Excessive Delay in Nutrient Release by Controlled-Release Fertilizers Can Reduce Chestnut Yield
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
2.2. Experimental Design and Plot Management
2.3. Field Measurements
2.4. Sampling Plant Tissues and Soils
2.5. Laboratory Analysis
2.6. Data Analysis
3. Results
3.1. Nutrient Concentration in Plant Tissues
3.2. SPAD Readings, FieldScout NDVI and Chlorophyll a Fluorescence
3.3. Soil Properties
3.4. Nut Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Carneiro-Carvalho, A.; Pereira, C.; Marques, T.; Martins, L.; Anjos, R.; Pinto, T.; Lousada, J.; Gomes-Laranjo, J. Potential of silicon fertilization in the resistance of chestnut plants to ink disease (Phytophthora cinnamomi). Int. J. Environ. Agric Biotechnol. 2017, 2, 2740–2753. [Google Scholar] [CrossRef]
- Gençer, N.S.; Mert, C. Studies on the gall characteristics of Dryocosmus kuriphilus in chestnut genotypes in Yalova and Bursa provinces of Turkey. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 47, 177–182. [Google Scholar] [CrossRef]
- Murolo, S.; Concas, J.; Romanazzi, G. Use of biocontrol agents as potential tools in the management of chestnut blight. Biol. Control 2019, 132, 102–109. [Google Scholar] [CrossRef]
- Gouveia, E. Doenças. In Manual de Boas Práticas do Castanheiro; Bento, A., Ribeiro, A.C., Eds.; Terras de Trás-os-Montes: Bragança, Portugal, 2020; pp. 191–203. [Google Scholar]
- Rosário, J.; Coelho, V.; Rodrigues, M.A.; Raimundo, S.; Afonso, S.; Arrobas, M.; Gouveia, E. Metalaxyl-M, phosphorous acid and potassium silicate applied as soil drenches show different chestnut seedling performance and protection against Phytophthora root rot. Eur. J. Plant Pathol. 2021, 161, 147–159. [Google Scholar] [CrossRef]
- Afonso, A.; Pereira, F.; Bento, A. Porta-enxertos e variedades. In Manual de Boas Práticas do Castanheiro; Bento, A., Ribeiro, A.C., Eds.; Terras de Trás-os-Montes: Bragança, Portugal, 2020; pp. 93–118. [Google Scholar]
- Santos, A.; Marrão, R.; Bento, A. Pragas. In Manual de Boas Práticas do Castanheiro; Bento, A., Ribeiro, A.C., Eds.; Terras de Trás-os-Montes: Bragança, Portugal, 2020; pp. 205–227. [Google Scholar]
- FAOSTAT. Production: Crops and Livestock Products. 2022. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 15 April 2022).
- Cabo, P.; Aguiar, C.F. Caracterização da região. In Manual de Boas Práticas do Castanheiro; Bento, A., Ribeiro, A.C., Eds.; Terras de Trás-os-Montes: Bragança, Portugal, 2020; pp. 17–29. [Google Scholar]
- Rodrigues, M.A.; Raimundo, S.; Pereira, A.; Arrobas, M. Large chestnut trees (Castanea sativa) respond poorly to liming and fertilizer application. J. Soil Sci. Plant Nutr. 2020, 20, 1261–1270. [Google Scholar] [CrossRef]
- Aguiar, C.F. Sistemática, morfologia, fenologia e biologia da reprodução. In Manual de Boas Práticas do Castanheiro; Bento, A., Ribeiro, A.C., Eds.; Terras de Trás-os-Montes: Bragança, Portugal, 2020; pp. 31–72. [Google Scholar]
- Martins, A.; Marques, G.; Borges, O.; Portela, E.; Lousada, J.; Raimundo, F.; Madeira, M. Management of chestnut plantations for a multifunctional land use under Mediterranean conditions: Effects on productivity and sustainability. Agrofor. Syst. 2011, 81, 175–189. [Google Scholar] [CrossRef]
- Patrício, M.A. Sistemas de condução e poda. In Manual de Boas Práticas do Castanheiro; Bento, A., Ribeiro, A.C., Eds.; Terras de Trás-os-Montes: Bragança, Portugal, 2020; pp. 149–170. [Google Scholar]
- Rodrigues, M.A.; Arrobas, M. Gestão do solo. In Manual de Boas Práticas do Castanheiro; Bento, A., Ribeiro, A.C., Eds.; Terras de Trás-os-Montes: Bragança, Portugal, 2020; pp. 119–129. [Google Scholar]
- Arrobas, M.; Rodrigues, M.A. Fertilização. In Manual de Boas Práticas do Castanheiro; Bento, A., Ribeiro, A.C., Eds.; Terras de Trás-os-Montes: Bragança, Portugal, 2020; pp. 131–148. [Google Scholar]
