Abstract
Ultraviolet B (UV-B) irradiation has been demonstrated to play a synergistic effect with wounding in enhancing the accumulation of phenolic antioxidants in carrots. However, little is known about the molecular mechanisms of UV-B treatment on wounded carrots. In this study, wounded carrots were exposed to different doses of UV-B light, then stored at 15 °C for 2 d. The results showed that the content of phenolic compounds in 1 KJ m−2 UV-B–treated samples was 415% and 247% higher than that of whole and wounded carrots, respectively. Based on this, 1 KJ m−2 was selected as the optimal dose of UV-B treatment and used for further analysis. In addition, UV-B treatment greatly enhanced the enzyme activity of phenylalanine ammonia lyase (PAL) and the contents of phenolic profiles, especially 3-O-caffeoylquinic acid (3-CQA). Transcriptome analysis found that UV-B treatment could accelerate the KEGG pathways involved in signal transduction and secondary substance metabolism. The differentially expressed genes (DEGs) in phenolics biosynthesis–related metabolic processes of shikimate pathway and phenylpropanoid biosynthesis were extensively upregulated by UV-B treatment. Our results provided fundamental information for a better understanding of the molecular regulation mechanism of UV-B treatment in promoting the accumulation of phenolic compounds in wounded carrots.
1. Introduction
Phenolic compounds represent the most active and abundant class of plant secondary metabolites, which can act as dietary antioxidants and disease-preventing molecules that have potential benefits for preventing a grand array of chronic diseases such as cardiovascular and cerebrovascular diseases, cancer, diabetes and neurodegenerative diseases [1,2]. The recommended daily requirement of phenolic compounds is estimated as high as 1 g, a value that is 10 times higher than vitamin C and 100 times higher than vitamin E and carotenoids, respectively [3]. In view of this, the use of feasible technologies to enhance the levels of polyphenols in food plants is beneficial for human health.
In the last decade, much research evidence has proven that mechanical injury resulting from fresh-cut processing can trigger the biosynthesis of phenolic components and thereby result in the increase in total phenolic content (TPC) in various horticultural crops, such as carrot, purple onion, stem lettuce and pitaya [4,5,6,7]. Among these crops, carrot receives more attention because of its observed high enhancement of phenolic compounds and has been recognized as a model system to further explore if other abiotic stresses can intensify this wound-induced biochemical reaction. It is well reported that many approaches including hormone, herbicide, hyperoxia storage, water stress and UV irradiation can strengthen the accumulation of phenolic antioxidants in wounded carrots [8,9,10,11,12]. By analyzing the effects of each additional stress strategy, we found that the effect of hormones seems limited, since the TPC of ethylene (ET)-treated shredded carrots stored for 6 d at 15 °C was only three times higher than its initial value [8]. Although the application of glyphosate induced a large accumulation of phenolic profiles in wounded carrot, it would not be allowed in the handling of edible materials [9]. Moreover, both hyperoxia storage and water stress brought a considerable enhancement of TPC in wounded carrots; however, their operating conditions were harsh and not easy to achieve [10,11]. In comparison with other abiotic stresses, UV radiation, especially UV-B, might be a relatively safer, simpler, cheaper and more efficient way to intensify the production of phenolic compounds in wounded carrots. As reported by Formica-Oliveira et al. [12], shredded carrots with UV-B irradiation attained the highest phenolic increments of 498% after 3 d storage at 15 °C. They also found that the single effect of UV-B was superior to UV-C alone or a combination of UV-B and UV-C.
To better manipulate the production of phenolic compounds in wounded produce, many regulatory mechanisms have been elucidated by carrot tissue. For instance, it has been demonstrated that the signal-molecules reactive oxygen species (ROS) play a vital role in the wound-induced accumulation of phenolic compounds in carrot, whereas ET and jasmonic acid (JA) are essential to modulate ROS levels [13]. Recently, this theory has been validated and moved forward, that UV radiation provokes the generation of ROS, thereby eliciting the cross-talk of ROS, ET and JA [14]. However, knowledge concerning molecular responses related to wound-induced accumulation of phenolic compounds in carrot still remains poor. The major objective of this manuscript was to understand the changes in transcription levels of wounded carrots in response to UV-B irradiation. Therefore, the optimal dose of UV-B was determined firstly according to the amount of phenolics in wounded carrots and used further for transcriptome analysis. Differentially expressed genes (DEGs) were identified in primary (shikimate pathway) and secondary (phenylpropanoid biosynthesis) metabolisms of phenolic compounds. The information presented herein will contribute to understanding the molecular mechanism of UV-induced phenolics accumulation in wounded carrot and guide the enhancement of dietary polyphenols in wounded produce.
2. Materials and Methods
2.1. Plant Materials and Treatments
The fresh carrots (Daucus carota L. cv. Sanhongqicun) were purchased from a local supermarket (Jinan, China) and immediately transferred to the laboratory. After cleaning, all carrots with no mechanical damage and similar size (the diameter of the middle part was 2–3 cm, the total length was 20–25 cm) were selected. The selected samples were conditioned overnight at 15 °C before the wounding process.
