Singh, E.; Pieterse, C.M.; Schenk, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef]
- Richard, B.; Qi, A.; Fitt, B.D. Control of crop diseases through Integrated Crop Management to deliver climate—Smart farming systems for low- and high-input crop production. Plant Pathol. 2022, 71, 187–206. [Google Scholar] [CrossRef]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B.P.A. Combining biocontrol agents with chemical fungicides for integrated plant fungal disease control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef]
- Corkley, I.; Fraaije, B.; Hawkins, N. Fungicide resistance management: Maximizing the effective life of plant protection products. Plant Pathol. 2022, 71, 150–169. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, Q.; Li, N.; Liu, D.; Yuan, Y. Transcriptome analysis of fungicide-responsive gene expression profiles in two Penicillium italicum strains with different response to the sterol demethylation inhibitor (DMI) fungicide prochloraz. BMC Genom. 2020, 21, 156. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, C.X.; Zhai, X.Y.; Jamieson, P.A.; Zhang, C.Q. Mutation in cyp51b and overexpression of cyp51a and cyp51b confer multiple resistant to DMIs fungicide prochloraz in Fusarium fujikuroi. Pest. Manag. Sci. 2021, 77, 824–833. [Google Scholar] [CrossRef]
- Degani, O.; Becher, P.; Gordani, A. Pathogenic interactions between Macrophomina phaseolina and Magnaporthiopsis maydis in mutually infected cotton sprouts. Agriculture 2022, 12, 255. [Google Scholar] [CrossRef]
