Alleviation Mechanism of Melatonin in Chickpea (Cicer arietinum L.) under the Salt Stress Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. MT Treatments
2.3. Salinity Treatments
2.4. Growth Parameters
2.5. CRV and Chlorophyll Content
2.6. Physiological Parameters
2.7. Antioxidant Enzyme Activity
2.8. Proline Content
2.9. H2O2 and MDA Content
2.10. Mineral Analysis
2.11. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gao, W.; Feng, Z.; Bai, Q.; He, J.; Wang, Y. Melatonin-mediated regulation of growth and antioxidant capacity in salt-tolerant naked oat under salt stress. Int. J. Mol. Sci. 2019, 20, 1176. [Google Scholar] [CrossRef]
- Soliman, M.; Elkelish, A.; Souad, T.; Alhaithloul, H.; Farooq, M. Brassinosteroid seed priming with nitrogen supplementa-tion improves salt tolerance in soybean. Physiol. Mol. Biol. Plants 2020, 26, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, J.M.; Porcel, R.; Azcón, C.; Aroca, R. Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: New challenges in physiological and molecular studies. J. Exp. Bot. 2012, 63, 4033–4044. [Google Scholar] [CrossRef] [PubMed]
- Baâtour, O.; Kaddour, R.; Mahmoudi, H.; Tarchoun, I.; Bettaieb, I.; Nasri, N.; Marzouk, B. Salt effects on Origanum majorana fatty acid and essential oil composition. J. Sci. Food Agric. 2011, 91, 2613–2620. [Google Scholar] [CrossRef]
- Bettaieb Rebey, I.; Bourgou, S.; Rahali, F.Z.; Msaada, K.; Ksouri, R.; Marzouk, B. Relation between salt tolerance and bio-chemical changes in cumin (Cuminum cyminum L.) seeds. J. Food Drug Anal. 2017, 25, 391–402. [Google Scholar] [CrossRef]
- Bahcesular, B.; Yildirim, E.D.; Karaçocuk, M. Seed priming with melatonin effects on growth, essential oil compounds and antioxidant activity of basil (Ocimum basilicum L.) under salinity stress. Ind. Crops Prod. 2020, 146, 112165. [Google Scholar] [CrossRef]
- Rasool, S.; Ahmad, A.; Siddiqi, T.O.; Ahmad, P. Changes in growth, lipid peroxidation and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta Physiol. Plant. 2013, 35, 1039–1050. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Wen, W.; Shi, Z.; Gu, Q.; Ahamed, G.J.; Guo, S. Hydrogen peroxide mediates spermidine-induced autophagy to alleviate salt stress in cucumber. Autophagy 2021, 17, 2876–2890. [Google Scholar] [CrossRef]
- Ekinci, M.; Yildirim, E.; Turan, M. Ameliorating effects of hydrogen sulfide on growth, physiological and biochemical characteristics of eggplant seedlings under salt stress. S. Afr. J. Bot. 2021, 143, 79–89. [Google Scholar] [CrossRef]
