If You Grow It, They Will Come: Ornamental Plants Impact the Abundance and Diversity of Pollinators and Other Flower-Visiting Insects in Gardens
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Site Preparation
2.2. Selection and Planting of Ornamental Garden Plants
2.3. Maintenance
2.4. Data Collection
2.5. Analysis
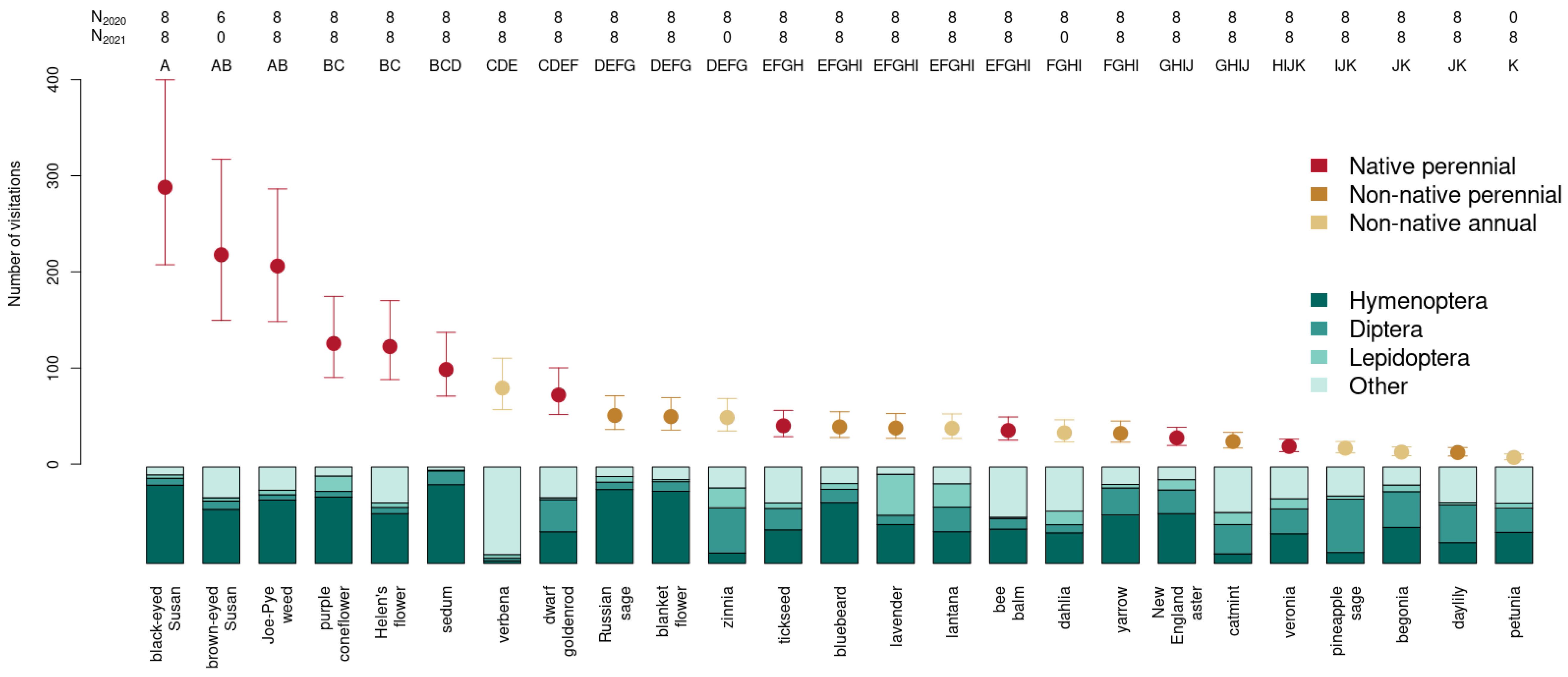
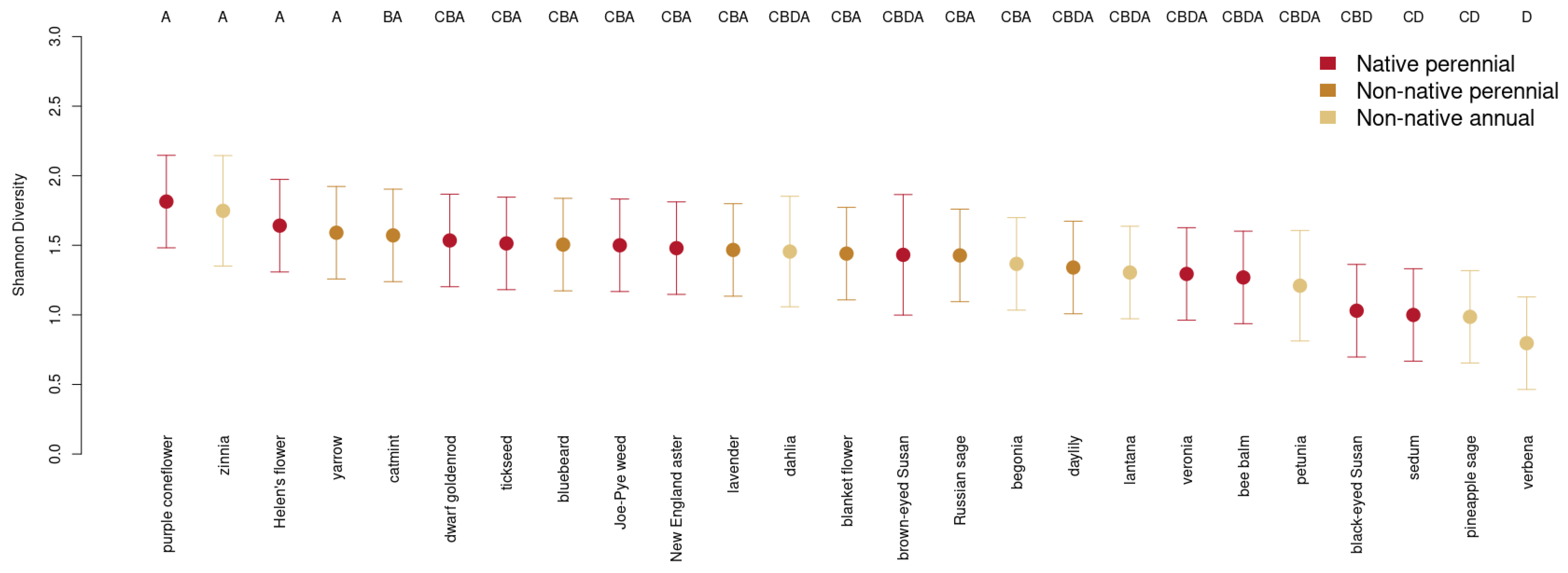
3. Results
3.1. Insect Weighted Abundance across Plant Species
| N | Apis Mellifera | Bombus Bimaculatus | Bombus Impatiens | Other Bumble Bees | Xylocopa Virginica | Anthidium Manicatum | Other wild Bees | Wasps | Hoverflies | Other Diptera | Papilionidae | Lycaenidae | Nymphalidae | Pieridae | Hesperiidae | Sphingidae | Other Lepidoptera | Hemiptera | Popillia Japonica | Other Coleoptera | Mantodea | Orthoptera | Odonata | Other Insects | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Native Perennial | |||||||||||||||||||||||||
| Joe-Pye weed | 16 | 196 | 0 | 1130 | 6 | 6 | 0 | 865 | 117 | 91 | 100 | 1 | 12 | 5 | 9 | 47 | 0 | 93 | 699 | 7 | 71 | 2 | 35 | 0 | 24 |
| bee balm | 16 | 32 | 0 | 0 | 0 | 0 | 17 | 175 | 10 | 27 | 46 | 0 | 0 | 0 | 0 | 6 | 0 | 3 | 48 | 230 | 25 | 1 | 7 | 2 | 28 |
| black-eyed Susan | 16 | 19 | 0 | 9 | 3 | 4 | 4 | 3726 | 221 | 258 | 97 | 0 | 3 | 2 | 11 | 76 | 0 | 83 | 246 | 11 | 66 | 1 | 18 | 3 | 42 |
| brown-eyed Susan | 6 | 5.3 | 0 | 13.3 | 5.3 | 0 | 0 | 1787 | 368 | 229.3 | 109.3 | 0 | 0 | 0 | 2.7 | 42.7 | 2.7 | 82.7 | 1000 | 16 | 29.3 | 8 | 21.3 | 2.7 | 146.7 |
| dwarf goldenrod | 16 | 6 | 0 | 4 | 0 | 0 | 0 | 200 | 183 | 160 | 241 | 0 | 3 | 0 | 1 | 2 | 1 | 18 | 136 | 6 | 48 | 2 | 12 | 0 | 174 |
| Helen’s flower | 16 | 648 | 0 | 11 | 2 | 3 | 0 | 350 | 15 | 67 | 63 | 0 | 6 | 0 | 2 | 48 | 0 | 43 | 309 | 7 | 19 | 3 | 30 | 7 | 354 |
| New England aster | 16 | 117 | 0 | 6 | 1 | 0 | 0 | 113 | 0 | 53 | 60 | 0 | 0 | 0 | 5 | 38 | 0 | 6 | 34 | 2 | 13 | 0 | 5 | 1 | 4 |
| purple coneflower | 16 | 706 | 24 | 202 | 89 | 7 | 2 | 368 | 13 | 51 | 69 | 0 | 1 | 3 | 16 | 287 | 0 | 21 | 104 | 7 | 39 | 0 | 7 | 1 | 28 |
| sedum | 16 | 31 | 0 | 11 | 1 | 17 | 30 | 1287 | 46 | 110 | 136 | 0 | 2 | 0 | 2 | 3 | 0 | 4 | 36 | 0 | 16 | 0 | 2 | 0 | 2 |