- Degani, O.; Dor, S.; Abraham, D.; Cohen, R. Interactions between Magnaporthiopsis maydis and Macrophomina phaseolina, the causes of wilt diseases in maize and cotton. Microorganisms 2020, 8, 249. [Google Scholar] [CrossRef]
- Khokhar, M.K.; Hooda, K.S.; Sharma, S.S.; Singh, V. Post flowering stalk rot complex of maize-Present status and future prospects. Maydica 2014, 59, 226–242. [Google Scholar]
- Buxton, E.; Perry, D. Pathogenic interactions between Fusarium oxysporum and Fusarium solani on peas. Trans. Br. Mycol. Soc. 1959, 42, 378–387. [Google Scholar] [CrossRef]
- Willsey, T.; Chatterton, S.; Heynen, M.; Erickson, A. Detection of interactions between the pea root rot pathogens Aphanomyces euteiches and Fusarium spp. using a multiplex qPCR assay. Plant Pathol. 2018, 67, 1912–1923. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Mnayer, D.; Tabanelli, G.; Stojanović-Radić, Z.; Sharifi-Rad, M.; Yousaf, Z.; Vallone, L.; Setzer, W.; Iriti, M. Plants of the genus Allium as antibacterial agents: From tradition to pharmacy. Cell. Mol. Biol. 2016, 62, 57–68. [Google Scholar]
- Demirci, F.; Bayraktar, H.; Babaliogullu, I.; Dolar, F.S.; Maden, S. In vitro and In vivo Effects of Some Fungicides against the Chickpea Blight Pathogen, Ascochyta rabiei. J. Phytopathol. 2003, 151, 519–524. [Google Scholar] [CrossRef]
