Aroma Profiling Analysis of Peach Flowers Based on Electronic Nose Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Peach Materials
2.2. Flower Aroma Measurement
2.3. Statistical Analysis
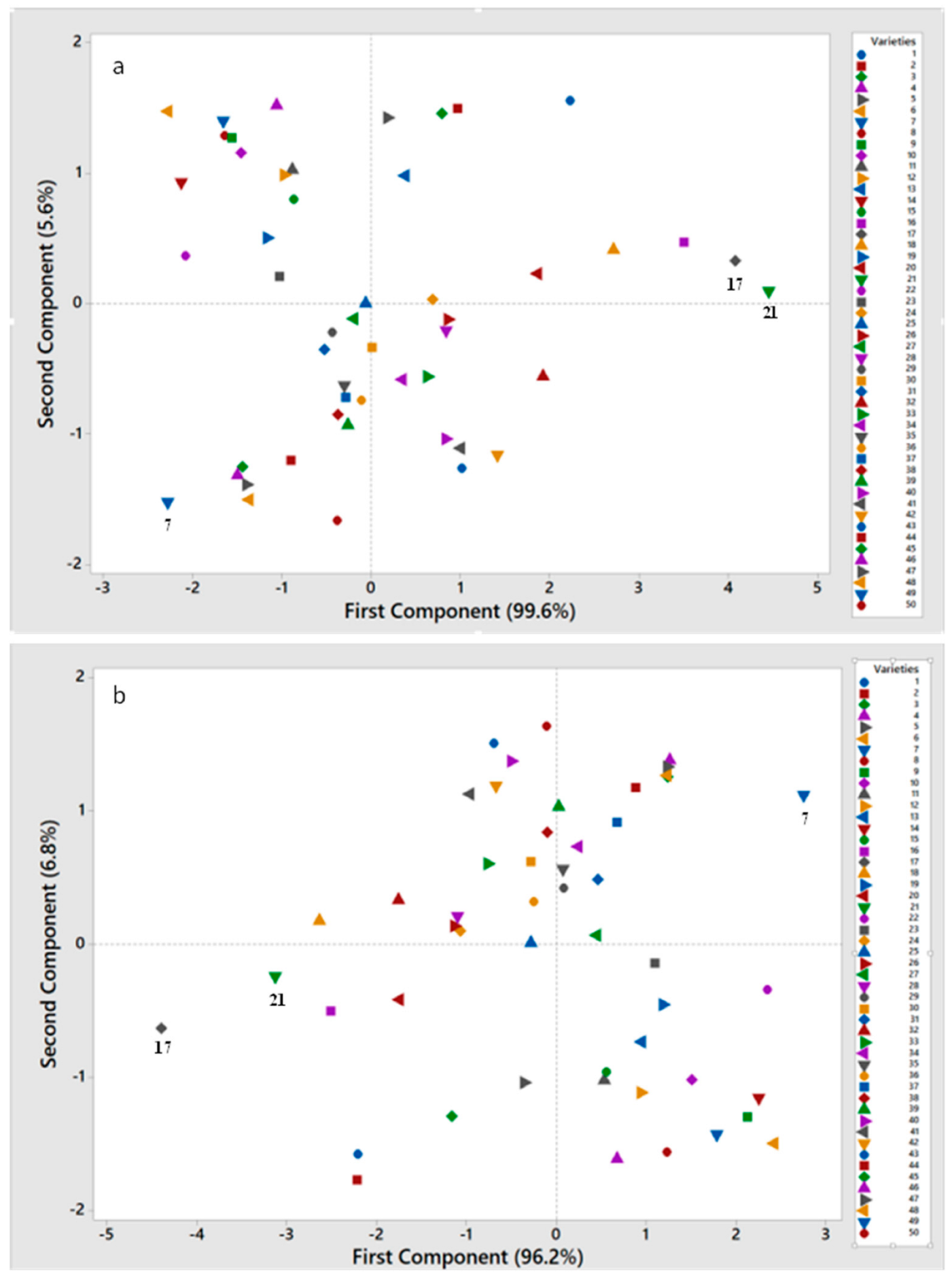
3. Results
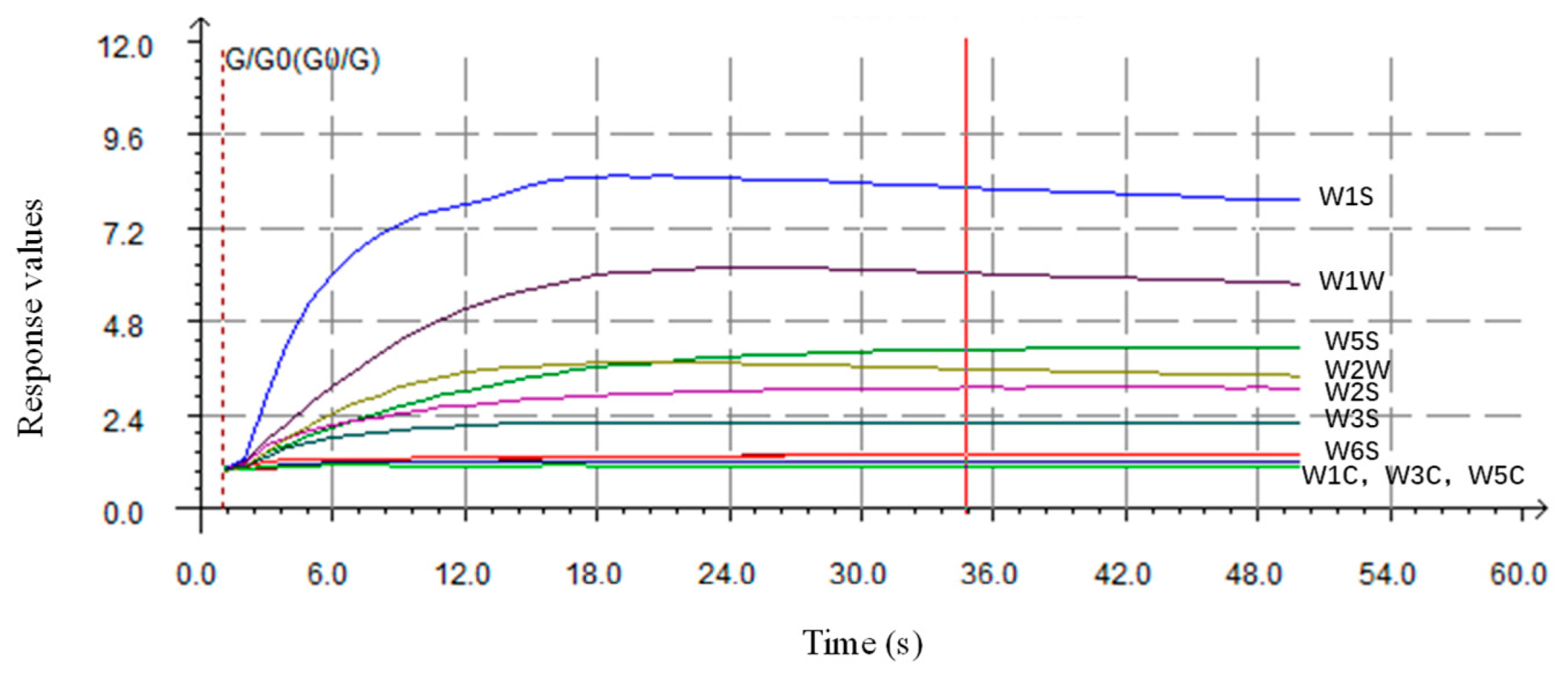
3.1. Response Values of the Sensors to Peach Flowers
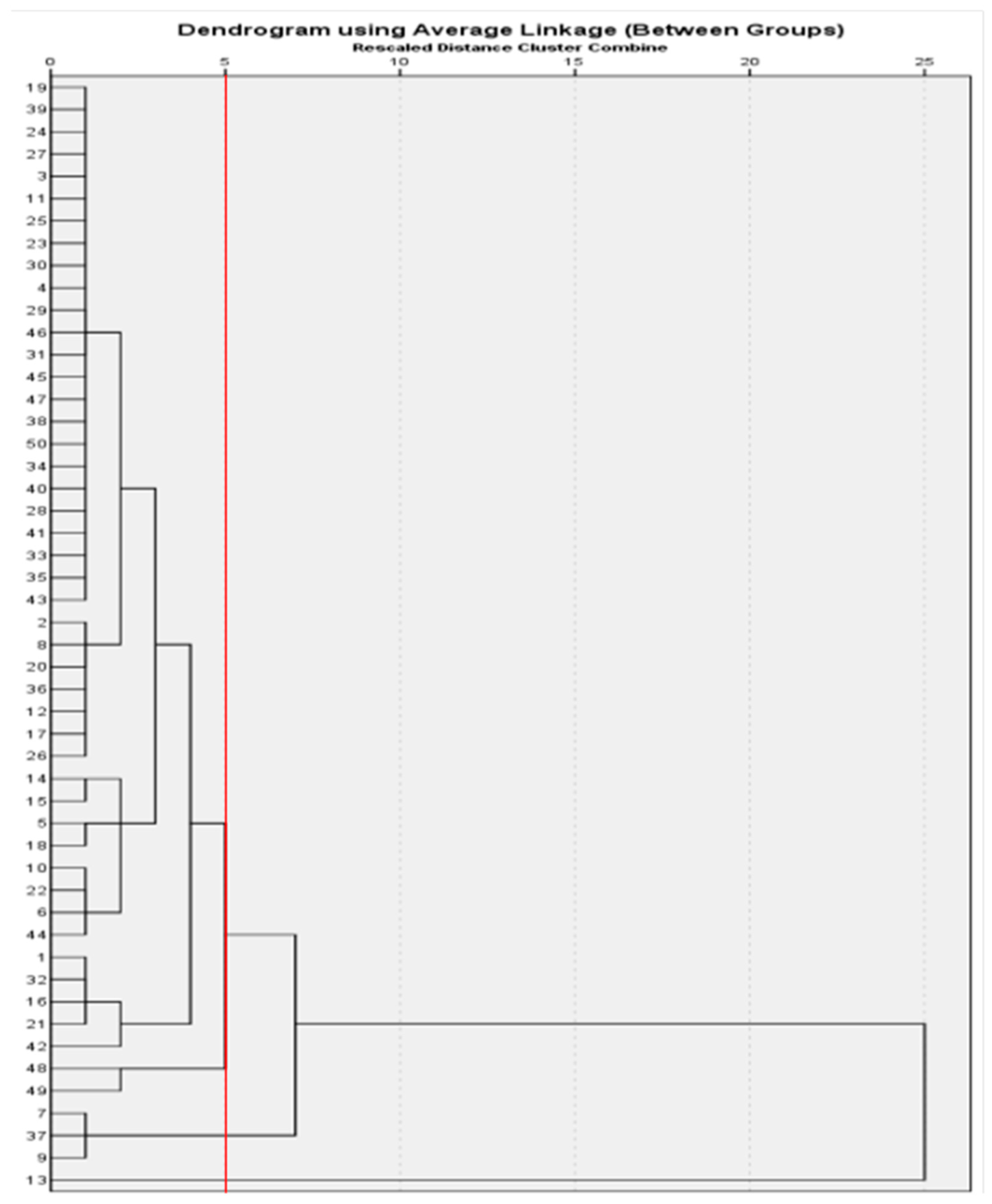
3.2. Cluster Analysis of the Tested Peach Germplasms Based on Their Blossom Aromas
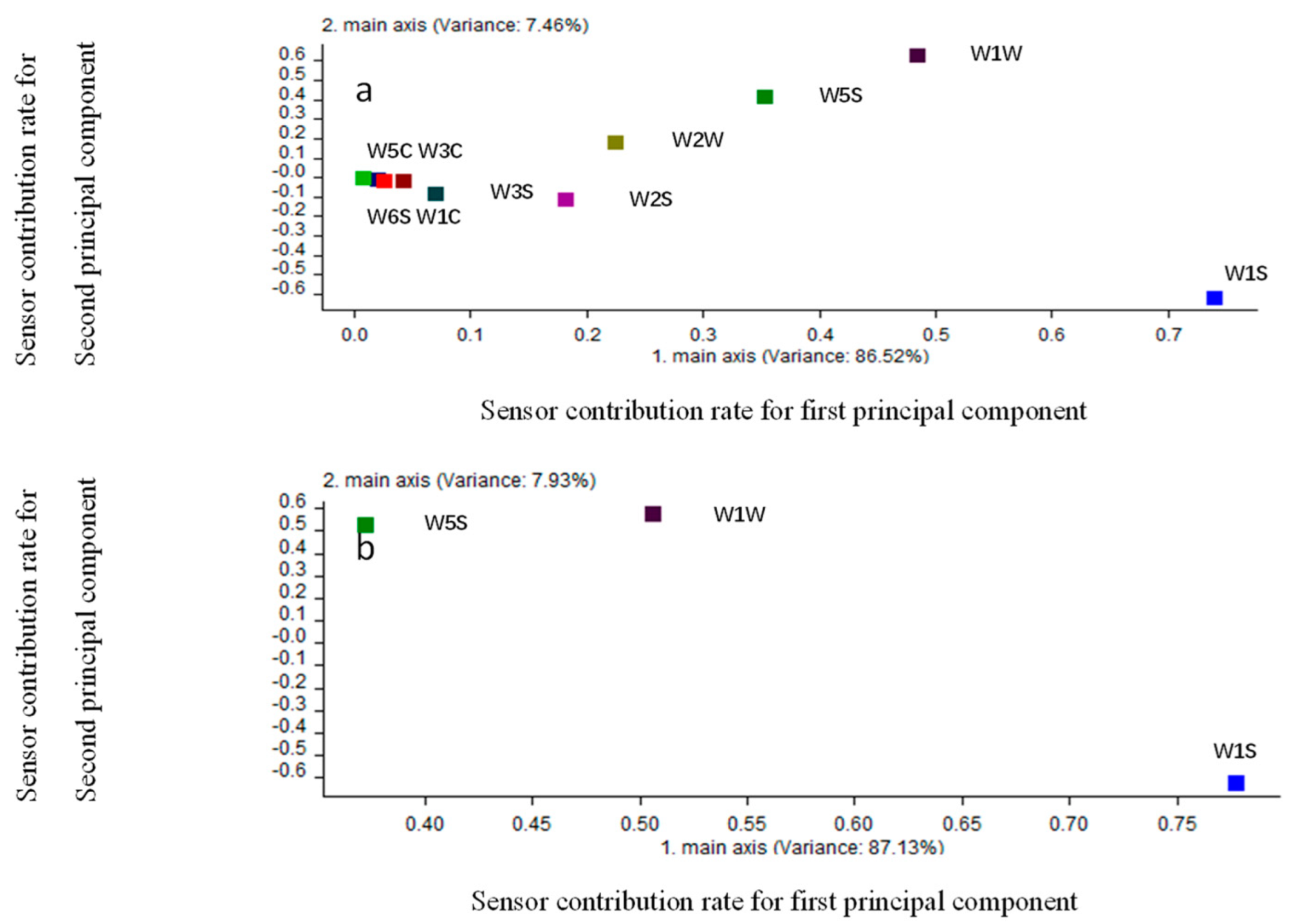
3.3. Analysis of the Main Sensors Used for Peach Blossom Aroma Detection
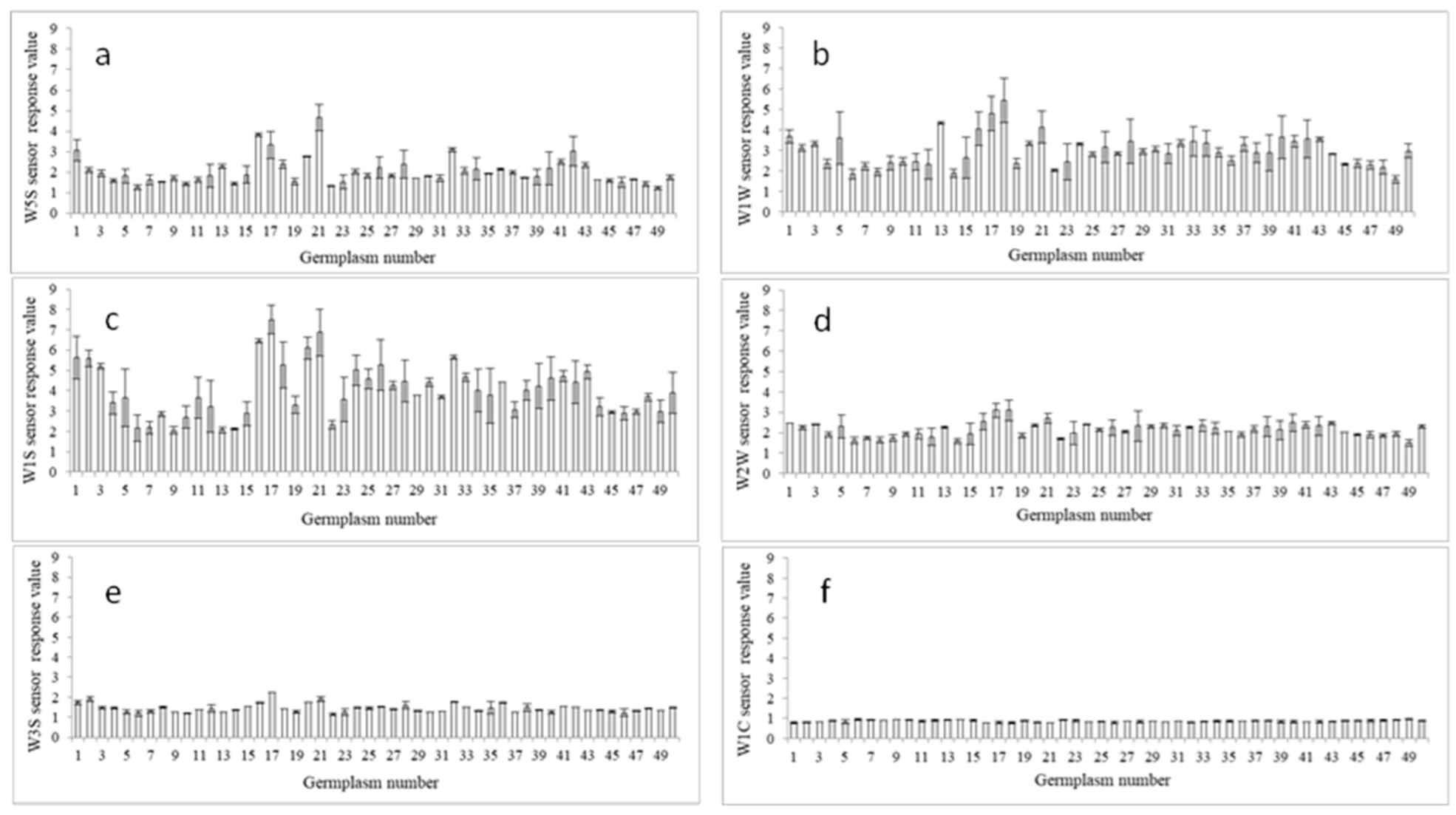
3.4. Aroma Components Analysis and Special Peach Germplasm Screening
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Raguso, R.A. Wake Up and Smell the Roses: The Ecology and Evolution of Floral Scent. Annu. Rev. Ecol. Syst. 2008, 39, 549–569. [Google Scholar] [CrossRef]
- Hadacek, F. Secondary Metabolites as Plant Traits: Current Assessment and Future Perspectives. Crit. Rev. Plant Sci. 2002, 21, 273–322. [Google Scholar] [CrossRef]
- Schiestl, F.P. Chemical and Functional Complexity in Flower Fragrance. CHIMIA Int. J. Chem. 2020, 74, 820. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.B.; Savita, S.; Neeta, R. Fragrance Stimulation Mechanisms of Flowers and their Regulation Under Environmental Constraints. J. Plant Growth Regul. 2022, 1–23. [Google Scholar] [CrossRef]