In the first experiment, whole carrots were cut into slices (0.4 cm in thickness) by a sharp stainless steel knife and then cut along the diameter into quarters. According to the method reported by Surjadinata and Cisneros-Zevallos [4], the calculated wounding intensity (surface area/weight) of quarter-slice was 0.63 m2 Kg−1. During the wounding process, the prepared wounded carrots were covered with plastic film to avoid moisture loss. After cutting, the wounded carrots were placed horizontally without overlap on a clean plastic plate below four UV-B lamps (T8, 20 W, Zhonghuo Purification Technology Co., Ltd., Nanjing, China). The peak emission of the UV-B lamp was 297 nm. The distance of the UV-B lamps from the wounded carrots was adjusted to reach the required intensity of 6.67 W m−2 by utilizing a digital radiometer (Photo-electric Instrument Factory of Beijing Normal University, Beijing, China). To select the optimal dose of UV-B irradiation, wounded carrots were irradiated on two sides with different doses of 0.25, 0.5, 1, 2, 3, 4 KJ m−2, respectively. The irradiation dose was achieved by changing the exposure time (1 KJ m−2 required 2.5 min exposure). Wounded carrots with no irradiation treatment were used as control. After UV-B treatments, all control and UV-B–treated samples were placed in rigid polypropylene boxes with holes in the surface and stored in a constant temperature and humidity chamber for 2 days. The storage condition was set as 15 °C, 95% relative humidity and no light. To estimate the contribution made by wounding, whole carrots were also used and placed in the chamber. The content of phenolic compounds was measured every 24 h to determine the optimal dose of UV-B, while other physiological parameters were evaluated by the selected dose. At each sampling point, fresh samples were taken from the chamber, frozen in liquid nitrogen, and then kept in polyethylene bags at −80 °C until use.
In the second experiment, the influence of UV-B irradiation on transcription levels of wounded carrots was investigated. According to the results of the first experiment, the dose of UV-B irradiation was selected as 1 KJ m−2. The quarters of carrot slices were obtained as described above. After the wounding process, one half of the samples were irradiated by UV-B light with the dose of 1 KJ m−2. Subsequently, the whole carrots, wounded carrots and UV-B–treated wounded carrots were stored in the same condition as the first experiment for 1 h. For each group, we prepared three biological replicates, totaling twelve carrots. After storage, all fresh tissues were sampled, frozen in liquid nitrogen, and saved in sterile tubes at −80 °C for RNA separation.
2.2. Total Soluble Phenolics (TSP) Content Analysis
Extraction of phenolic compounds was carried out according to the method of Han et al. [15]. Frozen tissues were ground in liquid nitrogen by a mortar (A11, IKA Co., Ltd., Staufen, Germany). Thereafter, the powder (5 g) was homogenized with 25 mL cold methanol, and the homogenate was kept in a 50 mL centrifuge tube at 4 °C for 12 h. Following centrifugation (13,000× g, 20 min), the clear supernatant was collected for subsequent measurement.
The TSP content of carrot was assessed using the Folin–Ciocalteu method. The reaction mixture was prepared in a glass tube by mixing the supernatant (0.5 mL) with the distilled water (1.5 mL) and the Folin–Ciocalteu reagent (1 mL). After standing for 10 min, 1 mL 7.5% (M/V) of Na2CO3 solution was added to the mixture to start the reaction. The tubes were immediately put in the water bath at 25 °C for 2 h before recording the absorbance at 765 nm by a spectrophotometer (V-1100d, AODA Instrument Co., Ltd., Beijing, China). The TSP content was presented as grams of chlorogenic acid (CA) per kilogram.
2.3. HPLC Analysis of Individual Phenolic Compounds
The procedure for the determination of phenolic profiles was adapted from Surjadinata and Cisneros-Zevallos [4] with some modifications. The same supernatant for TSP measurement was used for the identification and quantitation of phenolic profiles. The high-performance liquid chromatography (HPLC) system (Shimadzu LC-20A, Toyko, Japan) was equipped with a UV diode array detector (Shimadzu SPD-20A, Toyko, Japan). Briefly, 10 μL of filtered samples were injected in the HPLC system and separated on a 4.6 mm × 250 mm, 5 μL, C-18 reverse-phase column (Shimadzu InertSustain C18, Toyko, Japan) eluted with mobile phase A (water/HCl, pH 2.3) and phase B (acetonitrile) at a constant flow rate of 0.8 mL/min. The oven temperature was held at 40 °C. The gradient program was as follows: 0–10 min, 5–18% B; 10–15 min, 18–98% B; 15–30 min, 98% B; 30–31 min, 98–5% B; 31–40 min), 5% B. The phenolic profiles were measured at 280 and 320 nm. The identifications of individual phenolics were achieved based on their retention times and UV spectra, as compared with authentic standards and previous reports [16,17]. The concentrations of all individual compounds were calculated according to standard calibration curves, and the units were expressed as mg kg−1.
2.4. Phenylalanine Ammonia Lyase (PAL) Enzyme Activity Assay
The PAL activity was determined based on the method of Meng et al. [18] with slight modifications. Ground frozen powder of 2 g added with 0.2 g polyvinyl polypyrrolidone (PVPP) was extracted in 5 mL cold borax–borate buffer (50 mM, pH 8.8, containing 2 mmol L−1 EDTA and 5 mmol L−1 β-mercaptoethanol). Following centrifugation (13,000× g, 20 min), the clear supernatant was used for the enzyme assay. The reaction system contains 2 mL borax–borate buffer, 0.5 mL L-phenylalanine (20 mmol L−1) and 1 mL PAL crude enzyme. The mixture was incubated at 37 °C for 60 min. After incubation, 0.1 mL of HCl (6 mol L−1) was added to terminate the reaction. The absorbance at 290 nm was recorded before and after incubation. One unit of PAL activity was defined as 0.01 increase in absorbance per min per mass of tissue and expressed as U Kg−1.
