Sugar Metabolism and Photosynthesis of Tomatoes Irrigated with Water Treated with Low-Frequency Electromagnetic Resonance Fields in Different Fertigation Doses
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Area
2.2. Planting and Plant Management
2.3. Treatments
2.4. Water Treated with Very Low-Frequency Electromagnetic Resonance Fields (WVLF)
2.5. Irrigation and Fertigation Management
2.6. Determination of Total Sugar and Sucrose Contents
2.6.1. Determination of Total Sugars
2.6.2. Sucrose Determination
2.6.3. Determination of Photosynthetic Pigments
2.7. Gas Exchange
2.8. Chlorophyll Fluorescence
2.9. Determination of Production
2.10. Statistical Analysis
3. Results and Discussion
3.1. Sugar Metabolism
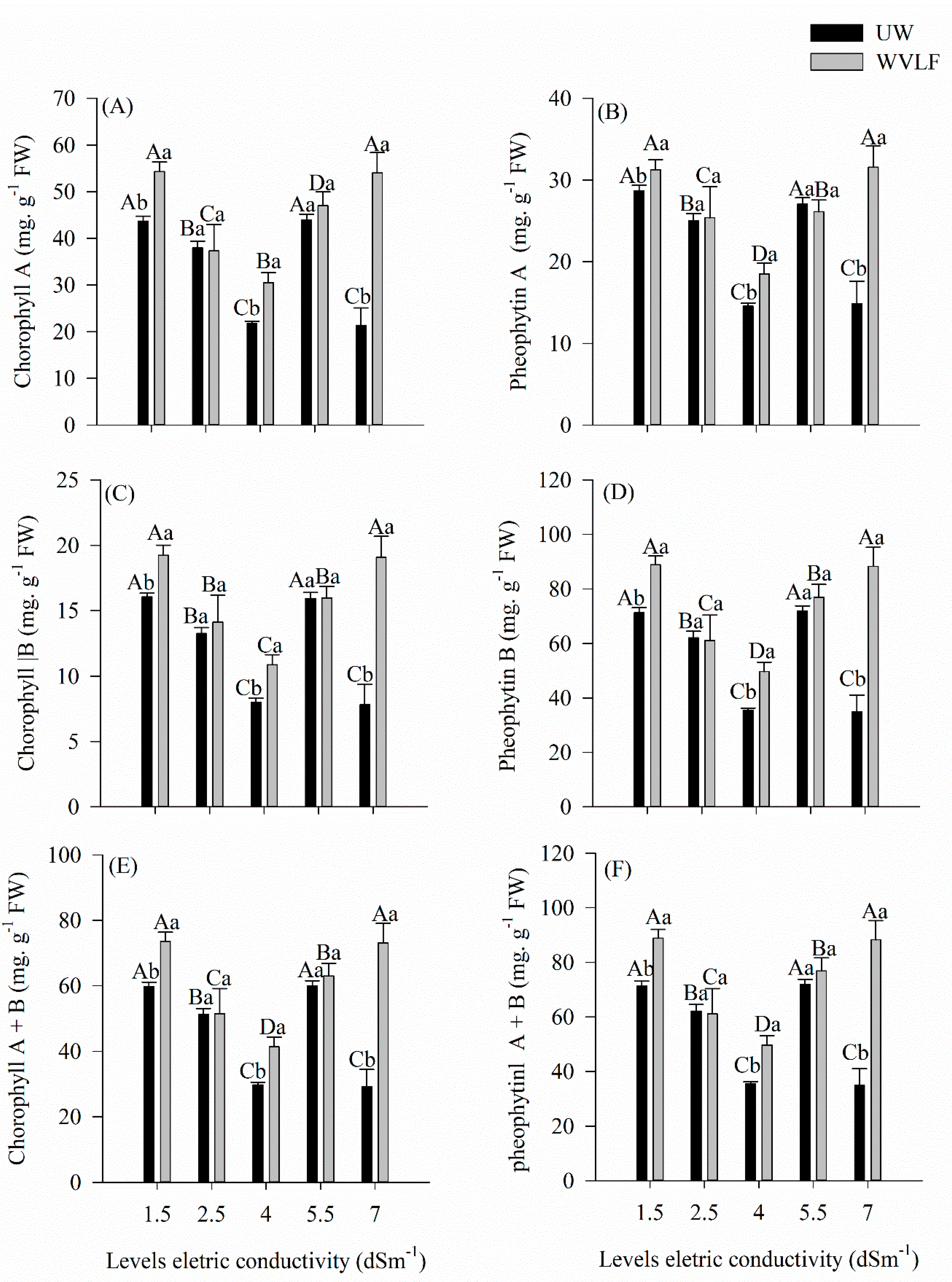
3.2. Photosynthetic Pigments
3.3. Gas Exchange
3.4. Chlorophyll Fluorescence A
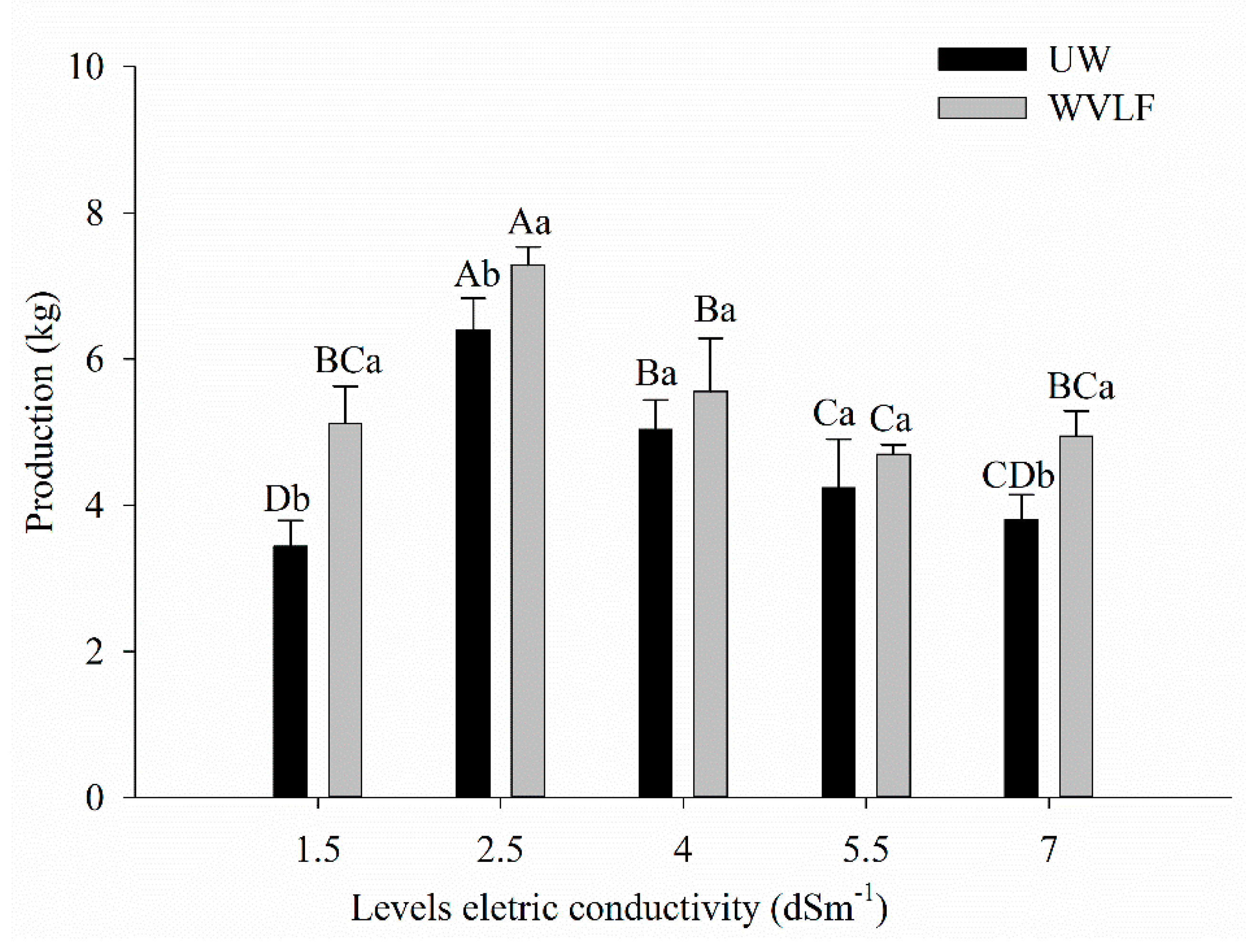
3.5. Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.-P.; Lutts, S. Tomato fruit development and metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yoshida, H.; Goto, E.; Hikosaka, S. Development of an irrigation method with a cycle of wilting–partial recovery using an image-based irrigation system for high-quality tomato production. Agronomy 2022, 12, 1410. [Google Scholar] [CrossRef]
- Dias, G.M.; Ayer, N.W.; Khosla, S.; Van Acker, R.; Young, S.B.; Whitney, S.; Hendricks, P. Life cycle perspectives on the sustainability of Ontario greenhouse tomato production: Benchmarking and improvement opportunities. J. Clean. Prod. 2017, 140, 831–839. [Google Scholar] [CrossRef]
- Junior, S.S.; Casagrande, J.G.; de Lima Toledo, C.A.; da Silva Ponce, F.; da Silva Ferreira, F.; Zanuzo, M.R.; Diamante, M.S.; Lima, G.P.P. Selection of thermotolerant Italian tomato cultivars with high fruit yield and nutritional quality for the consumer taste grown under protected cultivation. Sci. Hortic. 2022, 291, 110559. [Google Scholar] [CrossRef]
- Rouphael, Y.; Petropoulos, S.A.; Cardarelli, M.; Colla, G. Salinity as eustressor for enhancing quality of vegetables. Sci. Hortic. 2018, 234, 361–369. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Moles, T.M.; Pompeiano, A.; Huarancca Reyes, T.; Scartazza, A.; Guglielminetti, L. The efficient physiological strategy of a tomato landrace in response to short-term salinity stress. Plant Physiol. Biochem. 2016, 109, 262–272. [Google Scholar] [CrossRef]