- Haapalainen, M.; Latvala, S.; Kuivainen, E.; Qiu, Y.; Segerstedt, M.; Hannukkala, A. Fusarium oxysporum, F. proliferatum and F. redolens associated with basal rot of onion in Finland. Plant Pathol. 2016, 65, 1310–1320. [Google Scholar] [CrossRef]
- Dita, M.; Barquero, M.; Heck, D.; Mizubuti, E.S.; Staver, C.P. Fusarium wilt of banana: Current knowledge on epidemiology and research needs toward sustainable disease management. Front. Plant Sci. 2018, 9, 1468. [Google Scholar] [CrossRef]
- Cramer, C.S. Breeding and genetics of Fusarium basal rot resistance in onion. Euphytica 2000, 115, 159–166. [Google Scholar] [CrossRef]
- Taylor, A.; Teakle, G.R.; Walley, P.G.; Finch-Savage, W.E.; Jackson, A.C.; Jones, J.E.; Hand, P.; Thomas, B.; Havey, M.J.; Pink, D.A. Assembly and characterisation of a unique onion diversity set identifies resistance to Fusarium basal rot and improved seedling vigour. Theor. Appl. Genet. 2019, 132, 3245–3264. [Google Scholar] [CrossRef]
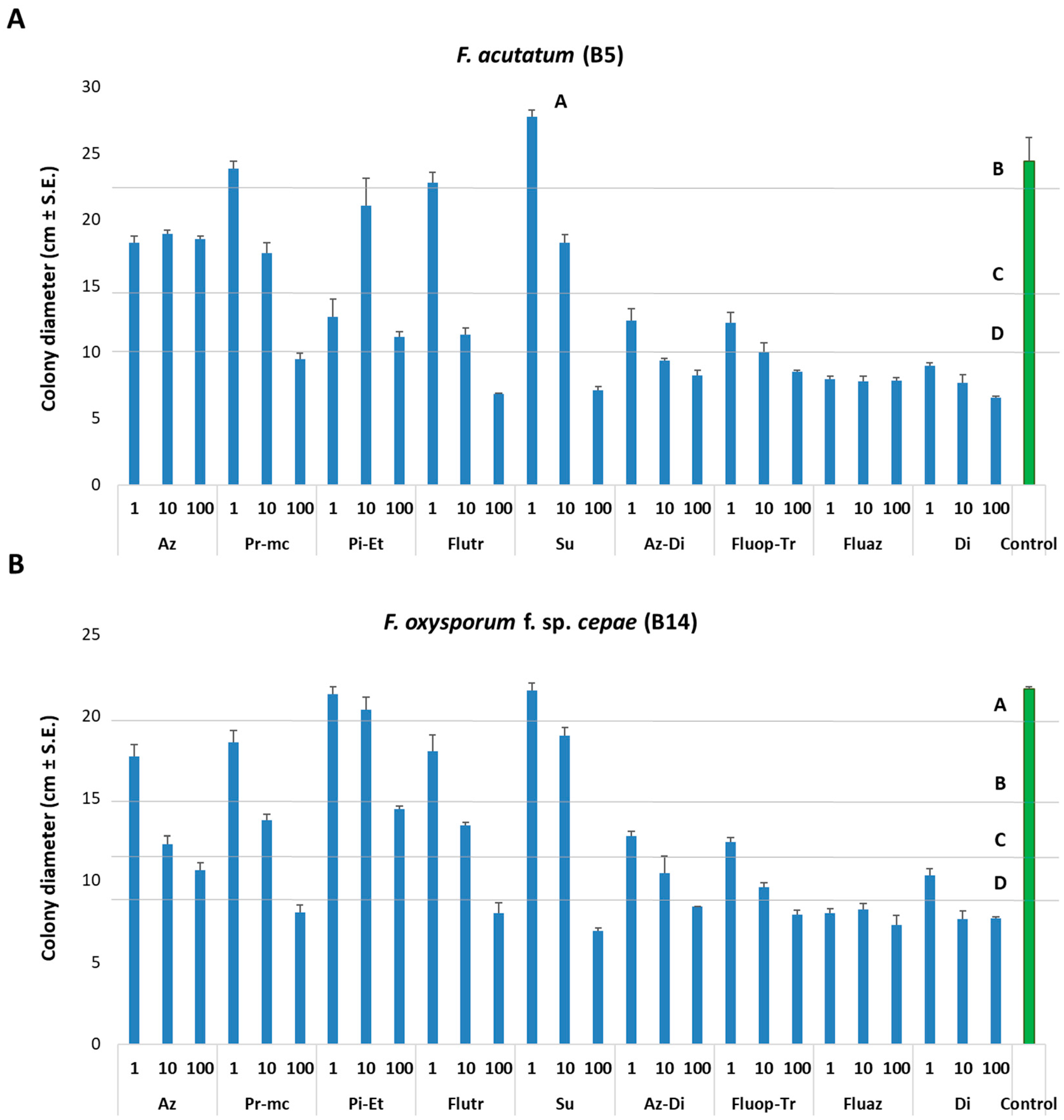



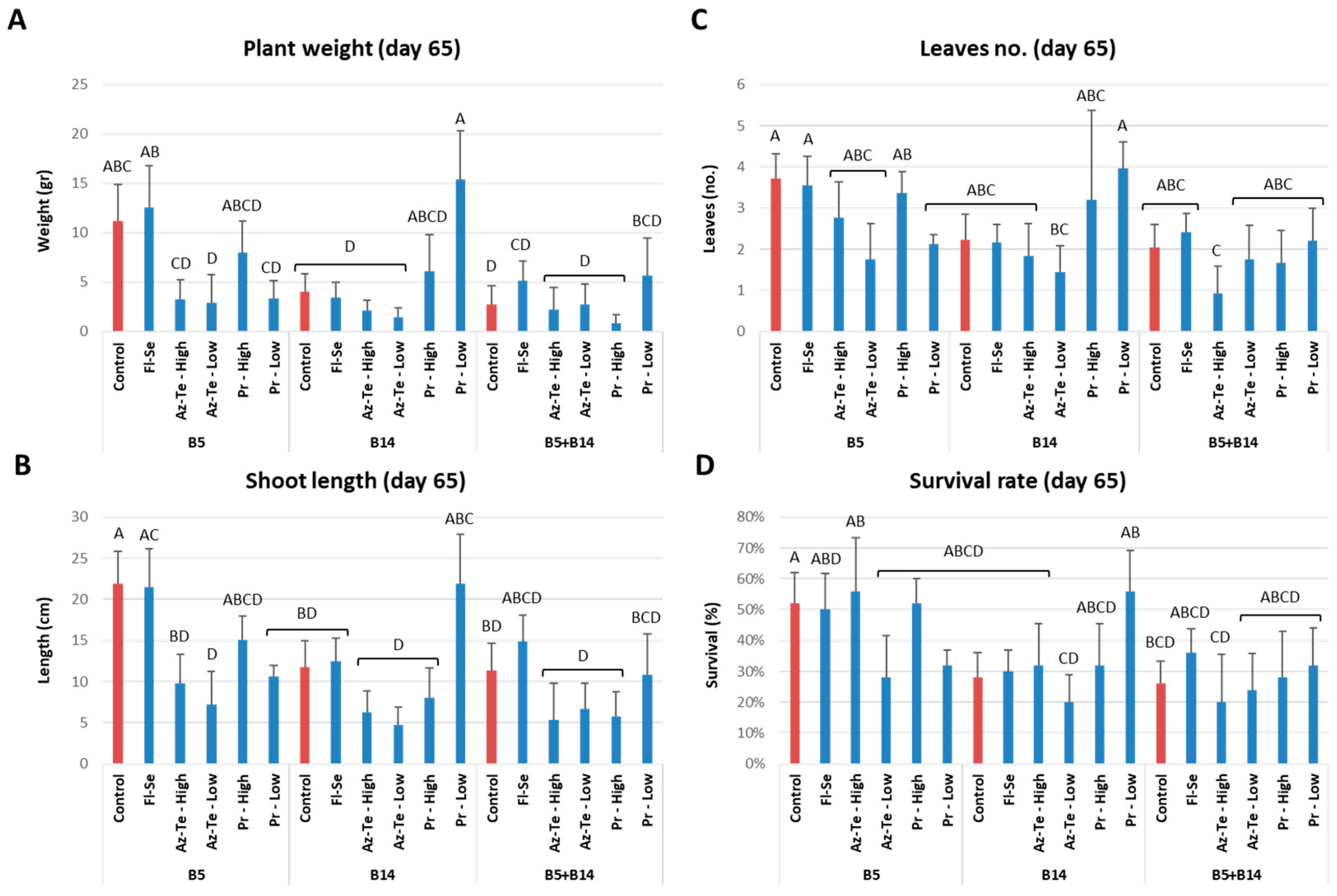
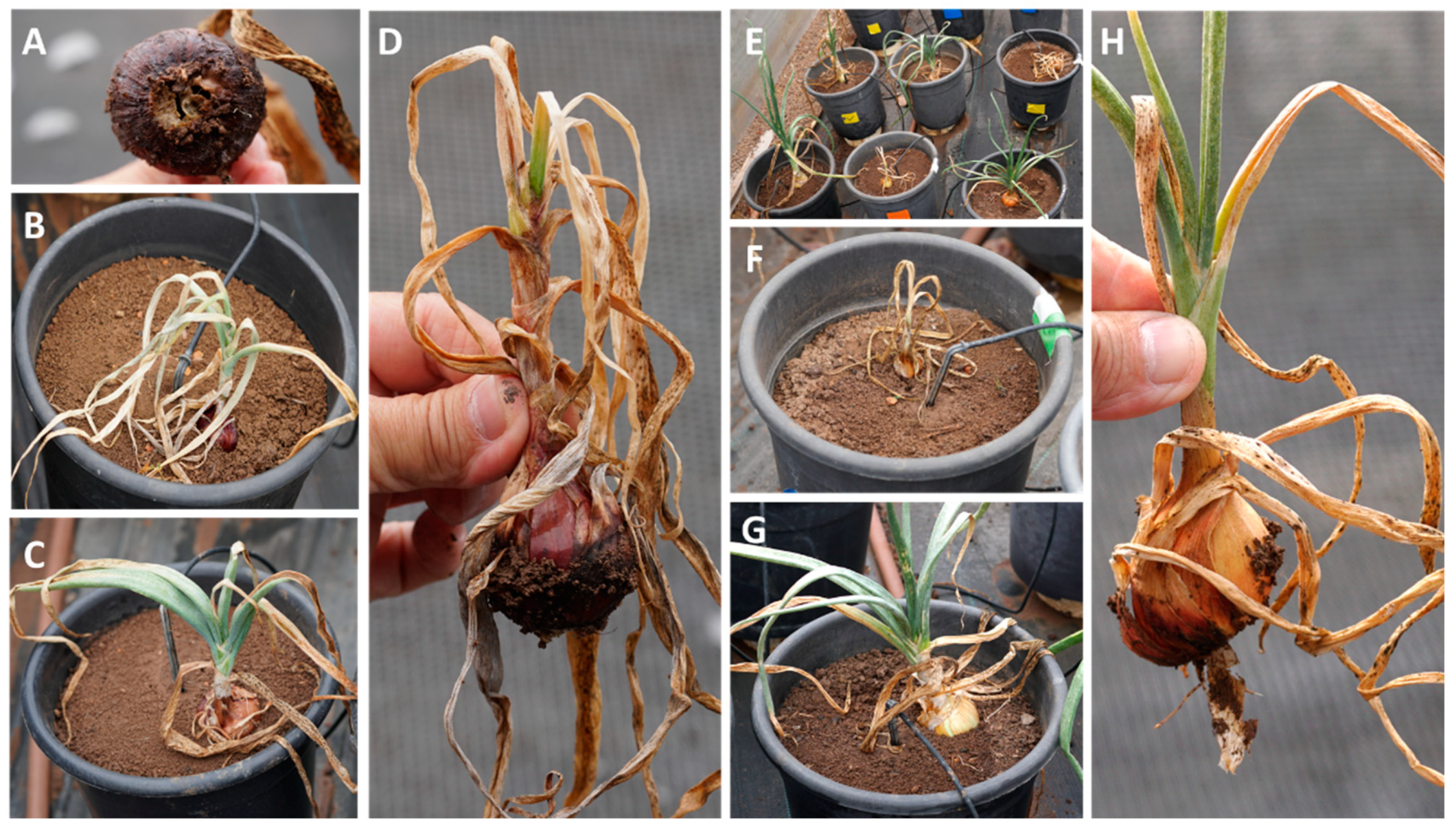
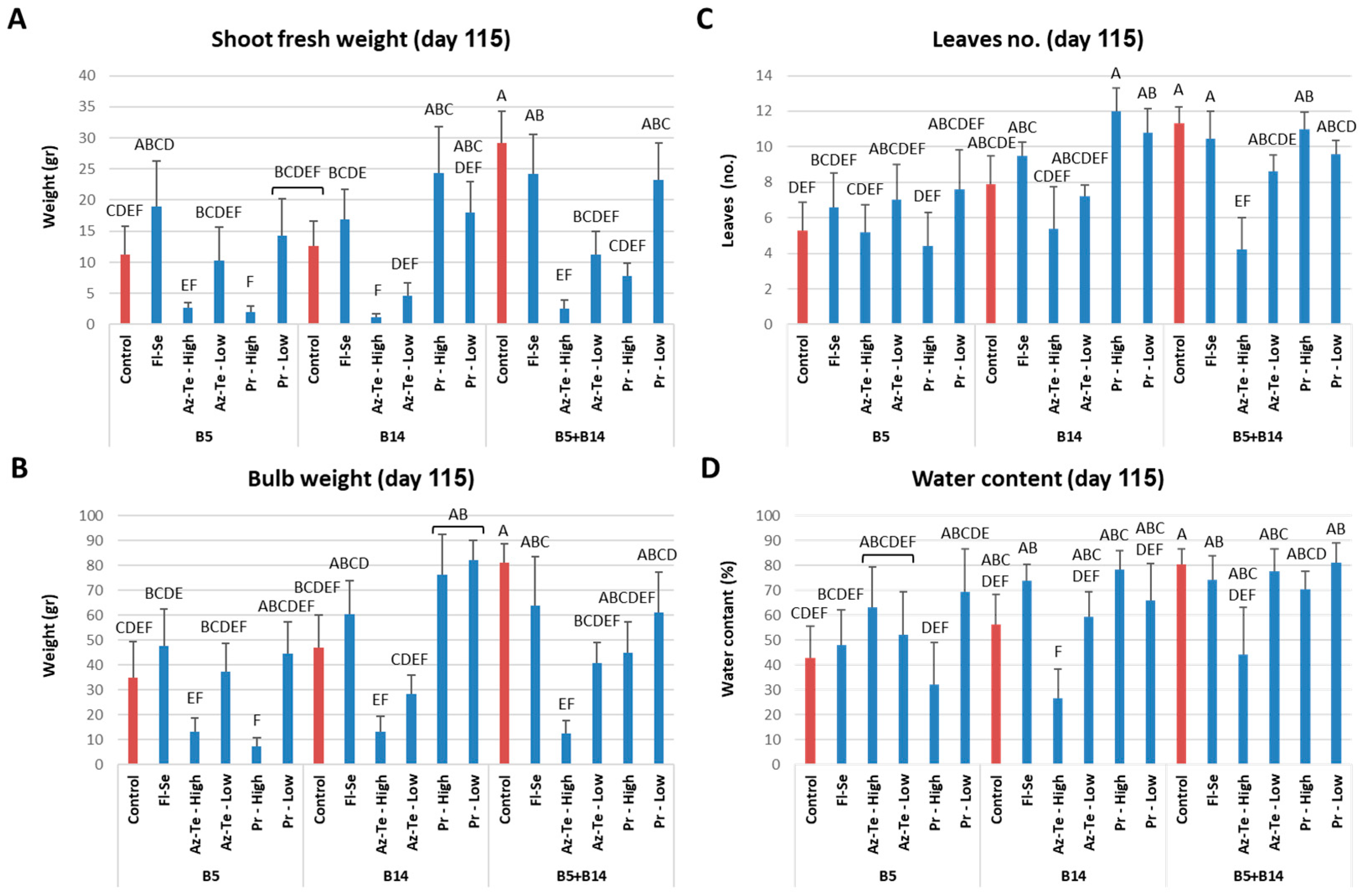

| Fungicide Name and Acronym | Manufacturer Supplier | Active Ingredient (Common Name) | Group Name | Chemical Group | Target Site of Action | AI (g/l) | Test 2 |
|---|---|---|---|---|---|---|---|
| Hosen (Flutr) | Cheminova (Lemvig, Denmark) Makhteshim Agan (Airport City, Israel) | Flutriafol | DMI-fungicides (demethylation inhibitors) | Triazoles | Disrupt C14- demethylation in sterol biosynthesis (erg11/cyp51) | 125 | Plates assay |