2.5. RNA Sequencing and Data Analyses
Frozen tissues obtained in the second experiment were used for RNA sequencing. For each group, three biological repeats were applied. Total RNA was extracted from each sample by a Trizol Kit (Magen Biotechnology Co., Ltd., Guangzhou, China). The enriched mRNA was fragmented with the ABclonal First Synthesis Reaction Buffer, and the mRNA fragments were used as templates for cDNA synthesis. The double-stranded cDNAs were then subjected to end repair, poly (A) addition and adapter ligation to prepare the pair-end library. Adapter-ligated cDNAs were used for PCR amplification. PCR products were purified by AMPure XP system, and library quality was evaluated by Agilent Bioanalyzer 4150 system. The cDNA was sequenced using Illumina Novaseq 6000/MGISEQ-T7 instrument by Shanghai Applied Protein Technology Co., Ltd., Shanghai, China. The generated raw reads were filtered by removing the impure sequences (adaptor reads and low-quality reads which containing more than 60% low-quality bases, or N ratio is greater than 5% reads) to obtain the clean reads for subsequent analyses.
The clean reads were mapped to a reference carrot transcriptome (https://www.ncbi.nlm.nih.gov/genome/?term=Daucus+carota, accessed on 6 May 2016) using HISAT2 software (v 2.2.1) [19] to obtain the mapped reads for subsequent analyses. The gene expression levels were normalized by the value of FPKM (fragments per kilobase of transcript per million mapped reads) using featureCounts software (v 2.0.1). Differential expression analysis was performed using the DESeq2 (http://bioconductor.org/packages/release/bioc/html/DESeq2.html, accessed on 9 May 2021) [20], and the DEGs between different samples were identified according to the two conditions of |log2fold change| > 1 and Padj (adjusted p-value) < 0.05. DEGs with KEGG orthology descriptions were mapped to their associated KEGG pathways. KEGG pathways (http://www.genome.jp/kegg/, accessed on 9 May 2021) [21] with p < 0.05 through Fisher’s exact test were regarded as significantly enhanced. Heat-mapping of phenylpropanoid metabolism-related DEGs was performed using R software (v 4.0.5) [22].
2.6. Quantitative Real-Time PCR (qRT-PCR) Analysis
Ten genes encoding the three key enzymes PAL, 4CL (4-coumaric acid coenzyme A ligase) and HCT (shikimate O-hydroxycinnamoyltransferase) in phenylpropanoid metabolism were selected for PCR verification. Total RNA was extracted by a Trizol Kit (Magen Biotechnology Co., Ltd., Guangzhou, China). The cDNA was synthesized using ReScriptTM II RT reagent Kit with gDNA Eraser (R712, Nobelab Biotechnology Co., Ltd., Beijing, China). The relative expression levels of selected genes were analyzed by qRT-PCR using SYBR® Premix Ex TaqTM (RR420A, TakaRa) in the Applied Biosystems 7500 Real-Time PCR System (Waltham, MA, USA). The reaction procedure was as follows: 95 °C for 10 min, then 95 °C for 15 s and 60 °C for 34 s, 40 cycles. The relative gene expression level was calculated using 2−ΔΔCT method. The selected genes and their sequences of primers are given in Supplementary Table S1.
2.7. Statistical Analysis
All data were processed by Microsoft Excel 2019, and the results were represented as mean ± standard deviation (SD) of three replicates. Data were analyzed by one-way analysis of variance (ANOVA), and statistically significant differences were detected based on least significant differences (LSD) tests at p < 0.05 using SPSS software (V 23, IBM CO., New York, NY, USA).
3. Results and Discussion
3.1. TSP Content
The TSP contents of whole, wounded and UV-B–treated carrots are shown in Figure 1. Wounding caused a dramatic increase in TSP contents in carrots with prolonged storage time, as compared with the intact ones. However, the application of UV-B irradiation markedly strengthened this increasing trend of wounded carrots, and significantly higher (p < 0.05) levels of TSP contents were observed in all doses of UV-B treatments after storage of 12 h. These results were consistent with the previous findings, demonstrating that UV-B can be used as an additional abiotic stress on wounded carrots to enhance their amounts of phenolic compounds [12,17,23]. As interpreted by Du et al. [23], UV-B can induce photobiological stress and activate the defense system in both whole and wounded plant species, thereby increasing the biosynthesis of UV-B absorbing compounds, mostly polyphenols. Over the course of storage at 15 °C, UV-B–treated samples with 1 KJ m−2 underwent a more rapid increase in TSP content, relative to that of other doses. At the end of storage, the maximum value of TSP was observed in 1 KJ m−2 UV-B–treated samples, which was 415% and 247% higher than that of whole and wounded carrots, respectively. Based on this, 1 KJ m−2 was selected as the optimal dose of UV-B treatment and used for further analysis.
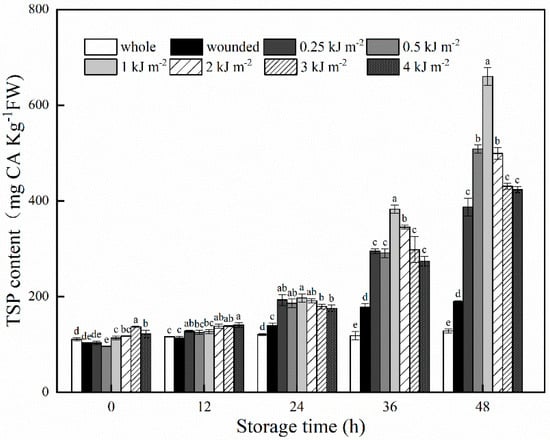
Figure 1.
TSP contents of whole, wounded and UV-B–treated carrots stored at 15 °C for 48 h. Data are expressed as the mean ± standard deviations of triplicate values. Lowercase letters represent significant difference (p < 0.05) among treatment factors at the same sampling time.