- Msimbira, L.A.; Smith, D.L. the roles of plant growth promoting microbes in enhancing plant tolerance to acidity and alkalinity stresses. Front. Sustain. Food Syst. 2020, 4, 106. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Han, Q.-H.; Huang, B.; Ding, C.-B.; Zhang, Z.-W.; Chen, Y.-E.; Hu, C.; Zhou, L.-J.; Huang, Y.; Liao, J.-Q.; Yuan, S.; et al. Effects of melatonin on anti-oxidative systems and photosystem ii in cold-stressed rice seedlings. Front. Plant Sci. 2017, 8, 785. [Google Scholar] [CrossRef] [PubMed]
- Mbarki, S.; Sytar, O.; Cerda, A.; Zivcak, M.; Rastogi, A.; He, X.; Zoghlami, A.; Abdelly, C.; Brestic, M. Strategies to mitigate the salt stress effects on photosynthetic apparatus and productivity of crop plants. In Salinity Responses and Tolerance in Plants; Springer International Publishing: Cham, Switzerland, 2018; Volume 1, pp. 85–136. [Google Scholar]
- Chourasia, K.N.; Lal, M.K.; Tiwari, R.K.; Dev, D.; Kardile, H.B.; Patil, V.U.; Kumar, A.; Vanishree, G.; Kumar, D.; Bhardwaj, V.; et al. Salinity stress in potato: Understanding physiological, biochemical and molecular responses. Life 2021, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Loudari, A.; Benadis, C.; Naciri, R.; Soulaimani, A.; Zeroual, Y.; El Gharous, M.; Kalaji, H.M.; Oukarroum, A. Salt stress affects mineral nutrition in shoots and roots and chlorophyll a fluorescence of tomato plants grown in hydroponic culture. J. Plant Interact. 2020, 15, 398–405. [Google Scholar] [CrossRef]
- Khatri, K.; Rathore, M.S. Salt and osmotic stress-induced changes in physio-chemical responses, PSII photochemistry and chlorophyll a fluorescence in peanut. Plant Stress 2022, 3, 100063. [Google Scholar] [CrossRef]
- Patel, M.K.; Kumar, M.; Li, W.; Luo, Y.; Burritt, D.J.; Alkan, N.; Tran, L.-S.P. Enhancing salt tolerance of plants: From metabolic reprogramming to exogenous chemical treatments and molecular approaches. Cells 2020, 9, 2492. [Google Scholar] [CrossRef]
- Surendran, U.; Sandeep, O.; Joseph, E.J. The impacts of magnetic treatment of irrigation water on plant, water and soil characteristics. Agric. Water Manag. 2016, 178, 21–29. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, C.; Wu, L.; Zhang, Q.; Zhu, L.; Zhao, R. Influence of alternating electromagnetic field and ultrasonic on calcium carbonate crystallization in the presence of magnesium ions. J. Cryst. Growth 2018, 499, 67–76. [Google Scholar] [CrossRef]