| Ortiva top(Az-Di) | Syngenta (Basel, Switzerland) Adama Makhteshim (Airport City, Israel) | Azoxystrobin | QoI-fungicides (quinone outside inhibitors) | Methoxy-acrylates | Respiration C3:cytochrome bc1(ubiquinol oxidase) at Qo site (cyt b gene) | 250 | Plates assay |
| Difenoconazole | DMI-fungicides (DeMethylation Inhibitors, SBI: Class I) | Triazoles | Sterol biosynthesis in membranes G1:C14- demethylase in sterol biosynthesis (erg11/cyp51) | 125 | |||
| Luna sensation (Fluop-Tr) | Bayer CropScience (Monheim am Rhein, Germany) Lidorr Chemicals Ltd. (Ramat Hasharon, Israel) | Fluopyram (Velum) | SDHI (succinate dehydrogenase inhibitors) | Pyridinyl-ethyl-benzamides | Respiration C2: complex II: succinate-dehydrogenase | 250 | Plates assay |
| Trifloxystrobin (Flint) | QoI-fungicides (Quinone outside Inhibitors) | Oximino acetates | Respiration C3: complex III: cytochrome bc1 (ubiquinol oxidase) at Qo site (cyt b gene) | 250 | |||
| Skipper (Di) | Syngenta (Basel, Switzerland) Tapazol Chemical Industries Ltd., (Beit Shemesh, Israel) | Difenoconazole | DMI-fungicides (DeMethylation Inhibitors, SBI: Class I) | Triazoles | Sterol biosynthesis in membranes G1: C14-demethylase in sterol biosynthesis (erg11/cyp51) | 250 | Plates assay |
| Beltanol (Su) | Probelte, S.A.U., Murcia, Spain Gadot Agro (Kidron, Israel) | Sulphate 8-Hydroxyquinoline | Sulphuric acid | 500 | Plates assay | ||