- Yildirim, E.; Ekinci, M.; Sahin, U.; Ors, S.; Turan, M.; Demir, İ.; Dursun, A.; Kotan, R. Improved water productivity in sum-mer squash under water deficit with PGPR and synthetic methylamine applications. Rhizosphere 2021, 20, 2198–2452. [Google Scholar] [CrossRef]
- Turan, M.; Ekinci, M.; Kul, R.; Boynueyri, F.G.; Yildirim, E. Mitigation of salinity stress in cucumber seedlings by exogenous hydrogen sulfide. Plant Res. 2022, 135, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Harde, R.; Madrid, J.A.; Tan, D.X.; Reiter, R.J. Melatonin, the circadian multioscillator system and health: The need for de-tailed analyses of peripheral melatonin signalling. J. Pineal Res. 2012, 34, 233–241. [Google Scholar] [CrossRef]
- Yan, Y.; Jing, X.; Tang, H.; Li, X.; Gong, B.; Shi, Q. Using transcriptome to discover a novel melatonin-induced sodic alkaline stress resistant pathway in Solanum lycopersicum L. Plant Cell Physiol. 2019, 60, 2051–2064. [Google Scholar] [CrossRef]
- Wei, J.; Li, D.X.; Zhang, J.R.; Shan, C.; Rengel, Z.; Song, Z.B.; Chen, Q. Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal Res. 2018, 65, e12500. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martín, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Zhang, M. Melatonin increases growth and salt tolerance of Limonium bicolor by improving photosynthetic and antioxidant capacity. BMC Plant Biol. 2022, 22, 16. [Google Scholar] [CrossRef]
- Tan, D.X.; Reiter, R.J. An evolutionary view of melatonin synthesis and metabolism related to its biological functions in plants. J. Exp. Bot. 2020, 71, 4677–4689. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef]
- Wang, Y.; Reiter, R.J.; Chan, Z. Phytomelatonin: A universal abiotic stress regulator. J. Exp. Bot. 2018, 69, 963–974. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, Z.; Zhang, X.; Zheng, S.; Wang, J.; Mo, J. Alleviating effects of exogenous melatonin on salt stress in cucumber. Sci. Hortic. 2020, 262, 109070. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liang, D.; Liao, M.A.; Lin, L. Effects of melatonin on the growth of radish seedlings under salt stress. In Proceedings of the 3rd International Conference on Renewable Energy and Environmental Technology (ICERE), Hanoi, Vietnam, 25–27 February 2017. [Google Scholar]
- Karakullukcu, E.; Adak, M. Determination of salinity tolerance of some chickpea (Cicer arietinum L.) varieties. J. Agrıcultural Scıences 2008, 14, 313–321. [Google Scholar] [CrossRef]