- Havlin, J.L.; Beaton, J.D.; Tisdale, S.L.; Nelson, W.L. Soil fertility and fertilizers. In An Introduction to Nutrient Management, 8th ed.; Pearson, Inc.: Upper Saddle River, NJ, USA, 2014. [Google Scholar]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Global Edition: London, UK, 2017. [Google Scholar]
- Yang, X.; Zhang, P.; Li, W.; Hu, C.; Zhang, X.; He, P. Evaluation of four seagrass species as early warning indicators for nitrogen overloading: Implications for eutrophic evaluation and ecosystem management. Sci. Total Environ. 2018, 635, 1132–1143. [Google Scholar] [CrossRef]
- Poikane, S.; Phillips, G.; Birk, S.; Free, G.; Kelly, M.G.; Willby, N.J. Deriving nutrient criteria to support ‘good’ ecological status in European lakes: An empirically based approach to linking ecology and management. Sci. Total Environ. 2019, 650, 2074–2084. [Google Scholar] [CrossRef]
- Coyne, M.S. Biological denitrification. In Nitrogen in Agricultural Systems; Schepers, J.S., Raun, W.R., Eds.; ASA: Madison, WI, USA; CSSA: Madison, WI, USA; SSSA: Madison, WI, USA, 2008; pp. 201–253. [Google Scholar]
- Pelster, D.E.; Larouche, F.; Rochette, P.; Chantigny, M.H.; Allaire, S.; Angers, D.A. Nitrogen fertilization but not soil tillage affects nitrous oxide emissions from a clay loam soil under a maize–soybean rotation. Soil Tillage Res. 2011, 3, 298–317. [Google Scholar] [CrossRef]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef]
- Tittonell, P. Ecological intensification of agriculture–sustainable by nature. Curr. Opin. Environ. Sustain. 2014, 8, 53–61. [Google Scholar] [CrossRef]
- IPMA (Instituto Português do Mar e da Atmosfera). Normais Climatológicas. Available online: https://www.ipma.pt/pt/oclima/normais.clima/ (accessed on 15 April 2022).
- Quinteiro, P.; Rafael, S.; Vicente, B.; Marta-Almeida, M.; Rocha, A.; Arroja, L.; Dias, A.C. Mapping green water scarcity under climate change: A case study of Portugal. Sci. Total Environ. 2019, 696, 134024. [Google Scholar] [CrossRef]
- IPCC 2022. Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar] [CrossRef]
- Silva, E.; Arrobas, M.; Gonçalves, A.; Martins, S.; Raimundo, S.; Pinto, L.; Brito, C.; Moutinho-Pereira, J.; Correia, C.M.; Rodrigues, M.A. A controlled-release fertilizer improved soil fertility but not olive tree performance. Nutr. Cycl. Agroecosys. 2021, 120, 1–15. [Google Scholar] [CrossRef]
- Fernández-Escobar, R. Fertilization. In El Cultivo del Olivo, 7th ed.; Barranco, D., Fernández-Escobar, R., Rallo, L., Eds.; Mundi-Prensa: Madrid, Spain, 2017; pp. 419–460. [Google Scholar]
- Arrobas, M.; Santos, D.; Ribeiro, A.; Pereira, E.; Rodrigues, M.A. Soil and foliar nitrogen and boron fertilization of almond trees grown under rainfed conditions. Eur. J. Agron. 2019, 106, 39–48. [Google Scholar] [CrossRef]
- Trenkel, M.E. Slow-and Controlled-Release and Stabilized Fertilizers. An Option for Enhancing Nutrient Use Efficiency in Agriculture; International Fertilizer Industry Association: Paris, France, 2010. [Google Scholar]
- Arrobas, M.; Parada, M.J.; Magalhães, P.; Rodrigues, M.A. Nitrogen-use efficiency and economic efficiency of slow-release N fertilisers applied to irrigated turfs in a Mediterranean environment. Nutr. Cycl. Agroecosys. 2011, 89, 329–339. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, Z.; He, X.; Wang, X.; Shi, X.; Zou, C.; Chen, X. The effects of controlled release urea on maize productivity and reactive nitrogen losses: A meta-analysis. Environ. Pollut. 2019, 246, 559–565. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Grade, V.; Barroso, V.; Pereira, A.; Cassol, L.C.; Arrobas, M. Chestnut response to organo-mineral and controlled-release fertilizers in rainfed growing conditions. J. Soil Sci. Plant Nutr. 2019, 20, 380–391. [Google Scholar] [CrossRef]
- Little, T.M.; Hills, F.J. Agricultural Experimentation: Design and analysis; John Wiley & Sons, Inc.: New York, NY, USA, 1978. [Google Scholar]
- Piekielek, W.P.; Fox, R.H.; Toth, J.D.; Macneal, K.E. Use of chlorophyll meter at the early dent stage of corn to evaluate nitrogen sufficiency. Agron. J. 1995, 87, 403–408. [Google Scholar] [CrossRef]