| tickseed | 16 | 13 | 0 | 0 | 0 | 0 | 0 | 211 | 22 | 135 | 23 | 0 | 0 | 0 | 0 | 12 | 0 | 29 | 118 | 2 | 29 | 8 | 22 | 1 | 80 |
| veronia | 16 | 7 | 0 | 21 | 0 | 1 | 0 | 66 | 6 | 73 | 12 | 0 | 1 | 0 | 0 | 18 | 0 | 16 | 82 | 0 | 14 | 0 | 3 | 0 | 8 |
| Non-native Perennial | |||||||||||||||||||||||||
| blanket flower | 16 | 63 | 0 | 217 | 0 | 1 | 0 | 340 | 13 | 58 | 27 | 0 | 0 | 0 | 3 | 13 | 0 | 2 | 78 | 0 | 8 | 2 | 8 | 0 | 12 |
| bluebeard | 16 | 102 | 0 | 253 | 1 | 14 | 1 | 150 | 5 | 86 | 27 | 0 | 1 | 2 | 22 | 17 | 0 | 9 | 97 | 0 | 19 | 1 | 10 | 3 | 10 |
| catmint | 16 | 9 | 0 | 3 | 0 | 1 | 6 | 18 | 5 | 115 | 12 | 0 | 3 | 0 | 28 | 19 | 1 | 2 | 23 | 0 | 3 | 7 | 142 | 1 | 21 |
| daylily | 16 | 8 | 0 | 1 | 0 | 0 | 0 | 29 | 6 | 30 | 50 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 44 | 0 | 16 | 0 | 7 | 0 | 7 |
| lavender | 16 | 44 | 4 | 52 | 9 | 4 | 71 | 67 | 3 | 55 | 7 | 0 | 1 | 0 | 156 | 104 | 0 | 7 | 23 | 0 | 6 | 1 | 9 | 0 | 7 |
| Russian sage | 16 | 328 | 0 | 133 | 1 | 28 | 15 | 277 | 7 | 70 | 8 | 0 | 0 | 0 | 19 | 29 | 3 | 9 | 50 | 0 | 8 | 1 | 7 | 13 | 20 |
| yarrow | 16 | 3 | 0 | 0 | 1 | 0 | 2 | 173 | 87 | 113 | 35 | 0 | 1 | 0 | 0 | 6 | 0 | 12 | 43 | 0 | 12 | 0 | 33 | 2 | 4 |
| Non-native Annual | |||||||||||||||||||||||||
| begonia | 16 | 4 | 0 | 21 | 0 | 0 | 1 | 54 | 1 | 57 | 24 | 0 | 0 | 0 | 3 | 9 | 0 | 3 | 24 | 0 | 8 | 0 | 8 | 0 | 0 |
| dahlia | 8 | 72 | 0 | 10 | 2 | 0 | 0 | 118 | 2 | 14 | 40 | 0 | 2 | 2 | 2 | 78 | 0 | 8 | 210 | 0 | 30 | 6 | 14 | 0 | 32 |
| lantana | 16 | 166 | 0 | 0 | 0 | 0 | 0 | 31 | 3 | 67 | 89 | 2 | 1 | 0 | 9 | 81 | 0 | 55 | 57 | 1 | 13 | 2 | 17 | 5 | 9 |
| petunia | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 38 | 0 | 20 | 10 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 24 | 0 | 10 | 0 | 10 | 0 | 0 |
| pineapple sage | 16 | 21 | 0 | 0 | 0 | 1 | 0 | 9 | 4 | 18 | 152 | 0 | 0 | 0 | 3 | 2 | 1 | 4 | 56 | 0 | 16 | 0 | 8 | 0 | 11 |
| verbena | 16 | 21 | 0 | 1 | 0 | 0 | 1 | 11 | 6 | 33 | 7 | 0 | 0 | 0 | 16 | 32 | 0 | 2 | 1109 | 0 | 10 | 0 | 5 | 0 | 147 |
| zinnia | 8 | 14 | 0 | 2 | 0 | 0 | 0 | 52 | 26 | 124 | 286 | 0 | 0 | 0 | 12 | 136 | 0 | 32 | 100 | 4 | 54 | 0 | 8 | 2 | 18 |
3.2. Honey Bee Abundance Correlates with Other Insect Pollinator Abundance