- Weeda, S.; Zhang, N.; Zhao, X.; Ndip, G.; Guo, Y.; Buck, G.A.; Ren, S. Arabidopsis transcriptome analysis reveals key roles of melatonin in plant defense systems. PLoS ONE 2014, 9, e93462. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, Q.; Zhang, H.; Cao, Y.; Weeda, S.; Ren, S.; Guo, Y.D. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2015, 66, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Moustafa-Farag, M.; Elkelish, A.; Dafea, M.; Khan, M.; Arnao, M.B.; Abdelhamid, M.T.; Ai, S. Role of melatonin in plant tolerance to soil stressors: Salinity, pH and heavy metals. Molecules 2020, 25, 5359. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Extraction of phtosynthetic tissues: Chlorophylls and carotenoids. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.2.1–F4.2.6. [Google Scholar] [CrossRef]
- Sahin, U.; Ekinci, M.; Ors, S.; Turan, M.; Yildiz, S.; Yildirim, E. Effects of individual and combined effects of salinity and drought on physiological, nutritional and biochemical properties of cabbage (Brassica oleracea var. capitata). Sci. Hortic. 2018, 240, 196–204. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Mertens, D. Plants preparation of laboratory sample. In Official Methods of Analysis, 18th ed.; Horwitz, W., Latimer, G.W., Eds.; AOAC: Gaithersburg, MD, USA, 2005; pp. 1–2. [Google Scholar]
- Mertens, D. Metal in plants and pet foods, In Official Methods of Analysis, 18th ed.; Horwitz, W., Latimer, G.W., Eds.; AOAC: Gaithersburg, MD, USA, 2005; pp. 3–4. [Google Scholar]
- SPSS Inc. SPSS® 18.0 Base User’s Guide; Prentice Hall: Chicago, IL, USA, 2010. [Google Scholar]
- Dadasoglu, E.; Ekinci, M.; Kul, R.; Shams, M.; Turan, M.; Yildirim, E. Nitric oxide enhances salt tolerance through regulating antioxidant enzyme activity and nutrient uptake in pea. Legume Res. 2021, 44, 41–45. [Google Scholar] [CrossRef]
- Shams, M.; Yildirim, E. Variations in response of CaPAO and CaATG8c genes, hormone, photosynthesis and antioxidative system in pepper genotypes under salinity stress. Sci. Hortic. 2021, 282, 110041. [Google Scholar] [CrossRef]
- Bajwa, V.S.; Shukla, M.R.; Sherif, S.M.; Murch, S.J.; Saxena, P.K. Role of melatonin in alleviating cold stress in Arabidopsis thaliana. J. Pineal Res. 2014, 56, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; He, Y.; Kou, H.; Ju, Z.; Gao, X.; Zhao, H. The effects of artificial light at night on Eurasian tree sparrow (Passer montanus): Behavioral rhythm disruption, melatonin suppression and intestinal microbiota alterations. Ecol. Indic. 2020, 108, 105–702. [Google Scholar] [CrossRef]