- Wang, Z.H.; Zhuang, E.J. Chinese Fruit (Peach); China Forestry Publishing House: Beijing, China, 2001; pp. 74–89. (In Chinese) [Google Scholar]
- Liu, J.C.; Yang, W.B.; Zhang, C.L.; Liu, H.; Lv, Z.Z.; Jiao, Z.G. Analysis of Nutritional and Functional Contituents in the Petals of Peach Blossom. J. Food Saf. Qual. 2016, 7, 3745–3751. (In Chinese) [Google Scholar]
- Zhang, J.J.; Yin, Z.H.; Chen, L.; Guo, Q.F.; Zhang, W. Analysis of Fat-soluble Constituents from Flowers of Amygdalus persica L. J. Henan Univ. 2019, 38, 158–160. (In Chinese) [Google Scholar]
- Messina, V.; Radice, S.; Baby, R.; de Reca Noemí, W. Variation of Odour Profile Detected in the Floral Stages of Prunus Persica (L) Batsch Using an Electronic Nose. AIP Conf. Proc. 2009, 1137, 465–468. [Google Scholar]
- Cao, H.; Li, Z.G.; Shen, D.L. GC/MS Fingerprinting of Aroma Components of Osmanthus Varieties. Acta Hortic. Sin. 2009, 36, 391–398. (In Chinese) [Google Scholar]
- Zhou, J.R.; Nie, D.J. Changes in Flower Aroma Compounds of Cultivars of Chimonanthus praecox (L.) Link and at Different Stages Relative to Chimonanthus Tea Quality. Acta Hortic. Sin. 2010, 37, 1621–1628. (In Chinese) [Google Scholar]