3.2. Phenolic Profiles
In order to figure out the changes of phenolic profiles that caused the observed increase in TSP content, the concentrations of individual phenolic compounds in whole, wounded and UV-B–treated carrots were determined by the HPLC system. The HPLC chromatograms (shown at 280 nm) are exhibited in Supplementary Figure S1. The identified phenolic profiles in whole carrots were 3-O-caffeoylquinic acid (3-CQA), ferulic acid (FA), 3,4-dicaffeoylquinic acid (3,4-diCQA) and 3,5-dicaffeoylquinic acid (3,5-diCQA). The results obtained herein were in accordance with previous reports that 3-CQA, and its isomers were the main phenolic compounds in carrot [10,12,16]. Notably, with prolonged storage time, phenolic profiles of wounded and UV-B–treated samples showed an additional presence of caffeic acid (CA) and 4,5-dicaffeoylquinic acid (4,5-diCQA) (Table 1). This finding was similar to Jacobo-Velázquez et al., who found that wounding alone or in combination with other abiotic stresses can induce the production of undetected components before [10]. Among these phenolic profiles, an evident change was observed in 3-CQA. Wounding induced a sharp increase in content of 3-CQA throughout storage, while the additional use of UV-B greatly improved this increase. After 48 h of storage, the content of 3-CQA in UV-B–treated samples was 396 mg kg−1, which was 17.2 times and 3.3 times that of whole and wounded samples. In comparation with 3-CQA, the concentrations of other phenolic profiles changed slightly and remained at relatively low levels during storage. As illustrated by Boerjan et al. [24], the biosynthesis of 3-CQA in wounded plants is part of the wound-healing process since it can be utilized as a precursor for lignin biosynthesis. Therefore, it was likely that UV-B further activated the defense reaction of wounded carrots and produced more 3-CQA, which mainly lead to the accumulation of TSP.

Table 1.
Individual phenolic compounds concentrations of whole, wounded and UV-B–treated carrots during storage at 15 °C for 48 h (mg Kg−1 FW).
3.3. Phenylalanine Ammonia Lyase (PAL) Enzyme Activity
The PAL activity of whole, wounded and UV-B–treated carrots is presented in Figure 2. Whole carrots showed a slight increase in PAL activity during storage; however, a great increase occurred in wounded carrots, as compared with the intact samples. The application of UV-B irradiation further strengthened this upward trend of PAL activity in wounded carrots, and significant differences (p < 0.05) were observed throughout storage time. The peak value of PAL activity in wounded carrots was observed at 24 h, whereas for UV-B–treated samples the highest levels were detected at 48 h. It is well known that PAL catalyzes the conversion of phenylalanine to trans-cinnamic acid, which is a key enzyme in the biosynthesis of phenolic compounds in plants [25]. Correspondingly, the variation trends of PAL activities were in accordance with the changes in TSP contents. This result confirmed the previous reports that UV-B irradiation played a synergistic effect with wounding in triggering an increased response on PAL activity and subsequently accelerated the production of phenolic compounds in carrots [17,23].
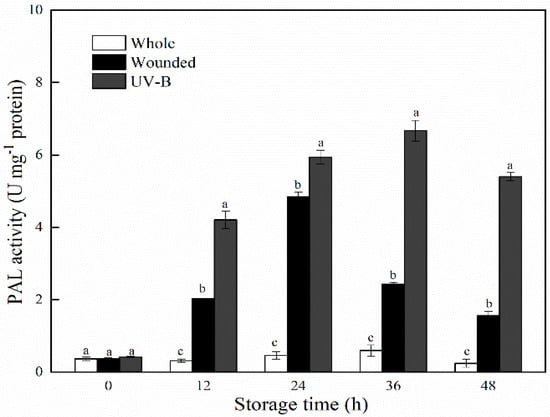
Figure 2.
PAL activities of whole, wounded and UV-B–treated carrots stored at 15 °C for 48 h. Data were expressed as the mean ± standard deviations of triplicate values. Lowercase letters represent significant difference (p < 0.05) among treatment factors at the same sampling time.
3.4. Summary of RNA-seq Data and Identification of DEGs
After storage at 15°C for 1 h, whole, wounded and UV-B–treated carrots were subjected to whole transcriptome sequencing. Whole carrots were used as control, marked with CK, while wounded and UV-B–treated carrots were marked with W and UV-B, respectively. As shown in Table 2, after sequencing and removing low-quality reads, a total of 812,462,796 clean reads were obtained, with approximately 90 million reads from each sample. Total mapped reads are the number of sequences that can be located on the genome, and unique mapped reads are those that map to only one position in the genome [26]. The number of total mapped reads and unique mapped reads were in the range of 76.1–85.3 million and 71.2–79.5 million, respectively. For each sample, the unique matching ratio was not less than 80%. In addition, approximately 94% of the acquired reads had quality scores >99.9 (Q30), and the percentage of GC content was over 43%. Overall, all these results indicated that the sequencing quality was high and could meet the requirement of further analysis.

Table 2.
Summary of RNA-seq data of whole (CK), wounded (W) and UV-B–treated (UV-B) carrots after storage at 15 °C for 1 h.