- Zlotopolski, V. The Impact of magnetic water treatment on salt distribution in a large unsaturated soil column. Int. Soil Water Conserv. Res. 2017, 5, 253–257. [Google Scholar] [CrossRef]
- Al-Douri, Y.; Hassan, S.M.; Batoo, K.M.; Raslan, E.H. Surface tension under magnetic field effect for nanoscaled water. Eur. Phys. J. Plus 2021, 136, 295. [Google Scholar] [CrossRef]
- El-Shafik El-Zawily, A.; Meleha, M.; El-Sawy, M.; El-Attar, E.-H.; Bayoumi, Y.; Alshaal, T. Application of magnetic field improves growth, yield and fruit quality of tomato irrigated alternatively by fresh and agricultural drainage water. Ecotoxicol. Environ. Saf. 2019, 181, 248–254. [Google Scholar] [CrossRef]
- Zhou, B.; Yang, L.; Chen, X.; Ye, S.; Peng, Y.; Liang, C. Effect of magnetic water irrigation on the improvement of salinized soil and cotton growth in Xinjiang. Agric. Water Manag. 2021, 248, 106784. [Google Scholar] [CrossRef]
- Khoshravesh, M.; Mostafazadeh-Fard, B.; Mousavi, S.F.; Kiani, A.R. Effects of magnetized water on the distribution pattern of soil water with respect to time in trickle irrigation. Soil Use Manag. 2011, 27, 515–522. [Google Scholar] [CrossRef]
- Putti, F.F.; Nogueira, B.B.; Vacaro de Souza, A.; Festozo Vicente, E.; Zanetti, W.A.L.; de Lucca Sartori, D.; Pigatto de Queiroz Barcelos, J. Productive and physico-chemical parameters of tomato fruits submitted to fertigation doses with water treated with very low-frequency electromagnetic resonance fields. Plants 2022, 11, 1587. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, B.L.; Grewal, H.S. Magnetic treatment of irrigation water: Its effects on vegetable crop yield and water productivity. Agric. Wat. Manag. 2009, 96, 1229–1236. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Wei, K.; Guo, Y.; Mu, W.; Sun, Y. Magnetic Water Treatment: An Eco-Friendly Irrigation Alternative to Alleviate Salt Stress of Brackish Water in Seed Germination and Early Seedling Growth of Cotton (Gossypium hirsutum L.). Plants 2022, 11, 1397. [Google Scholar] [CrossRef]
- Zhao, G.; Mu, Y.; Wang, Y.; Wang, L. Magnetization and oxidation of irrigation water to improve winter wheat (Triticum aestivum L.) production and water-use efficiency. Agric. Wat. Manag. 2022, 259, 107254. [Google Scholar] [CrossRef]