- Afonso, S.; Arrobas, M.; Ferreira, I.Q.; Rodrigues, M.A. Assessing the potential use of two portable chlorophyll meters in diagnosing the nutritional status of plants. J. Plant Nutr. 2018, 41, 261–271. [Google Scholar] [CrossRef]
- López-Bellido, R.J.; López-Bellido, L.; Fernández-García, P.; López-Bellido, J.M.; Munoz-Romero, V.; López-Bellido, P.L.; Calvache, S. Nitrogen remote diagnosis in a creeping bentgrass golf green. Eur. J. Agron. 2012, 37, 23–30. [Google Scholar] [CrossRef]
- Baker, N.R.; Oxborough, K. Chlorophyll fluorescence as a probe of photosynthetic productivity. In Chlorophyll Fluorescence: Signature of Photosynthesis; Papageorgiu, G.C., Govindgee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 66–79. [Google Scholar]
- Arrobas, M.; Afonso, S.; Ferreira, I.Q.; Moutinho-Pereira, M.; Correia, C.M.; Rodrigues, M.A. Liming and application of nitrogen, phosphorus, potassium and boron on a young plantation of Chestnut. Turk. J. Agric. 2017, 41, 441–451. [Google Scholar] [CrossRef]
- Temminghoff, E.E.J.M.; Houba, V.G. Plant Analysis Procedures, 2nd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004. [Google Scholar] [CrossRef]
- Van Reeuwijk, L.P. Procedures for Soil Analysis. Technical Paper 9; International Soil Reference Information Centre (ISRIC): Wageningen, The Netherlands, 2002. [Google Scholar]
- Lakanen, E.; Erviö, R. A comparison of eight extractants for the determination of plant available micronutrients in soils. Acta Agric. Fenn. 1971, 123, 223–232. [Google Scholar]
- Sharifi, M.; Zebarth, B.J.; Burton, D.L.; Grant, C.A.; Cooper, J.M. Evaluation of Some Indices of Potentially Mineralizable Nitrogen in Soil. Soil Sci. Soc. Am. J. 2007, 71, 1233–1239. [Google Scholar] [CrossRef]
- Baird, R.B.; Eaton, A.D.; Rice, E.W. Nitrate by ultraviolet spectropho-tometric method. In Standard Methods for the Examination of Water and Wastewater, 23rd ed.; Baird, R.B., Eaton, A.D., Rice, E.W., Eds.; American public Health Association: Washington, DC, USA; American Water Works Association: Washington, DC, USA; Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
- Portela, E.; Martins, A.; Pires, A.L.; Raimundo, F.; Marques, G. Cap 6–Práticas culturais no souto: O manejo do solo. Soil management practices in chestnut orchards. In Castanheiros; Gomes-Laranjo, J., Ferreira-Cardoso, J., Portela, E., Abreu, C.G., Eds.; Programa AGRO: Vila Real, Portugal; Universidade de Trás-os-Montes e Alto Douro: Vila Real, Portugal, 2007; Volume 499, pp. 207–264. [Google Scholar]
- Arrobas, M.; Afonso, S.; Rodrigues, M.A. Diagnosing the nutritional condition of chestnut groves by soil and leaf analyses. Sci. Hortic. 2018, 228, 113–121. [Google Scholar] [CrossRef]
- Basyouni, R.; Dunn, B.L.; Goad, C. Use of nondestructive sensors to assess nitrogen status in potted poinsettia (Euphorbia pulcherrima L. (Willd. ex Klotzsch)) production. Sci. Hortic. 2015, 192, 47–53. [Google Scholar] [CrossRef]
- Mahajan, G.; Pandey, R.N.; Kumar, D.; Datta, S.C.; Sahoo, R.N.; Parsad, R. Development of critical values for the leaf color chart, SPAD and FieldScout CM 1000 for fixed time adjustable nitrogen management in aromatic hybrid rice (Oryza sativa L.). Commun. Soil Sci. Plant Anal. 2014, 45, 1877–1893. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Dinis, L.-T.; Ferreira, H.; Pinto, G.; Bernardo, S.; Correia, C.M.; Moutinho-Pereira, J. Kaolin-based, foliar reflective film protects photosystem II structure and function in grapevine leaves exposed to heat and high solar radiation. Photosynthetica 2016, 54, 47–55. [Google Scholar] [CrossRef]
- Ferreira, I.Q.; Arrobas, M.; Moutinho-Pereira, J.M.; Correia, C.; Rodrigues, M.A. Olive response to potassium applications under different water regimes and cultivars. Nutr. Cycl. Agroecosys. 2018, 112, 387–401. [Google Scholar] [CrossRef]
- Opti-Sciences. Desktop Plant Stress Guide, 3rd ed.; Opti-Sciences: Hudson, NH, USA, 2014; Available online: www.optisci.com (accessed on 5 January 2014).