- Li, X.; Yu, B.; Cui, Y.; Yin, Y. Melatonin application confers enhanced salt tolerance by regulating Na+ and Cl− accumulation in rice. Plant Growth Regul. 2017, 83, 441–454. [Google Scholar] [CrossRef]
- Meng, J.F.; Xu, T.F.; Wang, Z.Z.; Fang, Y.L.; Xi, Z.M.; Zhang, Z.W. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: Antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 2014, 57, 200–212. [Google Scholar] [CrossRef]
- Ding, W.; Zhao, P.; Peng, J.; Zhao, Y.; Xu, J.W.; Li, T.; Yu, X. Melatonin enhances astaxanthin accumulation in the green mi-croalga Haematococcus pluvialis by mechanisms possibly related to abiotic stress tolerance. Algal. Res. 2018, 33, 256–265. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Samsampour, D.; Zahedi, S.M.; Zamanian, K.; Rahman, M.M.; Mostofa, M.G.; Tran, L.S.P. Melatonin alleviates drought impact on growth and essential oil yield of lemon verbena by enhancing antioxidant responses, mineral balance, and abscisic acid content. Physiol. Plant. 2021, 172, 1363–1375. [Google Scholar] [CrossRef]
- Marschner, P.; Rengel, Z. Nutrient availability in soils. In Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2012; pp. 315–330. [Google Scholar]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Suriyan, C.U.; Chalermpol, K. Proline accumulation, photosynthetic abilities and growth characters of sugarcane (Saccharum officinarum L.) plantlets in response to iso-osmotic salt and water-deficit stress. Agric. Sci. China 2009, 8, 51–58. [Google Scholar] [CrossRef]
- Wang, L.Y.; Liu, J.L.; Wang, W.X.; Sun, Y. Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 2016, 54, 19–27. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.; Zhou, L.; Pan, G.; Li, Z.; Cheng, F. Senescence-specific change in ROS scavenging enzyme activities and regulation of various SOD isozymes to ROS levels in psf mutant rice leaves. Plant Physiol. Biochem. 2016, 109, 248–261. [Google Scholar] [CrossRef]
- Liang, C.; Zheng, G.; Li, W.; Wang, Y.; Hu, B.; Wang, H.; Wu, H.; Qian, Y.; Zhu, X.G.; Tan, D.X. Melatonin delays leaf se-nescence and enhances salt stress tolerance in rice. J. Pineal Res. 2015, 59, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, H.J.; Zhao, B.; Sun, Q.Q.; Cao, Y.Y.; Li, R.; Wu, X.X.; Weeda, S.; Li, L.; Ren, S. The RNA-seq approach to discriminate gene expression profiles in response to melatonin on cucumber lateral root formation. J. Pineal Res. 2014, 56, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, X.; Li, C.; Wei, Z.; Liang, D.; Ma, F. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 2013, 54, 292–302. [Google Scholar] [CrossRef]