- Putti, F.F.; Vacaro de Souza, A.; Zanetti, W.A.L.; Bueno Nogueira, B.; Domingues Neto, F.J.; de Queiroz Barcelos, J.P.; de Lucca Sartori, D. Growth and Absorption Curve of Nutrients in Tomato Crop ‘BRS Imigrante’ Cultivar Grown in Coconut Fiber. J. Plant Nutr. 2022, 45, 2239–2250. [Google Scholar] [CrossRef]
- Mghaiouini, R.; Salah, M.; Monkade, M.; El Bouari, A. A new knowledge of water magnetism phenomenon. Arab. J. Sci. Eng. 2022, 47, 1129–1136. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- van Handel, E. Direct microdetermination of sucrose. Anal. Biochem. 1968, 22, 280–283. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- von Caemmerer, S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef]
- Schreiber, U. Chlorophyll fluorescence: New instruments for special applications. In Photosynthesis: Mechanisms and Effects; Springer: Dordrecht, The Netherlands, 1998; pp. 4253–4258. [Google Scholar]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Mukarram, M.; Choudhary, S.; Kurjak, D.; Petek, A.; Khan, M.M.A. Drought: Sensing, signalling, effects and tolerance in higher plants. Physiol. Plant. 2021, 172, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant. Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Tcherkez, G.; Limami, A.M. Net photosynthetic CO2 assimilation: More than just CO2 and O2 reduction cycles. New Phytol. 2019, 223, 520–529. [Google Scholar] [CrossRef]
- Agius, C.; von Tucher, S.; Rozhon, W. The Effect of salinity on fruit quality and yield of cherry tomatoes. Horticulturae 2022, 8, 59. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Recent advances in bacterial amelioration of plant drought and salt stress. Biology 2022, 11, 437. [Google Scholar] [CrossRef]
- Patel, M.; Fatnani, D.; Parida, A.K. Potassium deficiency stress tolerance in peanut (Arachis hypogaea) through ion homeostasis, activation of antioxidant defense, and metabolic dynamics: Alleviatory role of silicon supplementation. Plant Physiol. Biochem. 2022, 182, 55–75. [Google Scholar] [CrossRef]
- Wojciechowska, R.; Kunicki, E.; Długosz-Grochowska, O.; Kołton, A. Response of Broccoli Transplants to LED light during short and long-term storage. Agronomy 2020, 10, 1009. [Google Scholar] [CrossRef]
- Cui, H.; Liu, X.; Jing, R.; Zhang, M.; Wang, L.; Zheng, L.; Kong, L.; Wang, H.; Ma, F. Irrigation with magnetized water affects the soil microenvironment and fruit quality of eggplants in a covered vegetable production system in shouguang city, China. J. Soil Sci. Plant Nutr. 2020, 20, 2684–2697. [Google Scholar] [CrossRef]