- Rodrigues, M.A.; Afonso, S.; Ferreira, I.Q.; Arrobas, M. Response of stevia to nitrogen fertilization and harvesting regime in Northeastern Portugal. Arch. Agron. 2017, 63, 626–637. [Google Scholar] [CrossRef]
- Afonso, S.; Arrobas, M.; Rodrigues, M.A. Response of hops to algae-based and nutrient-rich foliar sprays. Agriculture 2021, 11, 798. [Google Scholar] [CrossRef]
- Arrobas, M.; Decker, J.V.; Feix, B.L.; Godoy, W.I.; Casali, C.A.; Correia, C.M.; Rodrigues, M.A. Biochar and zeolites did not improve phosphorus uptake or crop productivity in a field trial performed in an irrigated intensive farming system. Soil Use Manag. 2021, 38, 564–575. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Moller, I.S.; White, P. Functions of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Elsevier: London, UK, 2012; pp. 135–189. [Google Scholar]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of nutrients, micronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Elsevier: London, UK, 2012; pp. 191–248. [Google Scholar] [CrossRef]
- Portela, E.; Ferreira-Cardoso, J.; Louzada, J.; Gomes-Laranjo, J. Assessment of boron application in chestnuts: Nut yield and quality. J. Plant Nutr. 2015, 38, 973–987. [Google Scholar] [CrossRef]
- Ferreira, I.Q.; Rodrigues, M.A.; Arrobas, M. Soil and foliar applied boron in olive: Tree crop growth and yield, and boron remobilization within plant tissue. Span. J. Agric. Res. 2019, 17, e0901. [Google Scholar] [CrossRef]