After processing by the software DESeq2, the differentially expressed genes (DEGs) in carrots with different treatments were identified. As shown in Figure 3, a total of 4788 DEGs consisting of 3734 upregulated genes and 1054 downregulated genes were detected in the pairwise comparison of W versus CK, and a total of 6946 DEGs consisting of 5017 upregulated genes and 1929 downregulated genes were detected in the pairwise comparison of UV-B versus CK. This result indicated that numerous genes in carrot were activated immediately after wounding to meet the need of plant defense response, whereas UV-B treatment intensified the transcriptional changes of wounded carrots. As compared with wounded samples, a total of 3221 DEGs consisting of 1952 upregulated genes and 1269 downregulated genes were detected in UV-B–treated samples.
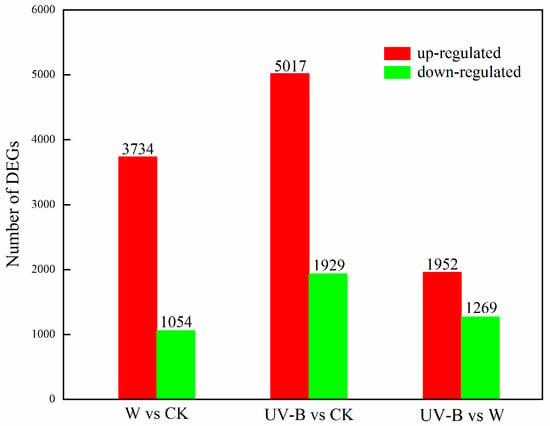
Figure 3.
Number of DEGs identified from carrots under different treatments.
3.5. Enrichment of KEGG Pathway Analysis
To further characterize the potential involvement of specific metabolic pathways in regulating the biosynthesis of phenolic compounds, all DEGs were investigated through the KEGG. The top 20 items of enriched KEGG pathways among different treatments were identified and are shown in Figure 4. In the comparison of W versus CK, the identified terms of enriched KEGG pathways were mainly classified into three categories, including signal transduction, primary substance metabolism and secondary substance metabolism, according to their functions (Figure 4A). Among them, “MAPK signaling pathway–plant” (ko04016, adjusted p-value = 8.31 × 10−7), “phenylpropanoid biosynthesis” (ko00940, adjusted p-value = 1.10 × 10−6) and “plant hormone signal transduction” (ko04075, adjusted p-value = 3.95 × 10−6) were the top three most highly enriched pathways. In a plant, the mitogen activated protein kinase (MAPK) signaling pathway has been reported to be related by the signal transduction of abiotic stresses and activation of stress-tolerance genes [27,28]. Moreover, the MAPK cascade could respond to the hormone signal and played synergistic roles with the hormone signaling pathway in plant defense responses [29]. In this study, these two pathways were activated promptly after wounding stress, which might play a primary signal role in modulating subsequent defense reaction. The pathway of “phenylpropanoid biosynthesis” was directly related to the biosynthesis of phenolic compounds. Here, approximately 37% of all background genes belonging to this pathway were greatly changed within 1 h after wounding, including 21 upregulated genes and 8 downregulated genes. When comparing UV-B versus W, in the first 10 items of enriched KEGG pathways, there were 10 items that could be found in W versus CK (Figure 4B). It was observed that UV-B treatment resulted in more upregulated genes in the first five items of “MAPK signaling pathway–plant” (ko04016, adjusted p-value = 5.07 × 10−6), “phenylpropanoid biosynthesis” (ko00940, adjusted p-value = 7.52 × 10−6), “metabolic pathways” (ko01100, adjusted p-value = 9.59 × 10−6), “biosynthesis of secondary metabolites” (ko01110, adjusted p-value = 1.63 × 10−4) and “plant hormone signal transduction” (ko04075, adjusted p-value = 1.12 × 10−2). This result might put forward the evidence that UV-B could further speed up the metabolic processes involved in signal transduction and secondary substance metabolism to synthesize more phenolic compounds in wounded carrots.
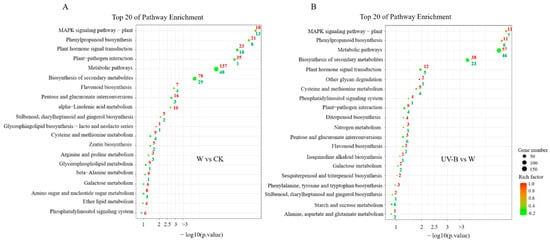
Figure 4.
KEGG pathway enrichment analysis of DEGs. (A) The pairwise comparison of W versus CK. (B) The pairwise comparison of UV-B versus W. Red color indicates the number of upregulated genes in each KEGG pathway; green indicates the number of down regulated genes in each KEGG pathway, respectively. Rich factor indicates the ratio of the number of DEGs located in each pathway to the number of all annotated genes in the same pathway.