3.3. Choice of Ornamental Garden Plants Significantly Impacts Insect Abundance
3.4. Choice of Ornamental Garden Plant Significantly Impacts Insect Diversity
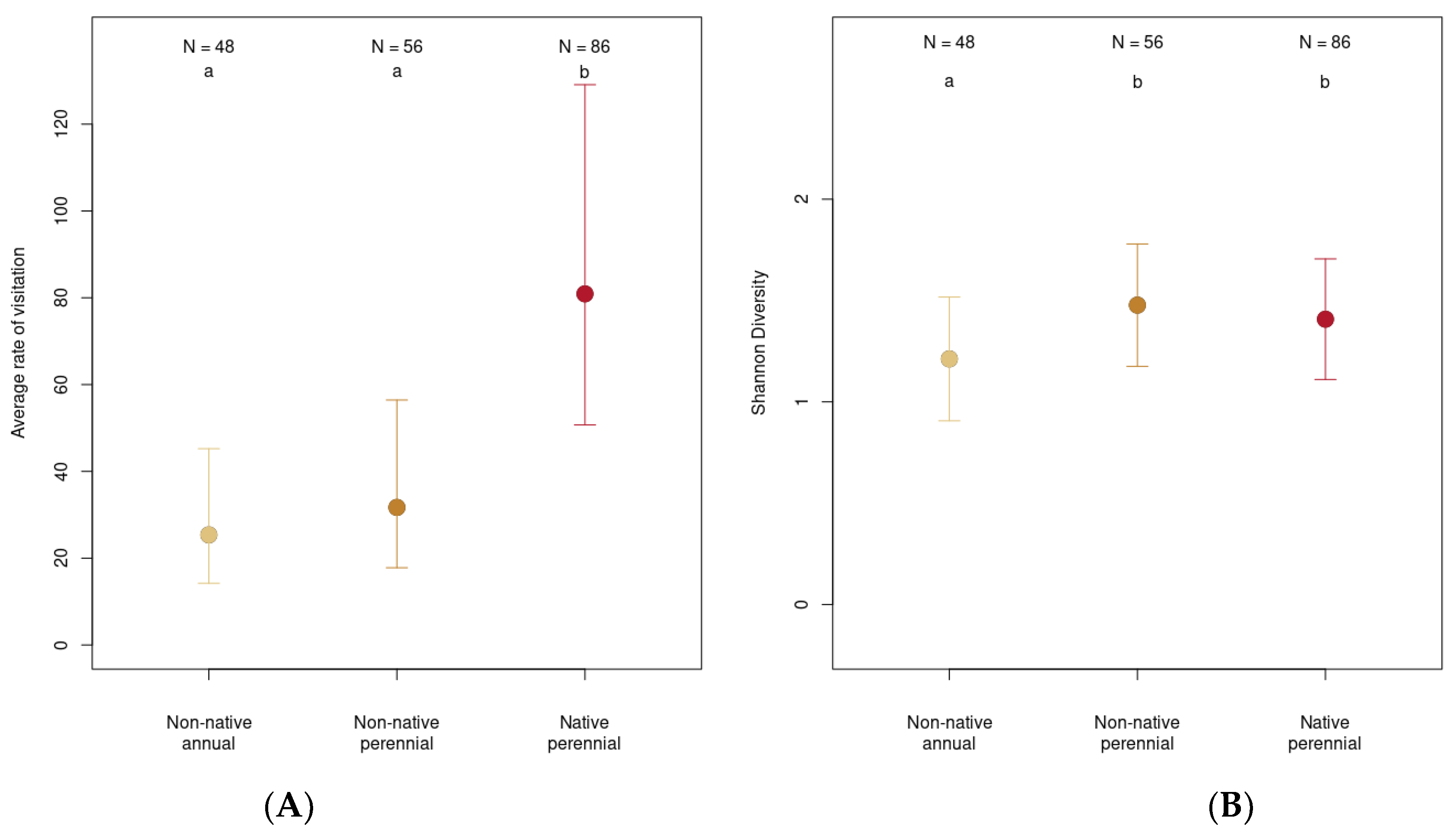
3.5. Native Perennials Brought Insect Abundance and Non-Native and Native Perennials Brought Insect Diversity
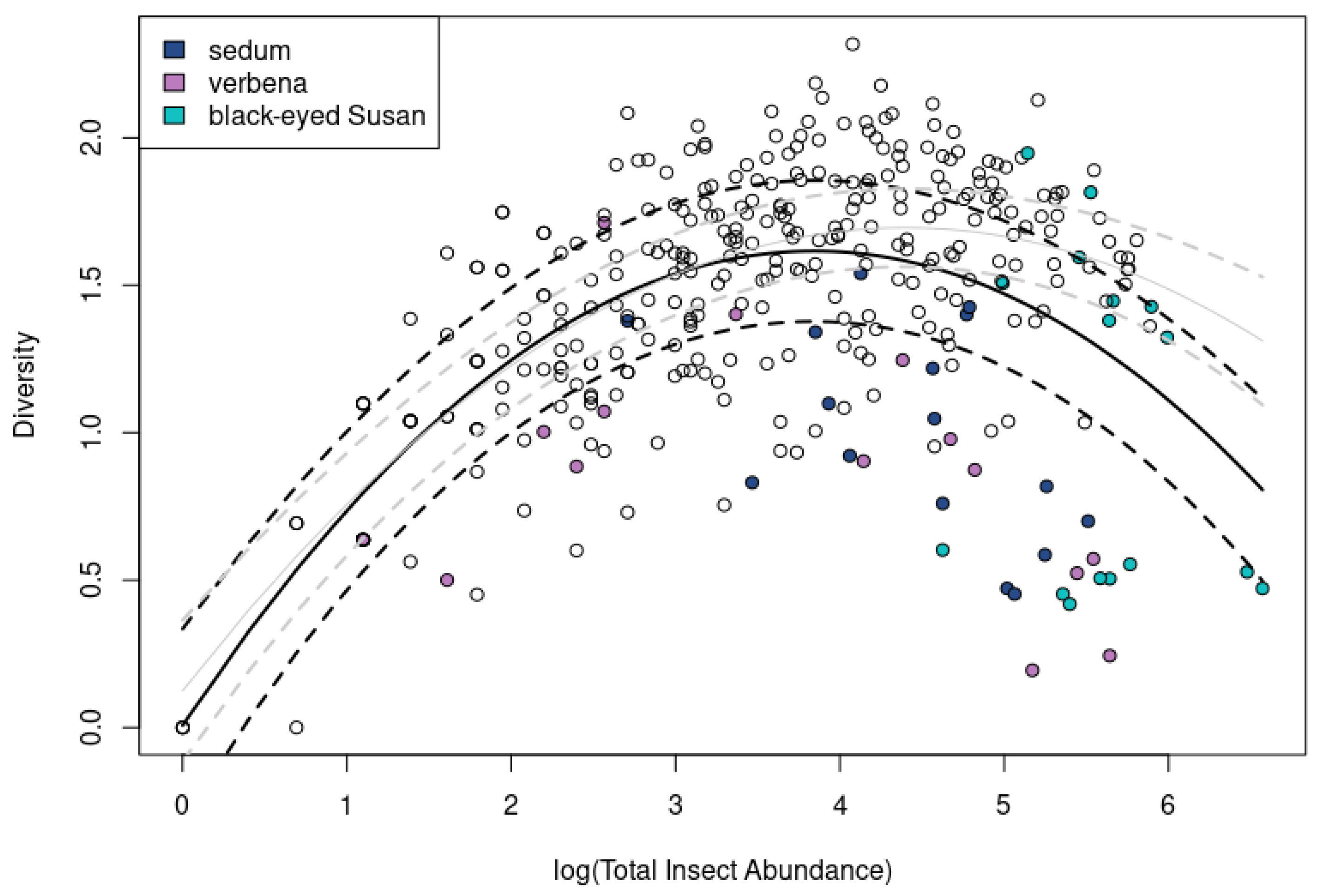
3.6. Insect Diversity Possesses a Curvilinear Relationship to Insect Abundance
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burkle, L.A.; Marlin, J.C.; Knight, T.M. Plant-pollinator interactions over 120 years: Loss of species, co-occurrence, and function. Science 2013, 339, 1611–1615. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Ssymank, A.; Sorg, M.; de Kroon, H.; Jongejans, E. Insect biomass decline scaled to species diversity: General patterns derived from a hoverfly community. Proc. Natl. Acad. Sci. USA 2021, 118, e2002554117. [Google Scholar] [CrossRef]
- Kosior, A.; Celary, W.; Olejniczak, P.; Fijał, J.; Król, W.; Solarz, W.; Płonka, P. The decline of the bumble bees and cuckoo bees (Hymenoptera: Apidae: Bombini) of Western and Central Europe. Oryx 2007, 41, 79–88. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Carr-Markell, M.K.; Demler, C.M.; Couvillon, M.J.; Schürch, R.; Spivak, M. Do honey bee (Apis mellifera) foragers recruit their nestmates to native forbs in reconstructed prairie habitats? PLoS ONE 2020, 15, e0228169. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.; Kleijn, D.; Williams, N.M.; Tschumi, M.; Blaauw, B.R.; Bommarco, R.; Campbell, A.J.; Dainese, M.; Drummond, F.A.; Entling, M.H. The effectiveness of flower strips and hedgerows on pest control, pollination services and crop yield: A quantitative synthesis. Ecol. Lett. 2020, 23, 1488–1498. [Google Scholar] [CrossRef]
- Fitch, G.; Wilson, C.J.; Glaum, P.; Vaidya, C.; Simao, M.-C.; Jamieson, M.A. Does urbanization favour exotic bee species? Implications for the conservation of native bees in cities. Biol. Lett. 2019, 15, 20190574. [Google Scholar] [CrossRef]
- Hopwood, J.L. The contribution of roadside grassland restorations to native bee conservation. Biol. Conserv. 2008, 141, 2632–2640. [Google Scholar] [CrossRef]