- Lopes, J.I.; Gonçalves, A.; Brito, C.; Martins, S.; Pinto, L.; Moutinho-Pereira, J.; Raimundo, S.; Arrobas, M.; Rodrigues, M.A.; Correia, C.M. Inorganic Fertilization at High N Rate Increased Olive Yield of a Rainfed Orchard but Reduced Soil Organic Matter in Comparison to Three Organic Amendments. Agronomy 2021, 11, 2172. [Google Scholar] [CrossRef]
- Sparrow, L.A.; Uren, N.C. Manganese oxidation and reduction in soils: Effects of temperature, water potential, pH and their interactions. Soil Res. 2014, 52, 483–494. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Ladeira, L.C.; Arrobas, M. Azotobacter-enriched organic manures to increase nitrogen fixation and crop productivity. Eur. J. Agron. 2018, 93, 88–94. [Google Scholar] [CrossRef]
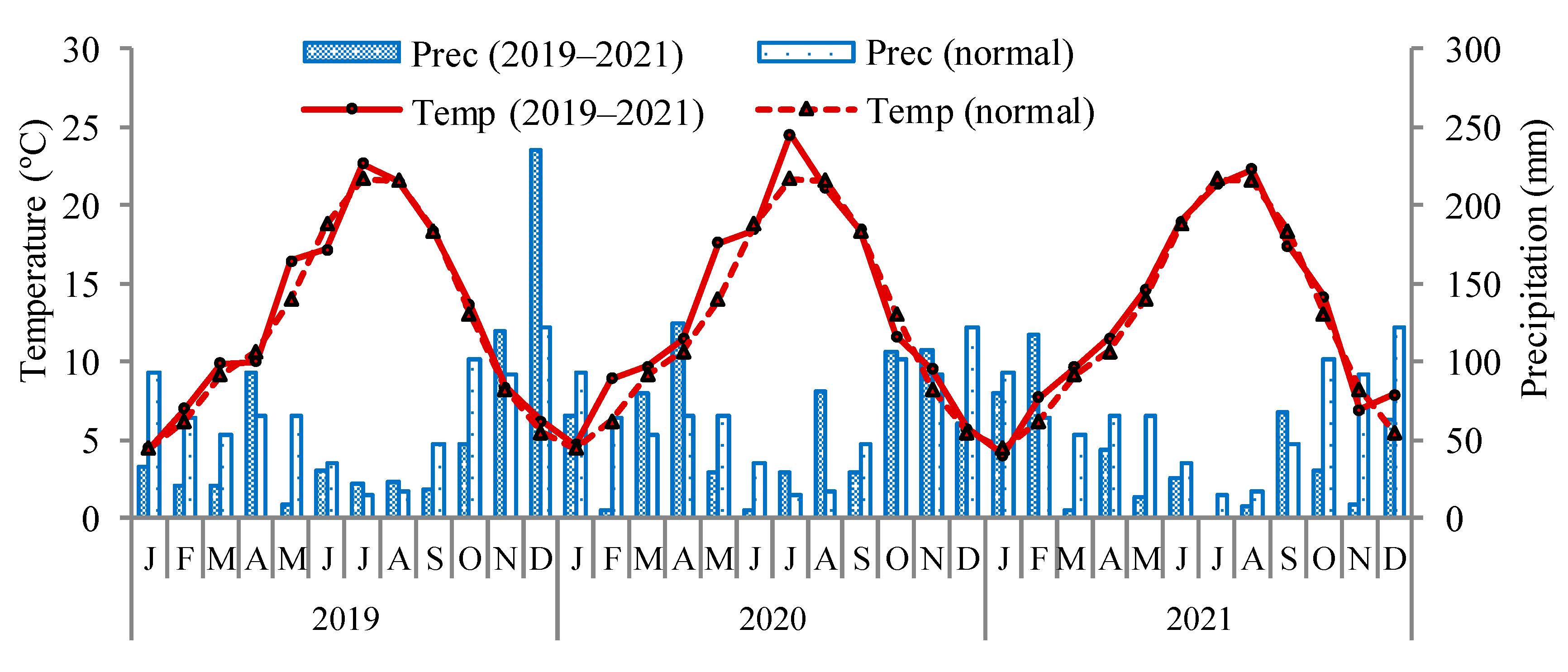
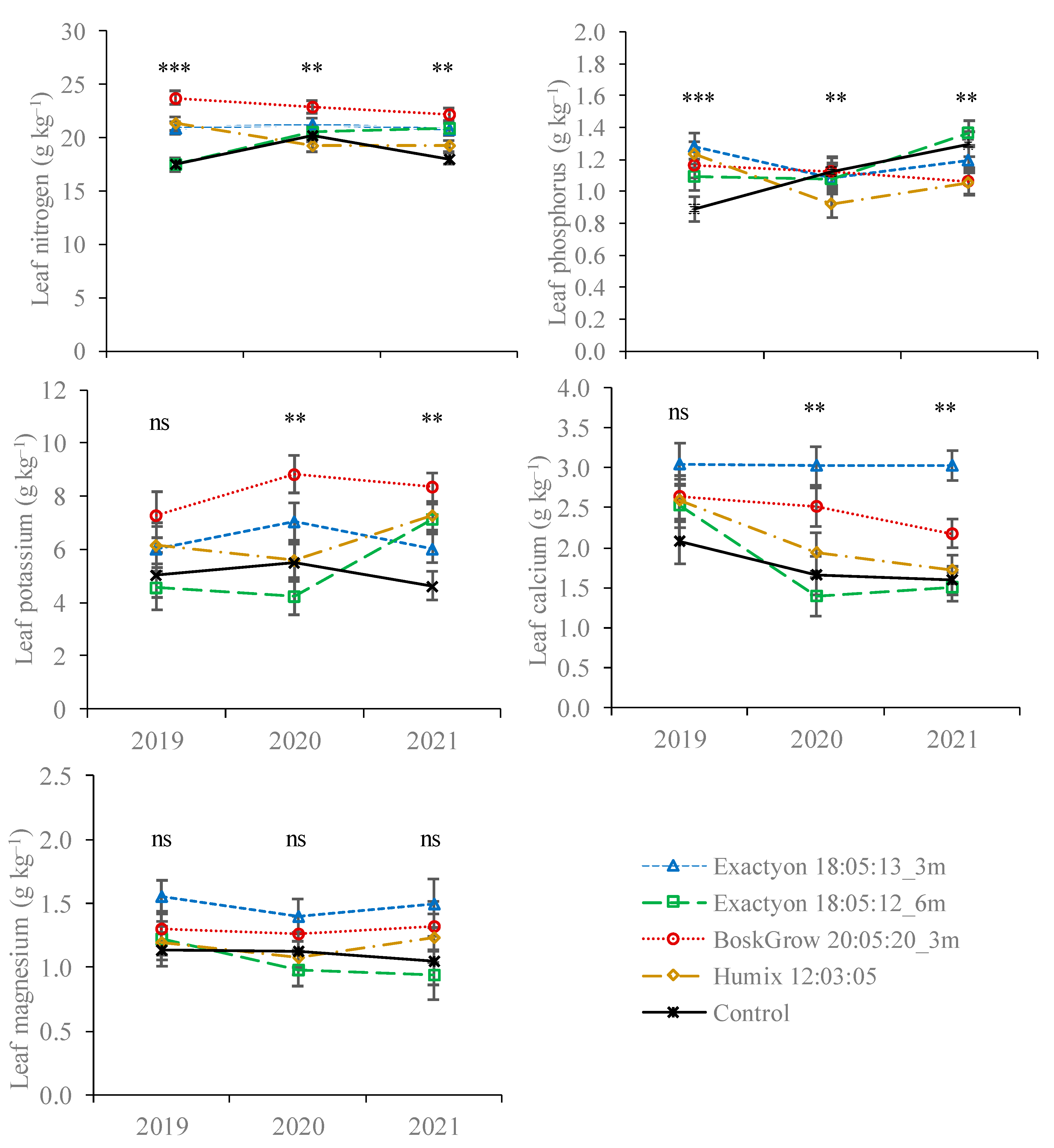
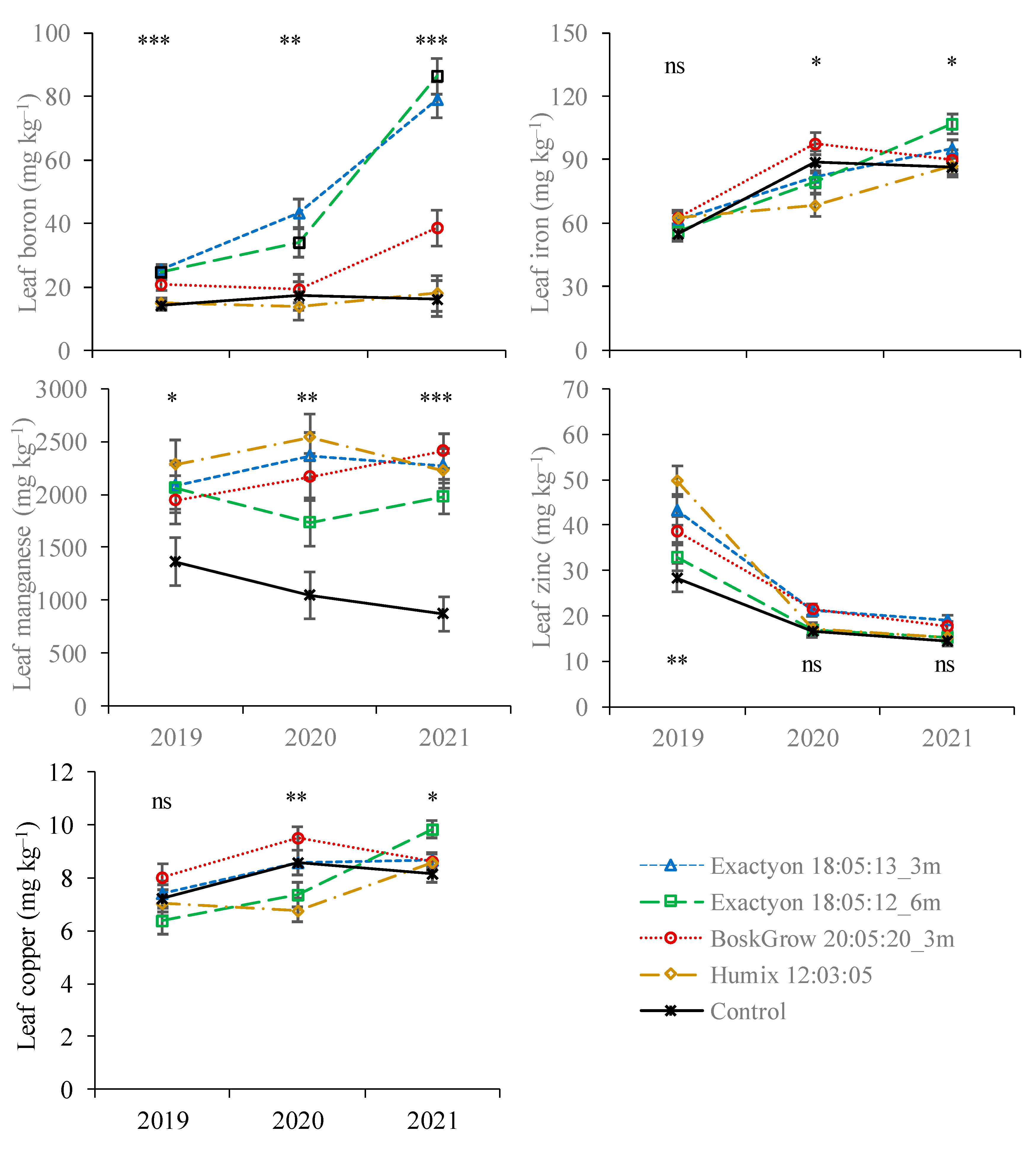

| Nitrogen | Phosphorus | Potassium | Calcium | Magnesium | Boron | Iron | Manganese | Zinc | Copper | |
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | g kg−1 | mg kg−1 | ||||||||
| 2019 | ||||||||||
| Exactyon 18:05:13_3m | 10.16 a * | 1.00 a | 4.31 a | 0.48 ab | 0.50 a | 13.4 b | 125.6 ab | 183.0 ab | 15.5 a | 8.6 a |
| Exactyon 18:05:12_6m | 9.56 a | 1.02 a | 3.92 a | 0.39 b | 0.44 a | 16.3 a | 109.9 ab | 166.9 ab | 15.3 a | 9.1 a |
| BoskGrow 20:05:20_3m | 10.33 a | 0.89 a | 4.40 a | 0.58 a | 0.57 a | 13.1 bc | 148.8 a | 140.3 bc | 15.9 a | 8.9 a |
| Humix 12:03:05 | 9.94 a | 1.04 a | 3.96 a | 0.47 ab | 0.51 a | 11.4 bc | 112.2 ab | 204.4 a | 17.0 a | 8.9 a |
| Control | 9.29 a | 1.01 a | 4.64 a | 0.48 ab | 0.51 a | 10.7 c | 93.6 b | 102.6 c | 15.7 a | 8.5 a |
| Probability | 0.4689 | 0.3460 | 0.4929 | 0.0375 | 0.3473 | <0.0001 | 0.0114 | 0.0006 | 0.8209 | 0.9231 |