3.6. DEGs Associated with the Biosynthesis of Phenolic Profiles
As a crucial precursor to the production of phenolic compounds, phenylalanine is produced predominantly in plastids via the shikimate pathway and is subsequently utilized to synthesize thousands of phenolic metabolites through the phenylpropanoid biosynthesis pathway [30]. Here, to visualize the molecular switches controlling phenylalanine formation and conversion, the partial metabolic pathways of the shikimate pathway and phenylpropanoid biosynthesis and the heat map of expression profiles of DEGs associated with these two pathways were generated (Figure 5). It was revealed that a total of 5 DEGs encoding 7 enzymes in the shikimate pathway and a total of 14 DEGs encoding 6 enzymes in the phenylpropanoid biosynthesis were identified. As compared with nonwounded carrots, wounding treatment significantly downregulated the expression of DEGs encoding enzymes of DAHP synthase, 3DD, SD and EPSP synthase, while it slightly enhanced the expression of DEGs encoding enzymes of ADT, PDT and TAT. This observation indicated that the shikimate pathway of carrots was inactive within a short time after wounding. However, UV-B treatment upregulated these DEGs in the shikimate pathway with the exception of the DEG encoding EPSP synthase, as compared with wounded carrots. The expression levels of DCAR_012164, which is encoded ADT and PDT, and DCAR_021258, which is encoded TAT in UV-B–treated samples, were significantly higher than those of intact and wounded samples. ADT and PDT catalyze the dehydration of prephenate to phenylpyruvate, and TAT catalyzes the transamination of phenylpyruvate to phenylalanine [31,32]. The upregulation of DEGs encoding these three enzymes suggested that UV-B stress can prompt the transcriptional levels of phenylalanine biosynthesis-related genes, thereby synthesizing more phenylalanine to provide raw materials for the production of phenolic compounds in wounded carrots. Furthermore, UV-B treatment brought a considerable upregulation of DEGs that were responsible for regulating the enzymes of PAL, 4CL, HCT and CSE in the pathway of phenylpropanoid biosynthesis, as compared with other two treatments. These DEGs included one PAL gene (DCAR_017697), one 4CL gene (DCAR_025617), two HCT genes (DCAR_004336, DCAR_016310) and two CSE genes (DCAR_025466, DCAR_016250). PAL and 4CL play an important role in catalyzing the formation of p-coumaroyl-CoA, whereas HCT is involved in the conversion of p-coumaroyl-CoA into p-CQA [33,34]. Following these steps, the caffeoylquinic acid is synthesized with the participation of C3′M [35]. In the current study, the increased accumulation of caffeoylquinic acid in UV-B–treated carrots might be closely related to the expression of these PAL-, 4CL- and HCT-related genes. In addition, it was worth noting that both wounding and UV-B treatments upregulated the expression level of CSE-related genes, which might explain why caffeic acid could be detected in wounded and UV-B–treated samples.
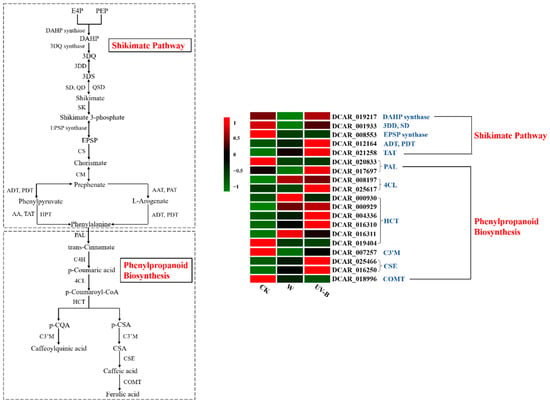
Figure 5.
Metabolic pathways of the shikimate pathway and phenylpropanoid biosynthesis. Heat map of expression profiles of DEGs associated with penylpropanoid biosynthesis in different samples after storage at 15 °C for 1 h. The DEGs were screened from two metabolic processes of the shikimate pathway and phenylpropanoid biosynthesis. Each column represents a treatment (CK, W and UV-B), and each row represents a screened DEG. The color scale indicates the expression value of each DEG: red color means high expression, and green color means low expression. E4P, D-erythrose 4-phosphate; PEP, phosphoenolpyruvate; DAHP, 2-dehydro-3-deoxy-D-arabino-heptolosonate-7-phosphate; 3DQ, 3-dehydroquinate; 3DD, 3-dehydroquinate dehydratase; 3DS, 3-dehydro-shikimate; SD, shikimate dehydrogenase; QD, quinate dehydrogenase; QSD, quinate/shikimate dehydrogenase; SK, shikimate kinase; EPSP, 5-enolpyruvylshikimate 3-phosphate; CS, chorismate synthase; CM, chorismate mutase; ADT, PDT, arogenate dehydratase/prephenate dehydratase; AAT, PAT, bifunctional aspartate aminotransferase and glutamate/aspartate-prephenate amino transferase; AA, aspartate aminotransferase; TAT, tyrosine aminotransferase; HPT, histidinol-phosphate aminotransferase; PAL, phenylalanine ammonium lyase; C4H, cinnamate-4-hydroxylase; 4CL, 4-coumarate coenzyme A ligase; HCT, shikimate O-hydroxycinnamoyltransferase; p-CQA, p-coumaroylquinic acid; p-CSA, p-coumaroylshikimic acid; C3′M, coumaroylquinate (coumaroylshikimate) 3’-monooxygenase; CQAs, caffeoylquinic acids; CSA, coumaroylshikimic acid; CSE, caffeoylshikimate esterase; COMT, caffeate O-methyl transferase.
3.7. Validation of DEGs Using qRT-PCR
To confirm the accuracy and reproducibility of the experimental data derived from RNA-Seq, 10 DEGs belonging to the three enzymes of PAL, 4CL and HCT were selected, and the expression level of each DEG was measured by qRT-PCR (Supplementary Figure S1). The symbol of each DEG has been described above. The results obtained from qRT-PCR were compared with the results of RNA-Seq by calculating log2 (fold change). As shown in Figure 6, the correlation analysis showed significantly positive correlation (R2 = 0.8394) between qPCR and RNA-seq, which verifies the reliability of RNA-seq.
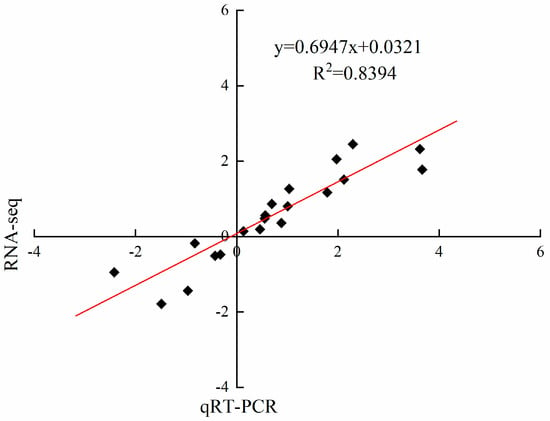
Figure 6.