- Zareei, E.; Zaare-Nahandi, F.; Hajilou, J.; Oustan, S. Eliciting effects of magnetized solution on physiological and biochemical characteristics and elemental uptake in hydroponically grown grape (Vitis vinifera L. cv. Thompson Seedless). Plant Physiol. Biochem. 2021, 167, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Hameed, A.; Akram, N.A.; Saleem, M.H.; Ashraf, M.; Ahmed, S.; Ali, S.; Abdullah Alsahli, A.; Alyemeni, M.N. Seed treatment with α-Tocopherol regulates growth and key physio-biochemical attributes in carrot (Daucus carota L.) plants under water limited regimes. Agronomy 2021, 11, 469. [Google Scholar] [CrossRef]
- Evelin, H.; Devi, T.S.; Gupta, S.; Kapoor, R. Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: Current understanding and new challenges. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Alamri, S.; Nasir Khan, M.; Corpas, F.J.; Al-Amri, A.A.; Alsubaie, Q.D.; Ali, H.M.; Kalaji, H.M.; Ahmad, P. Melatonin and calcium function synergistically to promote the resilience through ROS metabolism under arsenic-induced stress. J. Hazard. Mater. 2020, 398, 122882. [Google Scholar] [CrossRef]
- Sadeghipour, O. The effect of magnetized water on physiological and agronomic traits of cowpea (Vigna unguiculata L.). Int. J. Res. Chem. Metall. Civ. Eng. 2016, 3, 195–198. [Google Scholar] [CrossRef]
- Rawabdeh, H.; Shiyab, S.; Shibli, R. The Effect of irrigation by magnetically water on chlorophyll and macroelements uptake of pepper (Capsicum annuum L.). Jordan J. Agric. Sci. 2014, 10, 205–214. [Google Scholar]
- Mildaziene, V.; Ivankov, A.; Sera, B.; Baniulis, D. Biochemical and physiological plant processes affected by seed treatment with non-thermal plasma. Plants 2022, 11, 856. [Google Scholar] [CrossRef]
- Luo, F.; Cheng, S.-C.; Cai, J.-H.; Wei, B.-D.; Zhou, X.; Zhou, Q.; Zhao, Y.-B.; Ji, S.-J. Chlorophyll degradation and carotenoid biosynthetic pathways: Gene expression and pigment content in broccoli during yellowing. Food Chem. 2019, 297, 124964. [Google Scholar] [CrossRef]