- Jiang, C.; Cui, Q.; Feng, K.; Xu, D.; Li, C.; Zheng, Q. Melatonin improves antioxidant capacity and ion homeostasis and en-hances salt tolerance in maize seedlings. Acta Physiol. Plant 2016, 38, 82. [Google Scholar] [CrossRef]
- Ye, J.; Wang, S.; Deng, X.; Yin, L.; Xiong, B.; Wang, X. Melatonin increased maize (Zea mays L.) seedling drought tolerance by alleviating drought-induced photosynthetic inhibition and oxidative damage. Acta Physiol. Plant. 2016, 38, 48. [Google Scholar] [CrossRef]
- Hong, C.I.; Zámborszky, J.; Csikász-Nagy, A. Minimum criteria for DNA damage-induced phase advances in circadian rhythms. PLoS Comput. Biol. 2009, 5, e1000384. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Hayat, K.; Zhang, D.; Zhou, P. Small structures with big impact: Multi-walled carbon nanotubes enhanced remediation efficiency in hyperaccumulator Solanum nigrum L. under cadmium and arsenic stress. Chemosphere 2021, 276, 130. [Google Scholar] [CrossRef]
- Kishor, P.K.; Sangam, S.; Amrutha, R.N.; Laxmi, P.S.; Naidu, K.R.; Rao, K.S.; Sreenivasulu, N. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- El-Mashad, A.A.A.; Mohamed, H.I. Brassinolide alleviates salt stress and increases antioxidant activity of cowpea plants (Vigna sinensis). Protoplasma 2012, 249, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xie, Y.; Gu, Q.; Zhao, G.; Zhang, Y.; Cui, W.; Xu, S.; Wang, R.; Shen, W. The AtrbohF-dependent regulation of ROS signaling is required for melatonin-induced salinity tolerance in Arabidopsis. Free Radic. Biol. Med. 2017, 108, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Abdul Jaleel, C.; Salem, A.M.; Nabi, G.; Sharma, S. Roles of enzymatic and non-enzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Li, S.; Xu, Y.; Bi, Y.; Zhang, B.; Shen, S.; Jiang, T.; Zheng, X. Melatonin treatment inhibits gray mold and induces disease resistance in cherry tomato fruit during postharvest. Postharvest Biol. Technol. 2019, 157, 110–962. [Google Scholar] [CrossRef]
- Chen, Y.E.; Mao, J.J.; Sun, L.Q.; Huang, B.; Ding, C.B.; Gu, Y.; Liao, J.Q.; Hu, C.; Zhang, Z.W.; Yuan, S. Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol. Plant. 2018, 164, 349–363. [Google Scholar] [CrossRef]