- Shen, Z.; Liu, Q.R.; Zhou, J.R.; Zhou, F.L.; Liu, W.; Zhou, Y. Analysis of Volatile Flavor in the Processing of Flowery Black Tea Based on Electronic Nose. Farm Prod. Process. 2019, 03, 51–54. (In Chinese) [Google Scholar]
- Yan, J.; Cai, Z.X.; Zhang, M.H.; Xu, Z.Y.; Shen, Z.J.; Ma, R.J.; Yu, M.L. Evaluation of Aroma in Peach Fruit by Electronic Nose. J. Plant Genet. Resour. 2021, 22, 9. (In Chinese) [Google Scholar]
- Yan, J.; Zhang, M.; Peng, B.; Su, Z.; Xu, Z.; Cai, Z.; Yang, J.; Ma, R.; Yu, M.; Shen, Z. Predicting Chilling Requirement of Peach Floral Buds Using Electronic Nose. Sci. Hortic. 2021, 290, 110517. [Google Scholar] [CrossRef]
- Fukai, S.; Abe, Y. Discrimination of lily fragrance by use of an electronic nose. Acta Hortic. 2002, 572, 75–81. [Google Scholar] [CrossRef]
- Yan, H.; Fang, L.; Xia, Y.P.; Chen, K.S. Scent Profiling of Cymbidium Ensifolium by Electronic Nose. Sci. Hortic. 2011, 128, 306–310. [Google Scholar]
- Zhang, B.; Huang, Y.; Zhang, Q.; Liu, X.; Li, F.; Chen, K. Fragrance Discrimination of Chinese Cymbidium Species and Cultivars Using an Electronic Nose. Sci. Hortic. 2014, 172, 271–277. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kim, M.S.; Park, P.H.; Park, S.Y. Scent Analysis Using an Electronic Nose and Flowering Period of Potted Diploid and Tetraploid Cymbidium. Korean J. Hortic. Sci. Technol. 2016, 34, 163–171. [Google Scholar]
- Ray, H.; Bhattacharyya, N.; Ghosh, A.; Tudu, B.; Bandyopadhyay, R.; Ghosh, A.; Biswas, S.P.; Majumdar, S. Fragrance Profiling of Jasminum Sambac Ait. Flowers Using Electronic Nose. IEEE Sens. J. 2017, 17, 160–168. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zhao, F.; Rao, G.H.; Lin, H.Z.; Guo, Y.C.; Fu, T.P.; Weng, F.S.; Ye, N.X. Origin Difference Analysis of Aroma Components in Jasmine Tea Based on Electronic Nose and ATD-GC-MS. Sci. Technol. Food Ind. 2021, 42, 234–239. [Google Scholar]
- Wang, L.R.; Zhu, G.R. Descriptors and Data Standard for Peach (Prunus persica L.); China Agriculture Press: Beijing, China, 2005. (In Chinese) [Google Scholar]
- Gómez, A.H.; Wang, J.; Hu, G.; Pereira, A.G. Electronic nose technique potential monitoring mandarin maturity. Sens. Actuators B Chem. 2006, 113, 347–353. [Google Scholar] [CrossRef]
- Dobson, H. Floral Volatiles in Insect Biology. In Insect-Plant Interact; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Natalia, D.; Robert, A.; Raguso, J.W.; Jeannine, R.; Ross, E.P. Floral Scent Production in Clarkia Breweri. Plant Physiol. 1998, 116, 599. [Google Scholar]