- Wang, P.; Grimm, B. Connecting chlorophyll metabolism with accumulation of the photosynthetic apparatus. Trends Plant Sci. 2021, 26, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Hussain, S.; Anjum, M.A.; Khalid, M.F.; Saqib, M.; Zakir, I.; Hassan, A.; Fahad, S.; Ahmad, S. Oxidative stress. In Plant Abiotic Stress Tolerance; Springer International Publishing: Cham, Switzerland, 2019; pp. 191–205. [Google Scholar]
- de Melo, H.F.; de Souza, E.R.; Cunha, J.C. Fluorescence of chlorophyll a and photosynthetic pigments in Atriplex nummularia under abiotic stresses. Rev. Bras. Eng. Agrícola Ambient. 2017, 21, 232–237. [Google Scholar] [CrossRef]
- Al-Khazan, M.; Abdullatif, B.M.; Al-Assaf, N. Effects of magnetically treated water on water status, chlorophyll pigments and some elements content of Jojoba (Simmondsia chinensis L.) at different growth stages. Afr. J. Environ. Sci. Technol. 2011, 5, 722–731. [Google Scholar]
- Bezerra-Neto, E.; Coelho, J.B.M.; Jarma-Orozco, A.; Rodríguez-Páez, L.A.; Pompelli, M.F. Modulation of photosynthesis under salinity and the role of mineral nutrients in Jatropha curcas L. J. Agron. Crop Sci. 2022, 208, 314–334. [Google Scholar] [CrossRef]
- Bunce, J. Three methods of estimating me62sophyll conductance agree regarding its CO2 sensitivity in the Rubisco-Limited Ci range. Plants 2018, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Ospina-Salazar, D.I.; Benavides Bolaños, J.A.; Zúñiga-Escobar, O.; Muñoz-Perea, C.G. Fotosíntesis y rendimiento de biomasa en ají, rábano y maíz sometidos a agua tratada magnéticamente. Cienc. Tecnol. Agropecu. 2018, 19. [Google Scholar] [CrossRef]
- Ramos, J.G.; de Lima, G.S.; de Lima, V.L.A.; da Silva Paiva, F.J.; Nunes, K.G.; de Oliveira Pereira, M.; Fernandes, P.D.; Saboya, L.M.F. Foliar application of H2O2 as salt stress attenuator in ‘BRS Rubi do Cerrado’ sour passion fruit. Semin. Ciências Agrárias 2021, 42, 2253–2270. [Google Scholar] [CrossRef]
- Saddiq, M.S.; Iqbal, S.; Hafeez, M.B.; Ibrahim, A.M.H.; Raza, A.; Fatima, E.M.; Baloch, H.; Jahanzaib; Woodrow, P.; Ciarmiello, L.F. Effect of salinity stress on physiological changes in winter and spring wheat. Agronomy 2021, 11, 1193. [Google Scholar] [CrossRef]
- Shoukat, E.; Abideen, Z.; Ahmed, M.Z.; Gulzar, S.; Nielsen, B.L. Changes in growth and photosynthesis linked with intensity and duration of salinity in Phragmites karka. Environ. Exp. Bot. 2019, 162, 504–514. [Google Scholar] [CrossRef]