Correlation of fold change analyzed by RNA-Seq (x axis) and qRT-PCR (y axis). The values of RNA-Seq and qRT-PCR were derived from Supplementary Table S2 and Figure S1.
4. Conclusions
In summary, our study revealed that UV-B irradiation could effectively promote the accumulation of phenolic compounds in wounded carrots and pronouncedly improve the phenolics biosynthesis-related enzyme activity of PAL. HPLC analysis showed that 3-CQA was the predominant phenolic profile in wounded carrot, and UV-B treatment greatly enhanced its increasing trend during storage. Transcriptome sequencing and enrichment of KEGG pathway analysis showed that UV-B might further speed up the metabolic processes involved in signal transduction and secondary substance metabolism to synthesize more phenolic compounds in wounded carrots. According to the analysis of DEGs in the metabolic pathways of the shikimate pathway and phenylpropanoid biosynthesis, it was found that UV-B treatment induced an extensive upregulation of DEGs in these two pathways, especially the DEGs encoding ADT, PDT, TAT, PAL, 4CL, HCT and CSE. The results can provide a step for understanding the molecular regulation mechanism of UV-B treatment in inducing the accumulation of phenolic compounds in wounded carrots.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae8100896/s1, Figure S1: The relative expression levels of 10 selected DEGs; Table S1: Primers of the phenylpropanoid metabolism-related genes in carrot; Table S2: FPKM values of DEGs associated with the biosynthesis of phenolic profiles.
Author Contributions
Conceptualization, C.H.; methodology, M.F.; validation, W.Z., Y.T., Z.L. and C.H.; formal analysis, Z.L. and X.X.; investigation, W.Z.; resources, M.F.; data curation, Y.T. and X.X.; writing—original draft preparation, W.Z.; writing—review and editing, C.H.; supervision, M.F.; project administration, C.H.; funding acquisition, M.F. and C.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 31901754), the Innovation Pilot Project of Integration of Science, Education and Industry of Qilu University of Technology (No. 2022PY021, No. 2022PY1011) and the Youth Science and Technology Innovation Team of Shandong Province (No. 2019KJF010).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Condezo-Hoyos, L.; Gazi, C.; Pérez-Jiménez, J. Design of polyphenol-rich diets in clinical trials: A systematic review. Food Res. Int. 2021, 149, 110655. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of phenolic compounds in human disease: Current knowledge and future prospects. Molecules 2022, 27, 223. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef] [PubMed]
- Surjadinata, B.B.; Cisneros-Zevallos, L. Biosynthesis of phenolic antioxidants in carrot tissue increases with wounding intensity. Food Chem. 2012, 134, 615–624. [Google Scholar] [CrossRef]
- Berno, N.D.; Tezotto-Uliana, J.V.; Tadeu, D.S.D.C.; Kluge, R.A. Storage temperature and type of cut affect the biochemical and physiological characteristics of fresh-cut purple onions. Postharvest Biol. Technol. 2014, 93, 91–96. [Google Scholar] [CrossRef]
- Han, C.; Zhen, W.N.; Chen, Q.M.; Fu, M.R. UV-C irradiation inhibits surface discoloration and delays quality degradation of fresh-cut stem lettuce. LWT-Food Sci. Technol. 2021, 147, 111533. [Google Scholar] [CrossRef]
- Li, X.A.; Long, Q.H.; Gao, F.; Han, C.; Jin, P.; Zheng, Y.H. Effect of cutting styles on quality and antioxidant activity in fresh-cut pitaya fruit. Postharvest Biol. Technol. 2017, 124, 1–7. [Google Scholar] [CrossRef]
- Heredia, J.B.; Cisneros-Zevallos, L. The effect of exogenous ethylene and methyl jasmonate on pal activity, phenolic profiles and antioxidant capacity of carrots (Daucus carota) under different wounding intensities. Postharvest Biol. Technol. 2019, 51, 242–249. [Google Scholar] [CrossRef]
- Becerra-Moreno, A.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Plants as biofactories: Glyphosate-induced production of shikimic acid and phenolic antioxidants in wounded carrot tissue. J. Agric. Food Chem. 2012, 60, 11378–11386. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Martinez-Hernandez, G.B.; Rodriguez, S.; Cao, C.M.; Cisneros-Zevallos, L. Plants as biofactories: Physiological role of reactive oxygen species on the accumulation of phenolic antioxidants in carrot tissue under wounding and hyperoxia stress. J. Agric. Food Chem. 2011, 59, 6583–6593. [Google Scholar] [CrossRef]
- Becerra-Moreno, A.; Redondo-Gil, M.; Benavides, J.; Nair, V.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Combined effect of water loss and wounding stress on gene activation of metabolic pathways associated with phenolic biosynthesis in carrot. Front Plant Sci. 2015, 6, 837. [Google Scholar] [CrossRef] [PubMed]