- Chen, X.; Sun, C.; Laborda, P.; Zhao, Y.; Palmer, I.; Fu, Z.Q.; Liu, F. Melatonin treatment inhibits the growth of Xanthomonas oryzae pv. oryzae. Front. Microbiol. 2018, 9, 22–80. [Google Scholar] [CrossRef]
- Shi, H.; Jiang, C.; Ye, T.; Tan, D.X.; Reiter, R.J.; Zhang, H.; Liu, R.; Chan, Z. Comparative Physiological, Metabolomic, and Transcriptomic Analyses Reveal Mechanisms of Improved Abiotic Stress Resistance in Bermuda Grass [Cynodon dactylon (L). Pers.] by Exogenous Melatonin. J. Exp. Bot. 2015, 66, 681–694. [Google Scholar] [CrossRef]
- Khan, A.; Numan, M.; Khan, A.L.; Lee, I.J.; Imran, M.; Asaf, S.; Al-Harrasi, A. Melatonin: Awakening the defense mechanisms during plant oxidative stress. Plants 2020, 9, 407. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Alamri, S.; Alsubaie, Q.D.; Ali, H.M.; Ibrahim, A.A.; Alsadon, A. Potential roles of melatonin and sulfur in alleviation of lanthanum toxicity in tomato seedlings. Ecotoxicol. Environ. Saf. 2019, 180, 656–667. [Google Scholar] [CrossRef]
- Martinez, V.; Nieves-Cordones, M.; Lopez-Delacalle, M.; Rodenas, R.; Mestre, T.C.; Garcia-Sanchez, F.; Rivero, R.M. Tolerance to stress combination in tomato plants: New insights in the protective role of melatonin. Molecules 2018, 23, 535. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wang, Q.; Shah, F.A.; Liu, W.; Wang, D.; Huang, S.; Wu, L. Exogenous melatonin confers cadmium tolerance by coun-terbalancing the hydrogen peroxide homeostasis in wheat seedlings. Molecules 2018, 23, 799. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tan, D.X.; Liang, D.; Chang, C.; Jia, D.; Ma, F. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J. Exp. Bot. 2015, 66, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; Zhu, T.; Zhao, C.; Li, L.; Chen, M. The role of melatonin in salt stress responses. Int. J. Mol. Sci. 2019, 20, 1735. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Flowers, T.J.; Lauchli, A. Sodium versus potassium:substitution and compartmentation. In Encyclopedia of Plant Physiology New Series; Lauchli, A., Bieleski, R.L., Eds.; Springer-Verlag: Berlin, Germany, 1983; Volume 15, pp. 651–681. [Google Scholar]
- Chen, Q.; Qi, W.B.; Reiterc, R.J.; Wei, W.; Wang, B.M. Exogenously applied melatonin stimulates root growth and raises endogenous indoleacetic acid in roots of etiolated seedlings of Brassica juncea. J. Plant Physiol. 2009, 66, 324–328. [Google Scholar] [CrossRef]