- Radice, S.; Giordani, E.; Nencetti, V.; Bellini, E. Phenological expression in prunus salicina lindl. genotypes and its relationship with insect attraction and pollination. Acta Hortic. 2010, 874, 151–156. [Google Scholar] [CrossRef]
- Farré-Armengol, G.; Filella, I.; Llusià, J.; Niinemets, Ü.; Peñuelas, J. Optimum temperature for floral terpene emissions tracks the mean temperature of the flowering season. Funct. Plant Biol. 2015, 42, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, H.B.; Olsen, C.E. Influence of climatic factors on emission of flower volatiles in situ. Planta 1994, 192, 365–371. [Google Scholar] [CrossRef]
- Sagae, M.; Oyama-Okubo, N.; Ando, T.; Marchesi, E.; Nakayama, M. Effect of Temperature on the Floral Scent Emission and Endogenous Volatile Profile of Petunia axillaris. Biosci. Biotechnol. Biochem. 2008, 72, 110–115. [Google Scholar] [CrossRef]
- Cna’ani, A.; Muehlemann, J.K.; Ravid, J.; Masci, T.; Klempien, A.; Nguyen, T.T.; Dudareva, N.; Pichersky, E.; Vainstein, A. Petunia × Hybrida Floral Scent Production is Negatively Affected by Hightemperature Growth Conditions. Plant Cell Environ. 2015, 38, 1333–1346. [Google Scholar] [CrossRef]