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Díaz-López, V.; Artés, F.; Artés-Hernández, F. Effects of UV-B and UV-C combination on phenolic compounds biosynthesis in fresh-cut carrots. Postharvest Biol. Technol. 2017, 127, 99–104. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; González-Agüero, M.; Cisneros-Zevallos, L. Cross-talk between signaling pathways: The link between plant secondary metabolite production and wounding stress response. Sci. Rep. 2015, 5, 8608. [Google Scholar] [CrossRef] [PubMed]
- Surjadinata, B.B.; Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. Physiological role of reactive oxygen species, ethylene, and jasmonic acid on UV light induced phenolic biosynthesis in wounded carrot tissue. Postharvest Biol. Technol. 2021, 172, 111388. [Google Scholar] [CrossRef]
- Han, C.; Zuo, J.H.; Wang, Q.; Xu, L.J.; Zhai, B.Q.; Wang, Z.S.; Dong, H.Z.; Gao, L.P. Effects of chitosan coating on postharvest quality and shelf life of sponge gourd (luffa cylindrica) during storage. Sci. Hortic. 2014, 166, 1–8. [Google Scholar] [CrossRef]
- Cuéllar-Villarreal, M.D.R.; Ortega-Hernández, E.; Becerra-Moreno, A.; Welti-Chanes, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Effects of ultrasound treatment and storage time on the extractability and biosynthesis of nutraceuticals in carrot (Daucus carota). Postharvest Biol. Technol. 2016, 119, 18–26. [Google Scholar] [CrossRef]
- Avena-Bustillos, R.J.; Du, W.X.; Woods, R.; Olson, D.; Iii, A.P.B.; Mchugh, T.H. Ultraviolet-B light treatment increases antioxidant capacity of carrot products. J. Sci. Food Agric. 2012, 92, 2341–2348. [Google Scholar] [CrossRef]
- Meng, X.H.; Li, B.Q.; Liu, J.; Tian, S.P. Physiological responses and quality attributes of table grape fruit to chitosan preharvest spray and postharvest coating during storage. Food Chem. 2008, 106, 501–508. [Google Scholar] [CrossRef]
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef] [PubMed]
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Du, W.X.; Avena-Bustillos, R.J.; III, A.P.B.; McHugh, T.H. Effect of UV-B light and different cutting styles on antioxidant enhancement of commercial fresh-cut carrot products. Food Chem. 2012, 134, 1862–1869. [Google Scholar] [CrossRef] [PubMed]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.M.; Saltveit, M.E. Antioxidant capacity of lettuce leaf tissue increases after wounding. J. Agric. Food Chem. 2002, 50, 7536–7541. [Google Scholar] [CrossRef]
- Kakumanu, A.; Ambavaram, M.M.R.; Klumas, C.; Krishnan, A.; Batlang, U.; Myers, E.; Grene, R.; Pereira, A. Effects of drought on gene expression in maize reproductive and leaf meristem tissue revealed by rna-seq. Plant Physiol. 2012, 160, 846–867. [Google Scholar] [CrossRef]
- Pan, J.W.; Zhang, M.Y.; Kong, X.P.; Xin, X.; Liu, Y.K.; Zhou, Y.; Liu, Y.; Sun, L.P.; Li, D.Q. Zmmpk17, a novel maize group d map kinase gene, is involved in multiple stress responses. Planta 2012, 235, 661–676. [Google Scholar] [CrossRef]
- Hamel, L.P.; Nicole, M.C.; Sritubtim, S.; Marie-Josée, M.J.; Ellis, M.; Ehlting, J.; Beaudoin, N.; Barbazuk, B.; Klessig, D.; Lee, J. Ancient signals: Comparative genomics of plant mapk and mapkk gene families. Trends Plant Sci. 2006, 11, 192–198. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Shi, L.P.; Liu, Y.Y.; Tang, Q.; Shen, L.; Yang, S.; Cai, J.S.; Yu, H.X.; Wang, R.Z.; Wen, J.Y.; et al. Genome-wide identification and transcriptional expression analysis of mitogen-activated protein kinase and mitogen-activated protein kinase kinase genes in Capsicum annuum. Front. Plant Sci. 2015, 6, 780. [Google Scholar] [CrossRef]
- Yoo, H.; Shrivastava, S.; Lynch, J.H.; Huang, X.Q.; Widhalm, J.R.; Guo, L.Y.; Carter, B.C.; Qian, Y.C.; Maeda, H.A.; Ogas, J.P.; et al. Overexpression of arogenate dehydratase reveals an upstream point of metabolic control in phenylalanine biosynthesis. Plant J. 2021, 108, 737–751. [Google Scholar] [CrossRef]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef] [PubMed]
- Mehere, P.; Han, Q.; Lemkul, J.A.; Vavricka, C.J.; Robinson, H.; Bevan, D.R.; Li, J.Y. Tyrosine aminotransferase: Biochemical and structural properties and molecular dynamics simulations. Protein Cell 2010, 1, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Y.; Li, Y.; Feng, S.Q.; Zou, W.H.; Guo, K.; Fan, C.F.; Si, S.L.; Peng, L.C. Analysis of five rice 4-coumarate:coenzyme a ligase enzyme activity and stress response for potential roles in lignin and flavonoid biosynthesis in rice. Biochem. Biophys. Res. Commun. 2013, 430, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhou, P.N.; Gui, C.; Da, G.Z.; Gong, L.; Zhang, X.Q. Comparative transcriptome analysis of ampelopsis megalophylla for identifying genes involved in flavonoid biosynthesis and accumulation during different seasons. Molecules 2019, 24, 1267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Cheng, X.; Jin, Q.; Su, X.Q.; Li, M.L.; Yan, C.C.; Jiao, X.Y.; Li, D.H.; Lin, Y.; Cai, Y.P. Comparison of the transcriptomic analysis between two Chinese white pear (Pyrus bretschneideri Rehd.) genotypes of different stone cells contents. PLoS ONE 2017, 12, e0187114. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).