| Octave (Pr-mc) | BASF (Ludwigshafen, Germany) Merhav Agro Ltd. (Herzliah, Israel) | Prochloraz present as the manganese chloride complex | DMI-fungicides (demethylation inhibitors) | Imidazoles | C14-demethylation in sterol biosynthesis (erg11/cyp51) | 500 | Plates assay |
| Terraclor super X (Pi-Et) | Amvac (Los Angeles, CA, USA) Luxembourg Industries Ltd. (Tel Aviv, Israel) | Pintachloronitrobenzene (PCNB) | AH-fungicides (aromatic hydrocarbons) (chlorophenyls, nitroanilines) | Aromatic hydrocarbons | Cell peroxidation (proposed) | 232 | Plates assay |
| Etridiazole | Heteroaromatics | 1,2,4-thiadiazoles | Cell peroxidation (proposed) | 58 | |||
| Ohayo (Fluaz) | Phyteurop (Montreuil-Bellay, France) Luxembourg Industries Ltd. (Tel Aviv, Israel) | Fluazinam | QiI-Quinone inside inhibitors | 2,6-dinitro-anilines | Respiration C5: uncouplers of oxidative phosphorylation | 500 | Plates assay |
| Amistar (Az) | Syngenta (Basel, Switzerland) AdamaMakhteshim (Airport City, Israel) | Azoxystrobin | QoI-fungicides (quinone outside inhibitors) | Methoxy-acrylates | Respiration C3:cytochrome bc1(ubiquinol oxidase) at Qo site (cyt b gene) | 250 | Plates assay |
| Sportak (Pr) | Merhav Agro Ltd. (Herzliah Israel) Makhteshim Agan (Airport City, Israel) | Prochloraz | DMI-fungicides (demethylation inhibitors) | Imidazoles | C14-demethylation in sterol biosynthesis (erg11/cyp51) | 450 | Full Season plants |