- Meyer, S.; Unternährer, D.; Arlettaz, R.; Humbert, J.-Y.; Menz, M.H. Promoting diverse communities of wild bees and hoverflies requires a landscape approach to managing meadows. Agric. Ecosyst. Environ. 2017, 239, 376–384. [Google Scholar] [CrossRef]
- Wilson, C.J.; Jamieson, M.A. The effects of urbanization on bee communities depends on floral resource availability and bee functional traits. PLoS ONE 2019, 14, e0225852. [Google Scholar] [CrossRef]
- Winfree, R.; Bartomeus, I.; Cariveau, D.P. Native pollinators in anthropogenic habitats. Annu. Rev. Ecol. Evol. System. 2011, 42, 1–22. [Google Scholar] [CrossRef]
- Buri, P.; Arlettaz, R.; Humbert, J.-Y. Delaying mowing and leaving uncut refuges boosts orthopterans in extensively managed meadows: Evidence drawn from field-scale experimentation. Agric. Ecosyst. Environ. 2013, 181, 22–30. [Google Scholar] [CrossRef]
- Buri, P.; Humbert, J.-Y.; Arlettaz, R. Promoting pollinating insects in intensive agricultural matrices: Field-scale experimental manipulation of hay-meadow mowing regimes and its effects on bees. PLoS ONE 2014, 9, e85635. [Google Scholar] [CrossRef]
- Larson, J.L.; Kesheimer, A.J.; Potter, D.A. Pollinator assemblages on dandelions and white clover in urban and suburban lawns. J. Insect Conserv. 2014, 18, 863–873. [Google Scholar] [CrossRef]
- Garbuzov, M.; Fensome, K.A.; Ratnieks, F.L.W. Public approval plus more wildlife: Twin benefits of reduced mowing of amenity grass in a suburban public park in Saltdean, UK. Insect Conserv. Divers. 2015, 8, 107–119. [Google Scholar] [CrossRef]
- Campbell, B.; Khachatryan, H.; Rihn, A. Pollinator-friendly plants: Reasons for and barriers to purchase. HortTechnology 2017, 27, 831–839. [Google Scholar] [CrossRef]
- Kalaman, H.; Knox, G.W.; Wilson, S.B.; Wilber, W. A master gardener survey: Promoting pollinator-friendly plants through education and outreach. HortTechnology 2020, 30, 163–167. [Google Scholar] [CrossRef]
- Goddard, M.A.; Dougill, A.J.; Benton, T.G. Scaling up from gardens: Biodiversity conservation in urban environments. Trends Ecol. Evol. 2010, 25, 90–98. [Google Scholar] [CrossRef]