- Kaya, C.; Okant, M.; Ugurlar, F.; Alyemeni, M.N.; Ashraf, M.; Ahmad, P. Melatonin-mediated nitric oxide improves tolerance to cadmium toxicity by reducing oxidative stress in wheat plants. Chemosphere 2019, 225, 627–638. [Google Scholar] [CrossRef]
- Sun, L.; Li, X.; Wang, Z.; Sun, Z.; Zhu, X.; Liu, S.; Wang, Y. Cold priming induced tolerance to subsequent low temperature stress is enhanced by melatonin application during recovery in wheat. Molecules 2018, 23, 1091. [Google Scholar] [CrossRef]
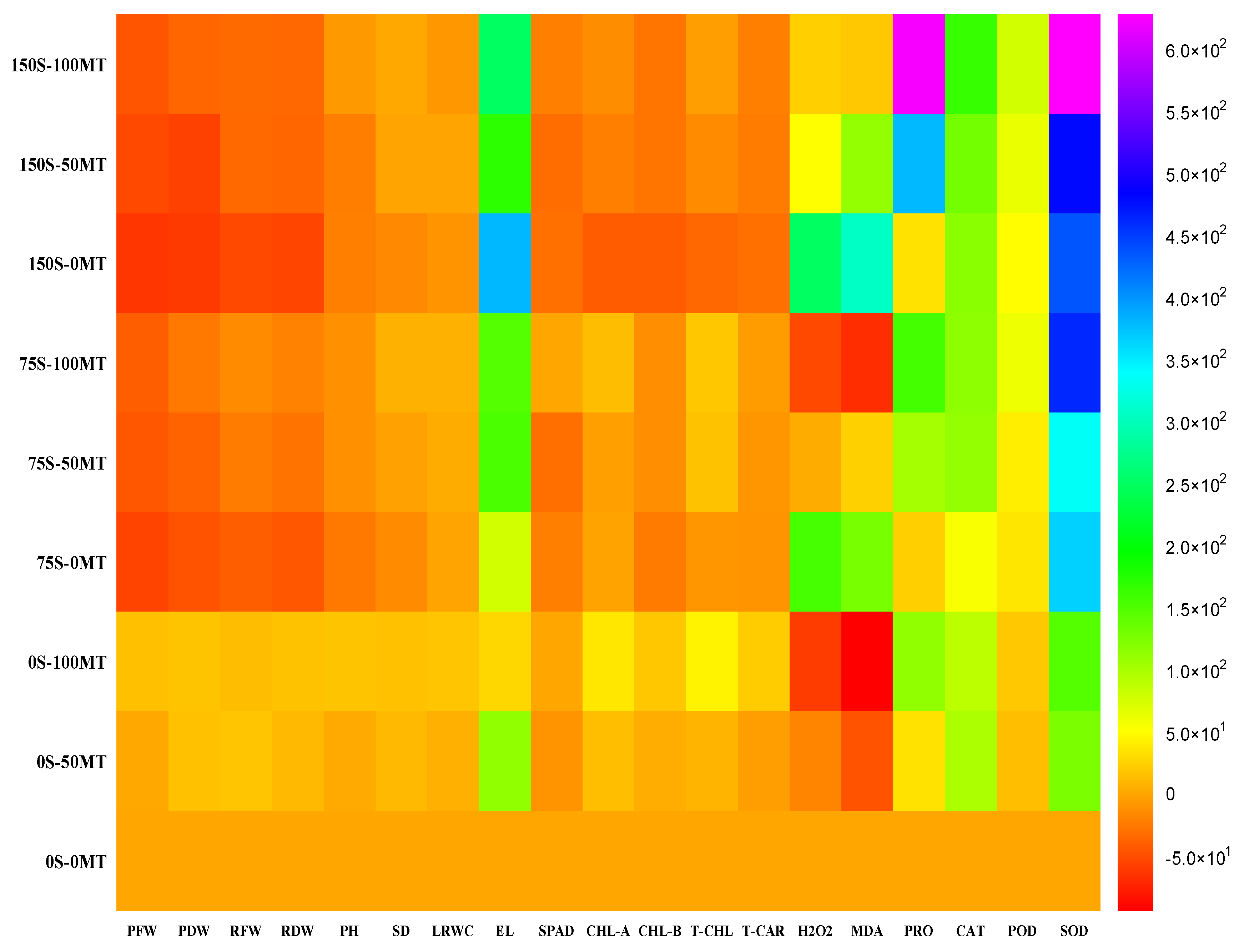
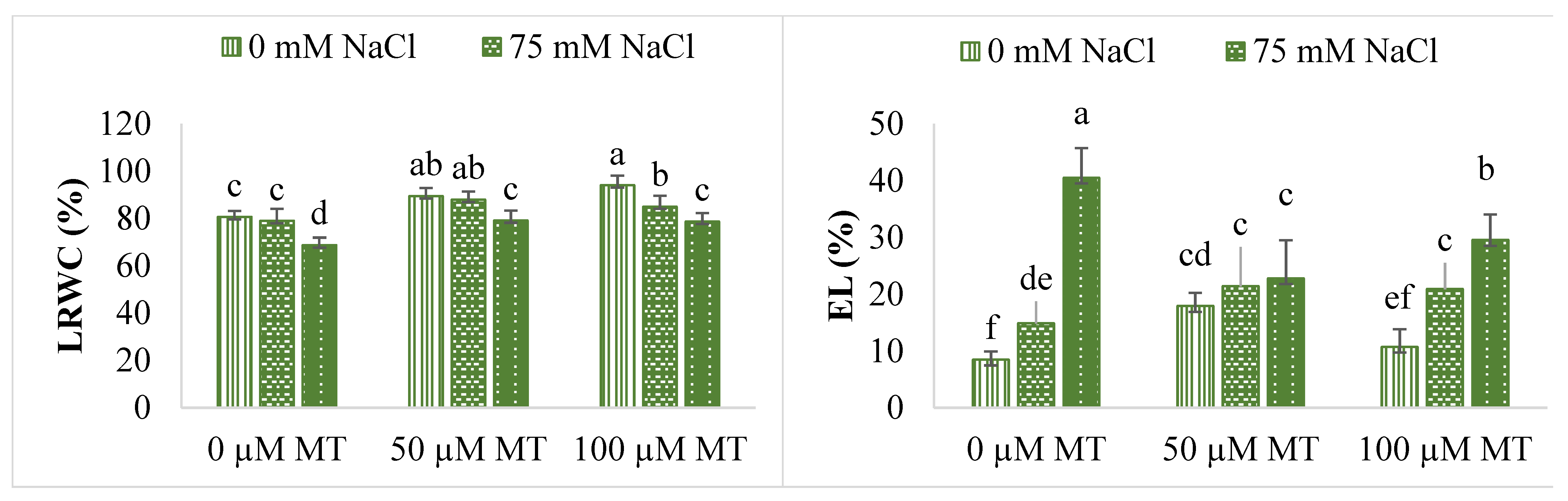


| NaCl (mM) | MT (µM) | Plant Fresh Weight (g plant−1) | Plant Dry Weight (g plant−1) | Root Fresh Weight (g plant−1) | Root Dry Weight (g plant−1) | Plant Height (cm) | Stem Diameter (mm) |
|---|---|---|---|---|---|---|---|
| 0 | 0 | 6.58 ± 0.12 b | 1.19 ± 0.05 b | 6.49 ± 0.6 b | 0.59 ± 0.03 b | 26.8 ± 1.2 b | 3.54 ± 0.05 b,c |
| 50 | 6.61 ± 0.14 b | 1.36 ± 0.07 a | 7.56 ± 0.3 a | 0.65 ± 0.02 a | 27.27 ± 1.3 b | 3.91 ± 0.06 a,b | |
| 100 | 7.48 ± 0.11 a | 1.38 ± 0.05 a | 7.28 ± 0.0.4 a | 0.68 ± 0.02 a | 31.19 ± 1.1 a | 4.06 ± 0.03 a | |
| 75 | 0 | 2.89 ± 0.04 e | 0.62 ± 0.02 d | 3.78 ± 0.05 f | 0.32 ± 0.05 f | 19.84 ± 1.5 e | 2.98 ± 0.02 d |
| 50 | 3.59 ± 0.07 d | 0.73 ± 0.01 c,d | 4.88 ± 0.07 d | 0.42 ± 0.03 c,d | 23.42 ± 1.3 c,d | 3.41 ± 0.05 c | |
| 100 | 3.84 ± 0.09 cd | 0.87 ± 0.02 c | 5.45 ± 0.03 c | 0.47 ± 0.06 c | 23.33 ± 1.6 c,d | 3.74 ± 0.02 a,c | |
| 150 | 0 | 2.37 ± 0.3 e | 0.45 ± 0.02 e | 3.04 ± 0.02 g | 0.26 ± 0.03 g | 20.88 ± 1.4 d,e | 2.93 ± 0.01 d |
| 50 | 3.08 ± 0.3 d | 0.50 ± 0.043 | 4.20 ± 0.05 e | 0.37 ± 0.02 d | 20.57 ± 1.3 d,e | 3.48 ± 0.03 b,c | |
| 100 | 3.46 ± 0.4d | 0.75 ± 0.02 c | 4.25 ± 0.02 e | 0.38 ± 0.02 d | 24.73 ± 1.7 b,c | 3.57 ± 0.02 b,c |
| NaCl (mM) | MT (µM) | CRV (SPAD) | Chlorophyll a (mg g −1) | Chlorophyll b (mg g−1) | Total Chlorophyll (mg g−1) | Total Carotenoid Content (mg g−1) |
|---|---|---|---|---|---|---|
| 0 | 0 | 47.00 ± 2.2 a | 0.75 ± 0.1 c | 0.28 ± 0.03 b | 0.94 ± 0.03 c | 5.59 ± 0.12 b |
| 50 | 41.90 ± 2.4 b | 0.85 ± 0.3 a,b | 0.29 ± 0.02 b | 1.01 ± 0.06 b | 5.28 ± 0.15 b | |
| 100 | 47.00 ± 2.4 a | 1.02 ± 0.2 a | 0.33 ± 0.01 a | 1.34 ± 0.04 a | 6.74 ± 0.17 a | |
| 75 | 0 | 36.40 ± 1.8 c | 0.73 ± 0.07 c | 0.21 ± 0.02 d | 0.85 ± 0.03 c | 4.96 ± 0.14 c |
| 50 | 32.13 ± 1.9 d | 0.72 ± 0.04 c | 0.24 ± 0.04 c | 1.08 ± 0.07 b | 5.03 ± 0.16 c | |
| 100 | 46.77 ± 1.5 a | 0.84 ± 0.03 a,b | 0.24 ± 0.05 c | 1.11 ± 0.05 a,b | 5.25 ± 0.13 b | |
| 150 | 0 | 32.40 ± 1.1 d | 0.43 ± 0.04 f | 0.16 ± 0.06 e | 0.60 ± 0.07 d | 3.85 ± 0.15 e |