- Zribi, L.; Fatma, G.; Fatma, R.; Salwa, R.; Hassan, N.; Néjib, R.M. Application of chlorophyll fluorescence for the diagnosis of salt stress in tomato “Solanum lycopersicum (variety Rio Grande)”. Sci. Hortic. 2009, 120, 367–372. [Google Scholar] [CrossRef]
- Guidi, L.; Degl’Innocenti, E.; Carmassi, G.; Massa, D.; Pardossi, A. Effects of boron on leaf chlorophyll fluorescence of greenhouse tomato grown with saline water. Environ. Exp. Bot. 2011, 73, 57–63. [Google Scholar] [CrossRef]
- Gharbi, F.; Zribi, L.; Ben Daly, A.; Rejeb, S.; Hanchi, B. Photosynthetic responses of tomato leaves to salt and cadmium stresses: Growth and chlorophyll a fluorescence kinetic analyses. Polish J. Environ. Stud. 2018, 27, 2499–2508. [Google Scholar] [CrossRef]
- Adamski, J.M.; Rosa, L.M.G.; de Menezes Peixoto, C.R.; Pinheiro, C.L.; Fett, J.P.; Sperotto, R.A. Photosynthetic activity of indica rice sister lines with contrasting cold tolerance. Physiol. Mol. Biol. Plants 2020, 26, 955–964. [Google Scholar] [CrossRef] [PubMed]


| A 1 | gs 2 | Ci 3 | E 4 | WUE 5 | A/Ci 6 | ||
|---|---|---|---|---|---|---|---|
| Water type | UW 7 | 5.89 b | 0.16 b | 238 a | 3.21 b | 1.98 b | 0.02 b |
| WVLF 8 | 7.84 a | 0.21 a | 223 b | 3.70 a | 2.20 a | 0.03 a | |
| 1.5 (dS m−1) | UW | 5.37 Ab | 0.15 | 227 Aa | 2.79 Bb | 1.78B Ca | 0.023 b |
| WVLF | 9.50 Aa | 0.27 | 242 Aa | 5.34 Aa | 1.93 Ca | 0.039 a | |
| 2.5 (dS m−1) | UW | 6.27 Aa | 0.20 | 232 Aa | 2.59 Ba | 2.42 ABb | 0.027 a |
| WVLF | 6.73 Ba | 0.24 | 239 Aa | 2.33 Ca | 2.88 Aa | 0.028 a | |
| 4 (dS m−1) | UW | 5.60 Ab | 0.08 | 183 Bb | 2.15 Bb | 2.61 Aa | 0.030 a |
| WVLF | 7.85 ABa | 0.16 | 241 Aa | 3.17 Ca | 2.47 ABa | 0.030 a | |
| 5.5 (dS m−1) | UW | 6.15 Ab | 0.19 | 239 Aa | 3.87 Aa | 1.62 CDa | 0.020 b |
| WVLF | 8.53 Aa | 0.21 | 237 Aa | 4.36 BCa | 1.95 Ca | 0.035 a | |
| 7.0 (dS m−1) | UW | 6.07 Aa | 0.19 | 235 Aa | 3.30 Ab | 1.32 Db | 0.028 a |
| WVLF | 6.57 Ba | 0.15 | 229 Aa | 4.63 ABa | 2.00 BCa | 0.025 a | |
| CV (%) | UW | 10.12 | 24.26 | 4.59 | 12.54 | 9.55 | 11.64 |
| W.T. | WVLF | 3.10 * | 4.77 ** | 5.96 ** | 9.78 * | 10.45 ** | 25.43 ** |
| L.C. | UW | 58.46 ** | 6.66 ** | 14.40 ** | 21.46 ** | 31.52 ** | 2.30 ns |
| W.T. × L.C. | WVLF | 725 * | 2.62 | 8.81 ** | 16.78 ** | 5.09 * | 5.02 * |
| Fv/Fm 1 | Fv′/Fm′ 2 | qP 3 | qNR 4 | AETR 5 | ||
|---|---|---|---|---|---|---|
| Water type | UW 6 | 0.93 | 0.45 | 0.50 | 2.26 a | 116.72 b |
| WVLF 7 | 0.93 | 0.45 | 0.52 | 2.15 b | 130.52 a | |