| Standard error | 0.44 | 0.05 | 0.32 | 0.04 | 0.04 | 0.60 | 9.48 | 12.97 | 1.07 | 0.55 |
| 2021 | ||||||||||
| Exactyon 18:05:13_3m | 10.07 ab | 1.10 a | 7.16 a | 0.43 a | 1.10 a | 23.1 a | 197.7 a | 178.1 a | 17.7 a | 8.5 a |
| Exactyon 18:05:12_6m | 12.20 a | 1.21 a | 6.37 a | 0.43 a | 0.82 a | 25.5 a | 194.9 a | 162.2 a | 18.9 a | 9.2 a |
| BoskGrow 20:05:20_3m | 12.73 a | 1.06 a | 6.41 a | 0.40 a | 0.85 a | 26.2 a | 150.8 a | 187.1 a | 17.3 a | 8.5 a |
| Humix 12:03:05 | 8.53 b | 1.14 a | 7.70 a | 0.49 a | 0.90 a | 13.5 a | 183.2 a | 209.6 a | 17.5 a | 8.5 a |
| Control | 9.02 b | 1.08 a | 6.52 a | 0.40 a | 0.42 a | 16.7 a | 198.1 a | 94.6 b | 18.6 a | 9.8 a |
| Probability | 0.0048 | 0.7401 | 0.1104 | 0.6146 | 0.2731 | 0.0928 | 0.3138 | 0.0030 | 0.5420 | 0.5831 |
| Standard error | 0.47 | 0.08 | 0.32 | 0.04 | 0.19 | 2.93 | 16.01 | 9.89 | 0.74 | 0.65 |
| 2019 | 2021 | |
|---|---|---|
| Treatments | mg kg−1 | |
| Exactyon 18:05:13_3m | 22.0 a * | 35.3 a |
| Exactyon 18:05:12_6m | 20.3 a | 34.1 a |
| BoskGrow 20:05:20_3m | 22.4 a | 28.6 ab |
| Humix 12:03:05 | 21.9 a | 20.8 b |
| Control | 18.8 a | 22.6 b |
| Probability | 0.4728 | 0.0050 |
| Standard error | 1.34 | 1.67 |
| SPAD | NDVI | FV/FM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatments | 2019 | 2020 | 2021 | 2019 | 2020 | 2021 | 2019 | 2020 | 2021 |
| Exactyon 18:05:13_3m | 45.0 a * | 42.1 a | 49.2 a | 0.798 a | 0.783 a | 0.820 a | 0.819 a | 0.803 a | 0.803 a |
| Exactyon 18:05:12_6m | 42.4 b | 40.4 a | 47.3 ab | 0.775 ab | 0.770 a | 0.808 ab | 0.807 a | 0.811 a | 0.815 a |