- Davies, Z.G.; Fuller, R.A.; Loram, A.; Irvine, K.N.; Sims, V.; Gaston, K.J. A national scale inventory of resource provision for biodiversity within domestic gardens. Biol. Conserv. 2009, 142, 761–771. [Google Scholar] [CrossRef]
- Taylor, L. From ways of life to lifestyle: Theordinari-ization’of British gardening lifestyle television. Eur. J. Commun. 2002, 17, 479–493. [Google Scholar] [CrossRef]
- Kaluza, B.F.; Wallace, H.; Heard, T.A.; Klein, A.M.; Leonhardt, S.D. Urban gardens promote bee foraging over natural habitats and plantations. Ecol. Evol. 2016, 6, 1304–1316. [Google Scholar] [CrossRef] [PubMed]
- Baldock, K.C.; Goddard, M.A.; Hicks, D.M.; Kunin, W.E.; Mitschunas, N.; Morse, H.; Osgathorpe, L.M.; Potts, S.G.; Robertson, K.M.; Scott, A.V. A systems approach reveals urban pollinator hotspots and conservation opportunities. Nat. Ecol. Evol. 2019, 3, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Lanner, J.; Kratschmer, S.; Petrović, B.; Gaulhofer, F.; Meimberg, H.; Pachinger, B. City dwelling wild bees: How communal gardens promote species richness. Urban Ecosyst. 2020, 23, 271–288. [Google Scholar] [CrossRef]
- Erickson, E.; Adam, S.; Russo, L.; Wojcik, V.; Patch, H.; Grozinger, C. More than meets the eye? The role of annual ornamental flowers in supporting pollinators. Environ. Entomol. 2020, 49, 178–188. [Google Scholar] [CrossRef]
- Garbuzov, M.; Samuelson, E.E.W.; Ratnieks, F.L.W. Survey of insect visitation of ornamental flowers in Southover Grange garden, Lewes, UK. Insect Sci. 2015, 22, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Garbuzov, M.; Ratnieks, F.L. Using the British national collection of asters to compare the attractiveness of 228 varieties to flower-visiting insects. Environ. Entomol. 2015, 44, 638–646. [Google Scholar] [CrossRef]
- Harris, B.A.; Braman, S.K.; Pennisi, S.V. Influence of plant taxa on pollinator, butterfly, and beneficial insect visitation. HortScience 2016, 51, 1016–1019. [Google Scholar] [CrossRef]
- Garbuzov, M.; Ratnieks, F.L. Listmania: The strengths and weaknesses of lists of garden plants to help pollinators. BioScience 2014, 64, 1019–1026. [Google Scholar] [CrossRef]