| Azimut (Az-Te) | Adama Makhteshim (Be’er Sheva, Israel) | Azoxystrobin 12% | QoI-fungicides (quinone outside inhibitors) | Methoxy-acrylates | Respiration C3: cytochrome bc1 (ubiquinol oxidase) at Qo site (cyt b gene) | 120 | Full Season plants |
| Tebuconazole 20% | DMI-fungicides (DeMethylation Inhibitors) (SBI: Class I) | Triazoles | C14-demethylase in sterol biosynthesis (erg11/cyp51) | 200 | |||
| Vibrance (Fl-Se) | Syngenta (Basel, Switzerland) Gadot Agro (Kidron, Israel) | Fludioxonil 2.5% | PP-fungicides (PhenylPyrroles) | Phenylpyrroles | MAP/HistidineKinase in osmotic signal transduction (os-2, HOG1) | 25 | Full Season plants |
| Sedaxen 2.5% | SDHI (succinatedehydrogenase inhibitors) | pyrazole-4-carboxamides | Complex II: succinate-dehydrogenase | 25 |
| Date | Inoculation, Planting, and Sprouting Assessment | Days from Sowing |
|---|---|---|
| 12 January 2022 | 1st inoculation (sterilized infected wheat grains) | −8 |
| 20 January 2022 | Sowing and 2nd inoculation (2 discs per seed) | 0 |
| 10 February 2022 | 3rd inoculation (2 discs per sprout) | 21 |
| 20 March 2022 | Aboveground sprouting | 115 |
| Days from sprouting1 | ||
| 23 March 2022 | Emergence evaluation I | 3 |
| 3 April 2022 | Emergence evaluation II | 14 |
| 24 April 2022 | Emergence evaluation III | 35 |
| Pesticide treatments | ||
| 5 April 2022 | Pesticide I | 16 |
| 24 April 2022 | Pesticide II (20 days from Pesticide I) | 35 |
| 15 May 2022 | Pesticide III (20 days from Pesticide II) | 56 |
| 10 May 2022 | Bifenthrin (Talstar) spraying treatment 2 | 51 |