| BoskGrow 20:05:20_3m | 44.9 a | 42.3 a | 48.7 ab | 0.793 ab | 0.785 a | 0.818 a | 0.830 a | 0.800 a | 0.794 a |
| Humix 12:03:05 | 43.8 ab | 41.8 a | 46.9 ab | 0.780 ab | 0.780 a | 0.798 ab | 0.803 a | 0.808 a | 0.819 a |
| Control | 42.5 b | 41.4 a | 45.9 b | 0.763 b | 0.765 a | 0.785 b | 0.807 a | 0.799 a | 0.730 a |
| Probability | 0.0350 | 0.8880 | 0.0208 | 0.0261 | 0.1001 | 0.0430 | 0.2759 | 0.9636 | 0.1054 |
| Standard error | 0.67 | 1.40 | 0.68 | 0.007 | 0.006 | 0.008 | 0.009 | 0.016 | 0.022 |
| Organic Carbon | pH(H2O) | Phosphorus | Potassium | Calcium | Magnesium | Boron | Manganese | |
|---|---|---|---|---|---|---|---|---|
| Treatments | g kg−1 | mg P2O5 kg−1 | mg K2O kg−1 | cmol+ kg−1 | cmol+ kg−1 | mg kg−1 | mg kg−1 | |
| Exactyon 18:05:13_3m | 18.6 a * | 5.29 a | 55.2 ab | 261.3 ab | 2.00 a | 0.63 a | 1.90 a | 124.0 ab |
| Exactyon 18:05:12_6m | 20.1 a | 5.29 a | 63.9 a | 245.3 bc | 1.73 a | 0.56 ab | 1.32 b | 122.4 ab |
| BoskGrow 20:05:20_3m | 21.1 a | 5.16 a | 65.3 a | 329.3 a | 1.52 a | 0.54 ab | 0.80 c | 137.2 a |
| Humix 12:03:05 | 18.4 a | 4.93 a | 44.1 b | 187.0 c | 1.20 a | 0.51 ab | 0.30 d | 147.6 a |
| Control | 19.0 a | 5.31 a | 46.1 b | 220.7 bc | 1.57 a | 0.47 b | 0.47 d | 76.6 b |
| Probability | 0.1270 | 0.1456 | 0.0004 | 0.0006 | 0.1369 | 0.0288 | <0.0001 | 0.0090 |
| Standard error | 1.15 | 0.11 | 2.59 | 14.89 | 0.20 | 0.03 | 1.16 b | 10.94 |
| NH4+ Hot | NH4+ Cold | NH4+ Hyd | NO3− Cold | |
|---|---|---|---|---|
| Treatments | mg kg−1 | |||
| Exactyon 18:05:13_3m | 48.0 ab * | 28.3 a | 19.7 b | 213.5 a |
| Exactyon 18:05:12_6m | 68.6 a | 39.4 a | 29.3 a | 131.7 b |