- Garbuzov, M.; Ratnieks, F.L. Quantifying variation among garden plants in attractiveness to bees and other flower-visiting insects. Funct. Ecol. 2014, 28, 364–374. [Google Scholar] [CrossRef]
- Rollings, R.; Goulson, D. Quantifying the attractiveness of garden flowers for pollinators. J. Insect Conserv. 2019, 23, 803–817. [Google Scholar] [CrossRef]
- Erickson, E.; Patch, H.; Grozinger, C. Herbaceous perennial ornamental plants can support complex pollinator communities. Sci. Rep. 2021, 11, 17352. [Google Scholar] [CrossRef]
- Ratnieks, F.L.W.; Shackleton, K. Does the Waggle Dance Help Honey Bees to Forage at Greater Distances than Expected for their Body Size? Front. Ecol. Evol. 2015, 3, 31. [Google Scholar] [CrossRef]
- DiSabato-Aust, T. The Well-Tended Perennial Garden: The Essential Guide to Planting + Pruning Techniques, 3rd ed.; Timber Press, Inc.: Portland, OR, USA, 2017. [Google Scholar]
- Adamson, N.L.; Borders, B.; Cruz, J.K.; Jordan, S.F.; Gill, K.; Hopwood, J.; Lee-Mader, E.; Minnerath, A.; Vaughan, M. Pollinator Plants: Mid-Atlantic Region; Xerces Society: Portland, OR, USA, 2017. [Google Scholar]
- Daniel, J.B.; Glennon, B.; Gordon, S.; Wentz, C. Virginia Plant Establishment Guide; USDA-NRCS: Washington, DC, USA, 2011. [Google Scholar]
- Garbuzov, M.; Madsen, A.; Ratnieks, F.L. Patch size has no effect on insect visitation rate per unit area in garden-scale flower patches. Acta Oecologica 2015, 62, 53–57. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Morris, E.K.; Caruso, T.; Buscot, F.; Fischer, M.; Hancock, C.; Maier, T.S.; Meiners, T.; Müller, C.; Obermaier, E.; Prati, D.; et al. Choosing and using diversity indices: Insights for ecological applications from the German Biodiversity Exploratories. Ecol. Evol. 2014, 4, 3514–3524. [Google Scholar] [CrossRef]
- Hutcheson, K. A test for comparing diversities based on the Shannon formula. J. Theor. Biol. 1970, 29, 151–154. [Google Scholar] [CrossRef]
- Barr, D.J.; Levy, R.; Scheepers, C.; Tily, H.J. Random effects structure for confirmatory hypothesis testing: Keep it maximal. J. Mem. Lang. 2013, 68, 255–278. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least Squares Means. Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 25 September 2022).