| 50 | 31.50 ± 1.7 d | 0.58 ± 0.02 d,e | 0.20 ± 0.04 d | 0.79 ± 0.02 c | 4.21 ± 0.18 d | |
| 100 | 36.50 ± 1.3 c | 0.64 ± 0.05 d | 0.20 ± 0.04 d | 0.89 ± 0.01 c | 4.31 ± 0.11 d |
| NaCl (mM) | MT (µM) | H2O2 (mmol kg–1) | MDA (mmol kg–1) | Proline (mmol kg−1) | CAT (eu g−1) | POD (eu g−1) | SOD (eu g−1) |
|---|---|---|---|---|---|---|---|
| 0 | 0 | 4.55 ± 0.11 e | 2.16 ± 0.14 d | 0.09 ± 0.01 f | 849.12 ± 17.65 f | 10,284.93 ± 22.54 f | 540.62 ± 10.44 h |
| 50 | 3.66 ± 0.14 f | 1.13 ± 0.05 e | 0.12 ± 0.03 e | 1669.89 ± 18.13 d | 11,651.22 ± 24.62 e | 1211.77 ± 17.53 g | |
| 100 | 1.78 ± 0.16 h | 0.12 ± 0.02 f | 0.19 ± 0.02 c,d | 1592.38 ± 16.43 d | 12,210.89 ± 25.77 e | 1330.96 ± 16.40 f | |
| 75 | 0 | 11.56 ± 1.10 b | 4.88 ± 0.11 b | 0.11 ± 0.02 e,f | 1299.62 ± 12.55 e | 13,952.95 ± 29.48 d,e | 2511.04 ± 15.90 d |
| 50 | 4.66 ± 1014 e | 2.66 ± 0.06 c | 0.18 ± 0.04 c,d | 1790.30 ± 15.98 c | 14,391.24 ± 24.85 d | 2345.81 ± 18.56 e | |
| 100 | 2.10 ± 0.12 g | 0.67 ± 0.01 g | 0.23 ± 0.04 c | 1817.91 ± 19.23 c | 16,441.12 ± 25.71 b | 3029.99 ± 17.21 b | |
| 150 | 0 | 15.88 ± 1.14 a | 8.74 ± 1.12 a | 0.12 ± 0.01 e | 1832.22 ± 13.86 c | 15,268.32 ± 22.68 c | 2890.17 ± 19.05 c |
| 50 | 6.79± 0.11 c | 4.55± 0.23 b | 0.43 ± 0.02 b | 1944.96 ± 10.43 b | 16,629.69 ± 30.19 b | 3124.15 ± 14.88 b | |
| 100 | 5.56 ± 0.13 d | 2.56 ± 0.08 c | 0.65 ± 0.04 a | 2237.35 ± 11.77 a | 18,074.17 ± 20.80 a | 3930.75 ± 20.67 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dadasoglu, E.; Turan, M.; Ekinci, M.; Argin, S.; Yildirim, E. Alleviation Mechanism of Melatonin in Chickpea (Cicer arietinum L.) under the Salt Stress Conditions. Horticulturae 2022, 8, 1066. https://doi.org/10.3390/horticulturae8111066
Dadasoglu E, Turan M, Ekinci M, Argin S, Yildirim E. Alleviation Mechanism of Melatonin in Chickpea (Cicer arietinum L.) under the Salt Stress Conditions. Horticulturae. 2022; 8(11):1066. https://doi.org/10.3390/horticulturae8111066
Chicago/Turabian StyleDadasoglu, Esin, Metin Turan, Melek Ekinci, Sanem Argin, and Ertan Yildirim. 2022. "Alleviation Mechanism of Melatonin in Chickpea (Cicer arietinum L.) under the Salt Stress Conditions" Horticulturae 8, no. 11: 1066. https://doi.org/10.3390/horticulturae8111066
APA StyleDadasoglu, E., Turan, M., Ekinci, M., Argin, S., & Yildirim, E. (2022). Alleviation Mechanism of Melatonin in Chickpea (Cicer arietinum L.) under the Salt Stress Conditions. Horticulturae, 8(11), 1066. https://doi.org/10.3390/horticulturae8111066