- Yuan, J.L.; Jin, X.L.; Yu, Q.X.; Hu, X.X. Research Progress on Extraction and Identification of Floral Fragrance Components of Magnoliaceae. J. Hunan Ecol. Sci. 2022, 9, 96–105. (In Chinese) [Google Scholar]

| Number | Name | CO | ST | GT | UT | Number | Name | CO | ST | GT | UT |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Baibitao1 | C | Pp | I | O | 26 | Jiangtao | C | Pp | L | O |
| 2 | Baibitao | C | Pp | L | O | 27 | Juhuatao | C | Pp | L | O |
| 3 | Baihuabitao | C | Pp | L | O | 28 | Mantianhong | C | Pp | I | O |
| 4 | Baihuashanbitao | C | L | O | 29 | Taiguohuatao | T | Pp | L | O | |
| 5 | Baihuashantao | C | W | S | 30 | Tanchun | C | Pp | L | O | |
| 6 | Baihuazhuxingtao | A | Pp | I | O | 31 | Taohua2 | C | Pp | I | O |
| 7 | Yuntaishanshantao | C | W | S | 32 | Wubaotao | C | Pp | L | O | |
| 8 | Beizhizhuxingfen | C | Pp | B | O | 33 | Yingchun | C | Pp | L | O |
| 9 | Dazhuanggansutao | C | Pk | W | S | 34 | Yushuizaihuatao | C | Pp | L | O |
| 10 | Danbanbaihua | C | Pp | L | O | 35 | Sahongtao | C | Pp | L | O |
| 11 | Fenhuazhuxingtao | A | Pp | I | O | 36 | Zhufenchuizhi | C | Pp | L | O |
| 12 | Fenrou sebitao | C | Pp | L | O | 37 | Zhouxingshantao | C | W | O/S | |