- Wardhaugh, C.W. How many species of arthropods visit flowers? Arthropod-Plant Interact. 2015, 9, 547–565. [Google Scholar] [CrossRef]
- Ollerton, J. Pollinators and Pollination: Nature and Society; Pelagic Publishing Ltd.: Exeter, UK, 2021. [Google Scholar]
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef]
- Colla, S.R.; Packer, L. Evidence for decline in eastern North American bumblebees (Hymenoptera: Apidae), with special focus on Bombus affinis Cresson. Biodivers. Conserv. 2008, 17, 1379–1391. [Google Scholar] [CrossRef]
- Theodorou, P.; Albig, K.; Radzevičiūtė, R.; Settele, J.; Schweiger, O.; Murray, T.E.; Paxton, R.J. The structure of flower visitor networks in relation to pollination across an agricultural to urban gradient. Funct. Ecol. 2017, 31, 838–847. [Google Scholar] [CrossRef]
- Goulson, D.; Hughes, W.; Derwent, L.; Stout, J. Colony growth of the bumblebee, Bombus terrestris, in improved and conventional agricultural and suburban habitats. Oecologia 2002, 130, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Pardee, G.L.; Philpott, S.M. Native plants are the bee’s knees: Local and landscape predictors of bee richness and abundance in backyard gardens. Urban Ecosyst. 2014, 17, 641–659. [Google Scholar] [CrossRef]
- Fukase, J.; Simons, A. Increased pollinator activity in urban gardens with more native flora. Appl. Ecol. Environ. Res. 2016, 14, 297–310. [Google Scholar] [CrossRef]
- Lowenstein, D.M.; Matteson, K.C.; Minor, E.S. Evaluating the dependence of urban pollinators on ornamental, non-native, and ‘weedy’ floral resources. Urban Ecosyst. 2019, 22, 293–302. [Google Scholar] [CrossRef]
- Kalaman, H.; Wilson, S.B.; Mallinger, R.E.; Knox, G.W.; Kim, T.; Begcy, K.; van Santen, E. Evaluation of Native and Nonnative Ornamentals as Pollinator Plants in Florida: II. Floral Resource Value. HortScience 2022, 57, 137–143. [Google Scholar] [CrossRef]
- Matteson, K.C.; Langellotto, G.A. Small scale additions of native plants fail to increase beneficial insect richness in urban gardens. Insect Conserv. Divers. 2011, 4, 89–98. [Google Scholar] [CrossRef]
- Garbuzov, M.; Alton, K.; Ratnieks, F.L. Most ornamental plants on sale in garden centres are unattractive to flower-visiting insects. PeerJ 2017, 5, e3066. [Google Scholar] [CrossRef]
- Steffan-Dewenter, I.; Tscharntke, T. Succession of bee communities on fallows. Ecography 2001, 24, 83–93. [Google Scholar] [CrossRef]
- Fussell, M.; Corbet, S.A. Flower usage by bumble-bees: A basis for forage plant management. J. Appl. Ecol. 1992, 29, 451–465. [Google Scholar] [CrossRef]
- Parrish, J.; Bazzaz, F. Difference in pollination niche relationships in early and late successional plant communities. Ecology 1979, 60, 597–610. [Google Scholar] [CrossRef]
- Corbet, S.A. Insects, plants and succession: Advantages of long-term set-aside. Agric. Ecosyst. Environ. 1995, 53, 201–217. [Google Scholar] [CrossRef]
- Willmer, P. Pollination and Floral Ecology; Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Tuell, J.K.; Fiedler, A.K.; Landis, D.; Isaacs, R. Visitation by wild and managed bees (Hymenoptera: Apoidea) to eastern US native plants for use in conservation programs. Environ. Entomol. 2014, 37, 707–718. [Google Scholar] [CrossRef]
- Weisenhorn, J. Annual Flowers That Attract Pollinators; University of Minnesota Extension: Minneapolis, MN, USA, 2021. [Google Scholar]
- Božanić Tanjga, B.; Ljubojević, M.; Đukić, A.; Vukosavljev, M.; Ilić, O.; Narandžić, T. Selection of Garden Roses to Improve the Ecosystem Services They Provide. Horticulturae 2022, 8, 883. [Google Scholar] [CrossRef]
- Strzałkowska-Abramek, M. Nectar and pollen production in ornamental cultivars of Prunus serrulata (Rosaceae). Folia Hortic. 2019, 31, 205–212. [Google Scholar] [CrossRef]
- Hicks, D.M.; Ouvrard, P.; Baldock, K.C.; Baude, M.; Goddard, M.A.; Kunin, W.E.; Mitschunas, N.; Memmott, J.; Morse, H.; Nikolitsi, M. Food for pollinators: Quantifying the nectar and pollen resources of urban flower meadows. PLoS ONE 2016, 11, e0158117. [Google Scholar] [CrossRef]
- Staab, M.; Pereira-Peixoto, M.H.; Klein, A.-M. Exotic garden plants partly substitute for native plants as resources for pollinators when native plants become seasonally scarce. Oecologia 2020, 194, 465–480. [Google Scholar] [CrossRef]
- Cubey, J.; Merrick, J. RHS Plant Finder 2022; Royal Horticultural Society: London, UK, 2022. [Google Scholar]
- NRCS USDA. The PLANTS Database; United States Department of Agriculture: Washington, DC, USA, 2022. [Google Scholar]
- Biesmeijer, J.C.; Slaa, J.E. The structure of eusocial bee assemblages in Brazil. Apidologie 2006, 37, 240–258. [Google Scholar] [CrossRef]
- von Frisch, K. The Dance Language and Orientation of Bees; Harvard University Press: Cambridge, MA, USA, 1967. [Google Scholar]