| Sampling and harvest | ||
| 24 May 2022 | Mid-season sampling and thinning | 65 |
| 13 July 2022 | Harvest and final sampling | 115 |
| Parameters | Winter (Post-Sprouting Period) | Spring-Summer (Growing Period) |
|---|---|---|
| Dates | 20 January–19 March | 20 March–13 July |
| Temperature (°C) | 9.5 ± 3.8 | 21.2 ± 7.2 |
| Humidity (%) | 74.0 ± 16.9 | 51.0 ± 24.8 |
| Precipitation (sum mm) | 100.1 | 42.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degani, O.; Dimant, E.; Gordani, A.; Graph, S.; Margalit, E. Prevention and Control of Fusarium spp., the Causal Agents of Onion (Allium cepa) Basal Rot. Horticulturae 2022, 8, 1071. https://doi.org/10.3390/horticulturae8111071
Degani O, Dimant E, Gordani A, Graph S, Margalit E. Prevention and Control of Fusarium spp., the Causal Agents of Onion (Allium cepa) Basal Rot. Horticulturae. 2022; 8(11):1071. https://doi.org/10.3390/horticulturae8111071
Chicago/Turabian StyleDegani, Ofir, Elhanan Dimant, Asaf Gordani, Shaul Graph, and Eliyahu Margalit. 2022. "Prevention and Control of Fusarium spp., the Causal Agents of Onion (Allium cepa) Basal Rot" Horticulturae 8, no. 11: 1071. https://doi.org/10.3390/horticulturae8111071
APA StyleDegani, O., Dimant, E., Gordani, A., Graph, S., & Margalit, E. (2022). Prevention and Control of Fusarium spp., the Causal Agents of Onion (Allium cepa) Basal Rot. Horticulturae, 8(11), 1071. https://doi.org/10.3390/horticulturae8111071