| 13 | Gansutao2 | C | Pk | W | S | 38 | Galaxy | A | Pp | I | F |
| 14 | Gansutao | C | Pk | W | S | 39 | Zijinhong1 | C | Pp | I | F |
| 15 | Hehuan erse | C | Pp | L | O | 40 | Flordaglo | A | Pp | I | F |
| 16 | Hongchuizhi | C | Pp | L | O | 41 | Sunraycer | A | Pp | I | F |
| 17 | Hongfenjiaren | C | Pp | I | O | 42 | Sunblaze | A | Pp | I | F |
| 18 | Honghuashantao | C | W | S | 43 | Nanshantiantao | C | Pp | L | F | |
| 19 | Honghuazhuxingtao | A | Pp | I | O | 44 | Shanganshantao | C | PdP | W | S |
| 20 | Hongyetao | C | Pp | L | O | 45 | Xiahui8 | C | Pp | I | F |
| 21 | Hua3 | C | Pp | I | O | 46 | Xiahui6 | C | Pp | I | F |
| 22 | Hua5 | C | Pp | I | O | 47 | Xiacui | C | Pp | I | F |
| 23 | Hua6 | C | Pp | I | O | 48 | Xinjiangpantao | C | Pf | L | F |
| 24 | Hua8 | C | Pp | I | O | 49 | Xinjianghuangrou | C | Pf | L | F |
| 25 | Riyuetao | C | Pp | L | O | 50 | Riben86 | J | Pp | I | F |
| Index | W1C | W5S | W3C | W6S | W5C | W1S | W1W | W2S | W2W | W3S |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 0.84 | 2.03 | 0.91 | 1.16 | 0.95 | 4.02 | 2.98 | 1.68 | 2.14 | 1.44 |
| Standard deviation | 0.05 | 0.67 | 0.02 | 0.06 | 0.02 | 1.28 | 0.77 | 0.35 | 0.34 | 0.21 |
| Variation amplitude | 0.8–0.9 | 1.3–4.7 | 0.9–1.0 | 1.1–1.4 | 0.9–1.0 | 2.1–7.5 | 1.6–5.4 | 1.2–2.8 | 1.5–3.1 | 1.2–2.2 |