| BoskGrow 20:05:20_3m | 42.8 ab | 26.4 a | 16.3 b | 146.6 b |
| Humix 12:03:05 | 37.8 ab | 20.4 a | 17.5 b | 50.1 c |
| Control | 23.1 b | 10.7 a | 12.4 b | 62.6 c |
| Probability | 0.0120 | 0.0813 | 0.0007 | <0.0001 |
| Standard error | 6.97 | 6.24 | 1.81 | 12.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrobas, M.; Belotto, L.B.; Marchetti, J.A.; Barroso, V.; Raimundo, S.; Cassol, L.C.; Correia, C.M.; Rodrigues, M.Â. Excessive Delay in Nutrient Release by Controlled-Release Fertilizers Can Reduce Chestnut Yield. Horticulturae 2022, 8, 1067. https://doi.org/10.3390/horticulturae8111067
Arrobas M, Belotto LB, Marchetti JA, Barroso V, Raimundo S, Cassol LC, Correia CM, Rodrigues MÂ. Excessive Delay in Nutrient Release by Controlled-Release Fertilizers Can Reduce Chestnut Yield. Horticulturae. 2022; 8(11):1067. https://doi.org/10.3390/horticulturae8111067
Chicago/Turabian StyleArrobas, Margarida, Leonardo Bomfim Belotto, Juliana Aparecida Marchetti, Valdemar Barroso, Soraia Raimundo, Luís César Cassol, Carlos Manuel Correia, and Manuel Ângelo Rodrigues. 2022. "Excessive Delay in Nutrient Release by Controlled-Release Fertilizers Can Reduce Chestnut Yield" Horticulturae 8, no. 11: 1067. https://doi.org/10.3390/horticulturae8111067
APA StyleArrobas, M., Belotto, L. B., Marchetti, J. A., Barroso, V., Raimundo, S., Cassol, L. C., Correia, C. M., & Rodrigues, M. Â. (2022). Excessive Delay in Nutrient Release by Controlled-Release Fertilizers Can Reduce Chestnut Yield. Horticulturae, 8(11), 1067. https://doi.org/10.3390/horticulturae8111067