- Couvillon, M.J. The dance legacy of Karl von Frisch. Insect. Soc. 2012, 59, 297–306. [Google Scholar] [CrossRef]
- Garbuzov, M.; Couvillon, M.J.; Schürch, R.; Ratnieks, F.L.W. Honey bee dance decoding and pollen-load analysis shows limited foraging on oilseed rape—A potential source of neonicotinoid contamination. Agric. Ecosyst. Environ. 2015, 203, 62–68. [Google Scholar] [CrossRef]
- Balfour, N.J.; Ratnieks, F.L. Using the waggle dance to determine the spatial ecology of honey bees during commercial crop pollination. Agric. For. Entomol. 2017, 19, 210–216. [Google Scholar] [CrossRef]
- Couvillon, M.J.; Ratnieks, F.L.W. Environmental consultancy: Dancing bee bioindicators to evaluate landscape “health”. Front. Ecol. Evol. 2015, 3. [Google Scholar] [CrossRef]
- Couvillon, M.J.; Schürch, R.; Ratnieks, F.L.W. Dancing bees communicate a foraging preference for rural lands under High Level Agri-Environment Schemes. Curr. Biol. 2014, 24, 1212–1215. [Google Scholar] [CrossRef] [PubMed]
- Mallinger, R.E.; Gaines-Day, H.R.; Gratton, C. Do managed bees have negative effects on wild bees?: A systematic review of the literature. PLoS ONE 2017, 12, e0189268. [Google Scholar] [CrossRef]
- Paini, D. Impact of the introduced honey bee (Apis mellifera)(Hymenoptera: Apidae) on native bees: A review. Austral Ecol. 2004, 29, 399–407. [Google Scholar] [CrossRef]
- Goulson, D. Effects of introduced bees on native ecosystems. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 1–26. [Google Scholar] [CrossRef]
- Angelella, G.M.; McCullough, C.T.; O’Rourke, M.E. Honey bee hives decrease wild bee abundance, species richness, and fruit count on farms regardless of wildflower strips. Sci. Rep. 2021, 11, 3202. [Google Scholar] [CrossRef]
- Balfour, N.J.; Garbuzov, M.; Ratnieks, F.L.W. Longer tongues and swifter handling: Why do more bumble bees (Bombus spp.) than honey bees (Apis mellifera) forage on lavender (Lavandula spp.). Ecol. Entomol. 2013, 38, 323–329. [Google Scholar] [CrossRef]
- Couvillon, M.J.; Schürch, R.; Ratnieks, F.L.W. Waggle Dance Distances as Integrative Indicators of Seasonal Foraging Challenges. PLoS ONE 2014, 9, e93495. [Google Scholar] [CrossRef]
- Garbuzov, M.; Schürch, R.; Ratnieks, F.W. Eating locally: Dance decoding demonstrates that urban honey bees in Brighton, UK, forage mainly in the surrounding urban area. Urban Ecosyst. 2014, 18, 411–418. [Google Scholar] [CrossRef]
- Sponsler, D.B.; Matcham, E.G.; Lin, C.-H.; Lanterman, J.L.; Johnson, R.M. Spatial and taxonomic patterns of honey bee foraging: A choice test between urban and agricultural landscapes. J. Urban Ecol. 2017, 3, juw008. [Google Scholar] [CrossRef]
- Couvillon, M.J.; Fensome, K.A.; Quah, S.K.L.; Schürch, R. Summertime blues: August foraging leaves honey bees empty-handed. Commun. Integr. Biol. 2014, 7, e28821. [Google Scholar] [CrossRef]
- Steele, T.N.; Schürch, R.; Ohlinger, B.D.; Couvillon, M.J. Apple orchards feed honey bees during, but even more so after, bloom. Ecosphere 2022, 13, e4228. [Google Scholar] [CrossRef]
- Erickson, E.; Junker, R.; Ali, J.; McCartney, N.; Patch, H.; Grozinger, C. Complex floral traits shape pollinator attraction to ornamental plants. Ann. Bot. 2022, 130, 561–577. [Google Scholar] [CrossRef]
- Marquardt, M.; Kienbaum, L.; Kretschmer, L.A.; Penell, A.; Schweikert, K.; Ruttensperger, U.; Rosenkranz, P. Evaluation of the importance of ornamental plants for pollinators in urban and suburban areas in Stuttgart, Germany. Urban Ecosyst. 2021, 24, 811–825. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmersheim, M.C.; Schürch, R.; O’Rourke, M.E.; Slezak, J.; Couvillon, M.J. If You Grow It, They Will Come: Ornamental Plants Impact the Abundance and Diversity of Pollinators and Other Flower-Visiting Insects in Gardens. Horticulturae 2022, 8, 1068. https://doi.org/10.3390/horticulturae8111068
Palmersheim MC, Schürch R, O’Rourke ME, Slezak J, Couvillon MJ. If You Grow It, They Will Come: Ornamental Plants Impact the Abundance and Diversity of Pollinators and Other Flower-Visiting Insects in Gardens. Horticulturae. 2022; 8(11):1068. https://doi.org/10.3390/horticulturae8111068
Chicago/Turabian StylePalmersheim, Michala C., Roger Schürch, Megan E. O’Rourke, Jenna Slezak, and Margaret J. Couvillon. 2022. "If You Grow It, They Will Come: Ornamental Plants Impact the Abundance and Diversity of Pollinators and Other Flower-Visiting Insects in Gardens" Horticulturae 8, no. 11: 1068. https://doi.org/10.3390/horticulturae8111068
APA StylePalmersheim, M. C., Schürch, R., O’Rourke, M. E., Slezak, J., & Couvillon, M. J. (2022). If You Grow It, They Will Come: Ornamental Plants Impact the Abundance and Diversity of Pollinators and Other Flower-Visiting Insects in Gardens. Horticulturae, 8(11), 1068. https://doi.org/10.3390/horticulturae8111068