| Xmax-xmin | 0.17 | 3.34 | 0.11 | 0.27 | 0.05 | 5.50 | 3.85 | 1.55 | 1.59 | 1.08 |
| Variable coefficient (%) | 5.9 | 33.0 | 2.1 | 5.2 | 2.1 | 31.8 | 25.8 | 20.8 | 15.9 | 14.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B.; Sun, M.; Cai, Z.; Su, Z.; Li, J.; Shen, Z.; Ma, R.; Yan, J.; Yu, M. Aroma Profiling Analysis of Peach Flowers Based on Electronic Nose Detection. Horticulturae 2022, 8, 875. https://doi.org/10.3390/horticulturae8100875
Zhao B, Sun M, Cai Z, Su Z, Li J, Shen Z, Ma R, Yan J, Yu M. Aroma Profiling Analysis of Peach Flowers Based on Electronic Nose Detection. Horticulturae. 2022; 8(10):875. https://doi.org/10.3390/horticulturae8100875
Chicago/Turabian StyleZhao, Bintao, Meng Sun, Zhixiang Cai, Ziwen Su, Jiyao Li, Zhijun Shen, Ruijuan Ma, Juan Yan, and Mingliang Yu. 2022. "Aroma Profiling Analysis of Peach Flowers Based on Electronic Nose Detection" Horticulturae 8, no. 10: 875. https://doi.org/10.3390/horticulturae8100875
APA StyleZhao, B., Sun, M., Cai, Z., Su, Z., Li, J., Shen, Z., Ma, R., Yan, J., & Yu, M. (2022). Aroma Profiling Analysis of Peach Flowers Based on Electronic Nose Detection. Horticulturae, 8(10), 875. https://doi.org/10.3390/horticulturae8100875