| 1.5 (dS m−1) | UW | 0.94 | 0.48 | 0.46 | 1.98 | 118.53 |
| WVLF | 0.93 | 0.47 | 0.47 | 2.01 | 118.51 | |
| 2.5 (dS m−1) | UW | 0.93 | 0.45 | 0.47 | 2.27 | 115.44 |
| WVLF | 0.94 | 0.48 | 0.44 | 2.08 | 126.71 | |
| 4 (dS m−1) | UW | 0.94 | 0.40 | 0.57 | 2.55 | 120.51 |
| WVLF | 0.94 | 0.44 | 0.55 | 2.24 | 137.17 | |
| 5.5 (dS m−1) | UW | 0.94 | 0.45 | 0.42 | 2.05 | 101.88 |
| WVLF | 0.92 | 0.42 | 0.57 | 2.14 | 126.76 | |
| 7.0 (dS m−1) | UW | 0.92 | 0.41 | 0.58 | 2.44 | 127.24 |
| WVLF | 0.94 | 0.45 | 0.55 | 2.28 | 143.44 | |
| CV (%) | - | 3.06 | 6.92 | 18.64 | 6.50 | 10.96 |
| W.T. | - | 0.68 ns | 1.51 ns | 0.21 ns | 4.53 * | 7.78 * |
| L.C. | - | 0.04 ns | 3.43 * | 1.60 ns | 8.63 ** | 2.32 ns |
| W.T × L.C. | - | 0.55 ns | 1.80 ns | 0.94 ns | 1.94 ns | 0.68 ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogueira, B.B.; Vicente, E.F.; Nunes Chaves, P.P.; Zanetti, W.A.L.; Ono, E.O.; da Silva, G.F.; dos Reis, A.R.; Putti, F.F. Sugar Metabolism and Photosynthesis of Tomatoes Irrigated with Water Treated with Low-Frequency Electromagnetic Resonance Fields in Different Fertigation Doses. Horticulturae 2022, 8, 868. https://doi.org/10.3390/horticulturae8100868
Nogueira BB, Vicente EF, Nunes Chaves PP, Zanetti WAL, Ono EO, da Silva GF, dos Reis AR, Putti FF. Sugar Metabolism and Photosynthesis of Tomatoes Irrigated with Water Treated with Low-Frequency Electromagnetic Resonance Fields in Different Fertigation Doses. Horticulturae. 2022; 8(10):868. https://doi.org/10.3390/horticulturae8100868
Chicago/Turabian StyleNogueira, Bianca Bueno, Eduardo Festozo Vicente, Prínscilla Pâmela Nunes Chaves, Willian Aparecido Leotti Zanetti, Elizabeth Orika Ono, Gustavo Ferreira da Silva, André Rodrigues dos Reis, and Fernando Ferrari Putti. 2022. "Sugar Metabolism and Photosynthesis of Tomatoes Irrigated with Water Treated with Low-Frequency Electromagnetic Resonance Fields in Different Fertigation Doses" Horticulturae 8, no. 10: 868. https://doi.org/10.3390/horticulturae8100868
APA StyleNogueira, B. B., Vicente, E. F., Nunes Chaves, P. P., Zanetti, W. A. L., Ono, E. O., da Silva, G. F., dos Reis, A. R., & Putti, F. F. (2022). Sugar Metabolism and Photosynthesis of Tomatoes Irrigated with Water Treated with Low-Frequency Electromagnetic Resonance Fields in Different Fertigation Doses. Horticulturae, 8(10), 868. https://doi.org/10.3390/horticulturae8100868








