Optimized Preparation of Methyl Salicylate Hydrogel and Its Inhibition Effect on Potato Tuber Sprouting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Inhibition Test of Potato Sprouting
2.3. Preparation of MeSA Hydrogel
2.3.1. Single-Factor Experiments
2.3.2. RSM Design for Optimization of Encapsulation Efficiency
2.3.3. Determination of Encapsulation Efficiency
2.4. Characterization of MeSA Hydrogel
2.4.1. Morphology and Size of the MeSA Hydrogel
2.4.2. Fourier-Transform Infrared (FTIR) Spectroscopy
2.4.3. Differential Scanning Calorimetry (DSC) Analysis
2.4.4. X-ray Diffraction (XRD) Spectroscopy
2.5. Release Properties of MeSA Hydrogel
2.6. Inhibition of MeSA Hydrogel on Potato Sprouting
2.7. Statistical Analysis
3. Results and Discussions
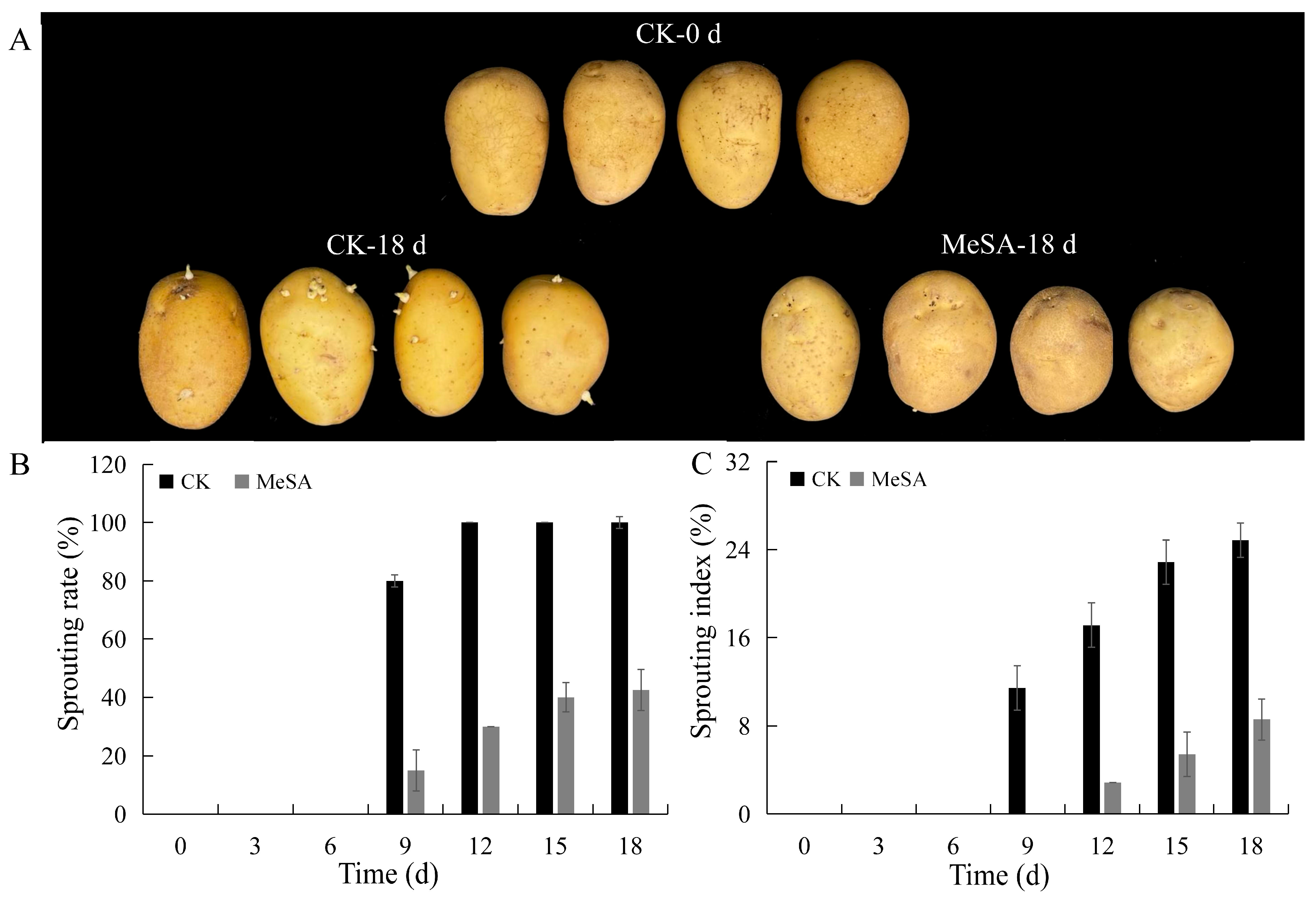
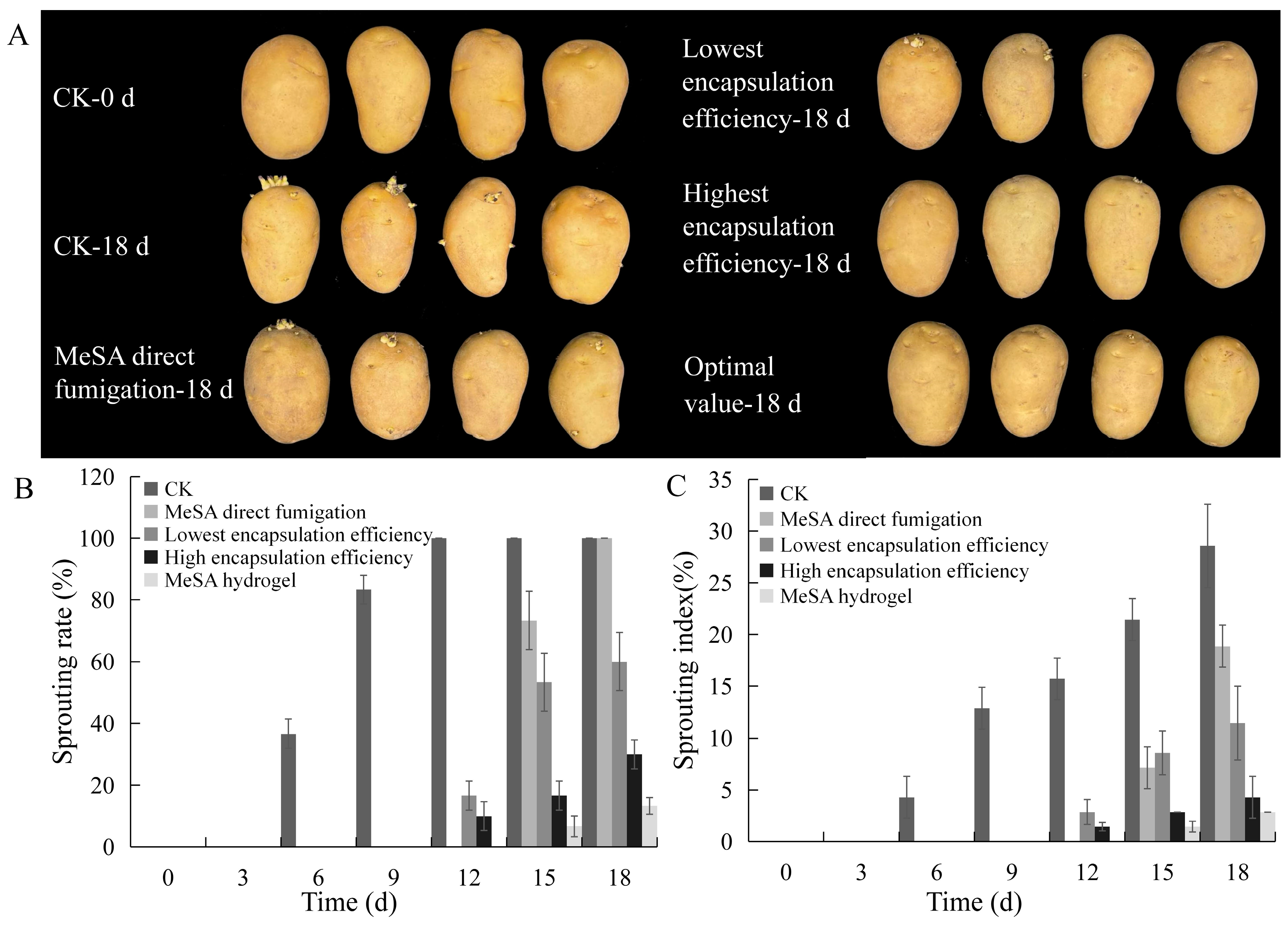
3.1. Inhibition Test of Potato Sprouting
3.2. Preparation and Characterization of MeSA Hydrogel
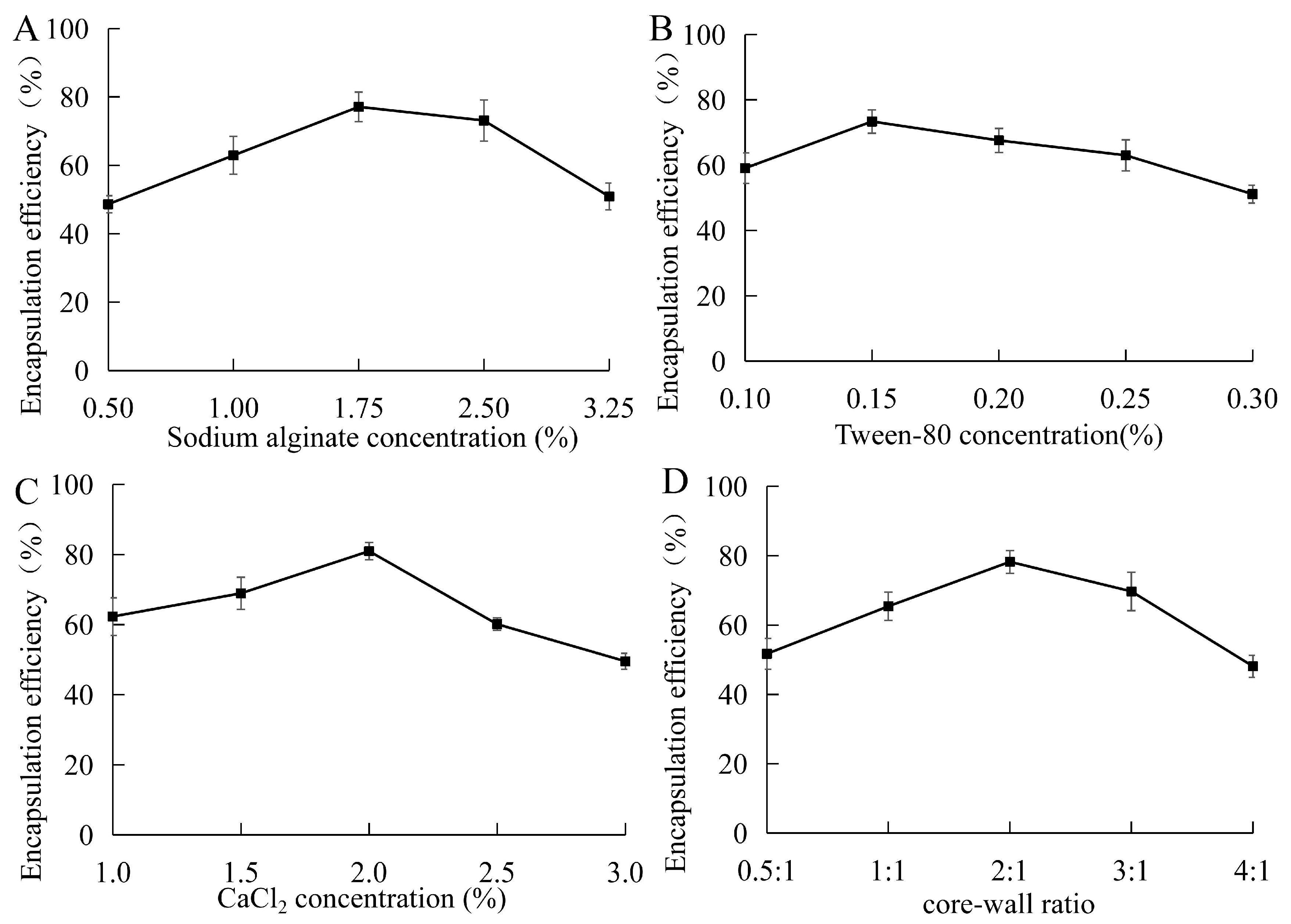
3.2.1. Single-Factor Experiments
3.2.2. Optimized Formulation of Encapsulation Efficiency
3.2.3. Accuracy of Predictive Models
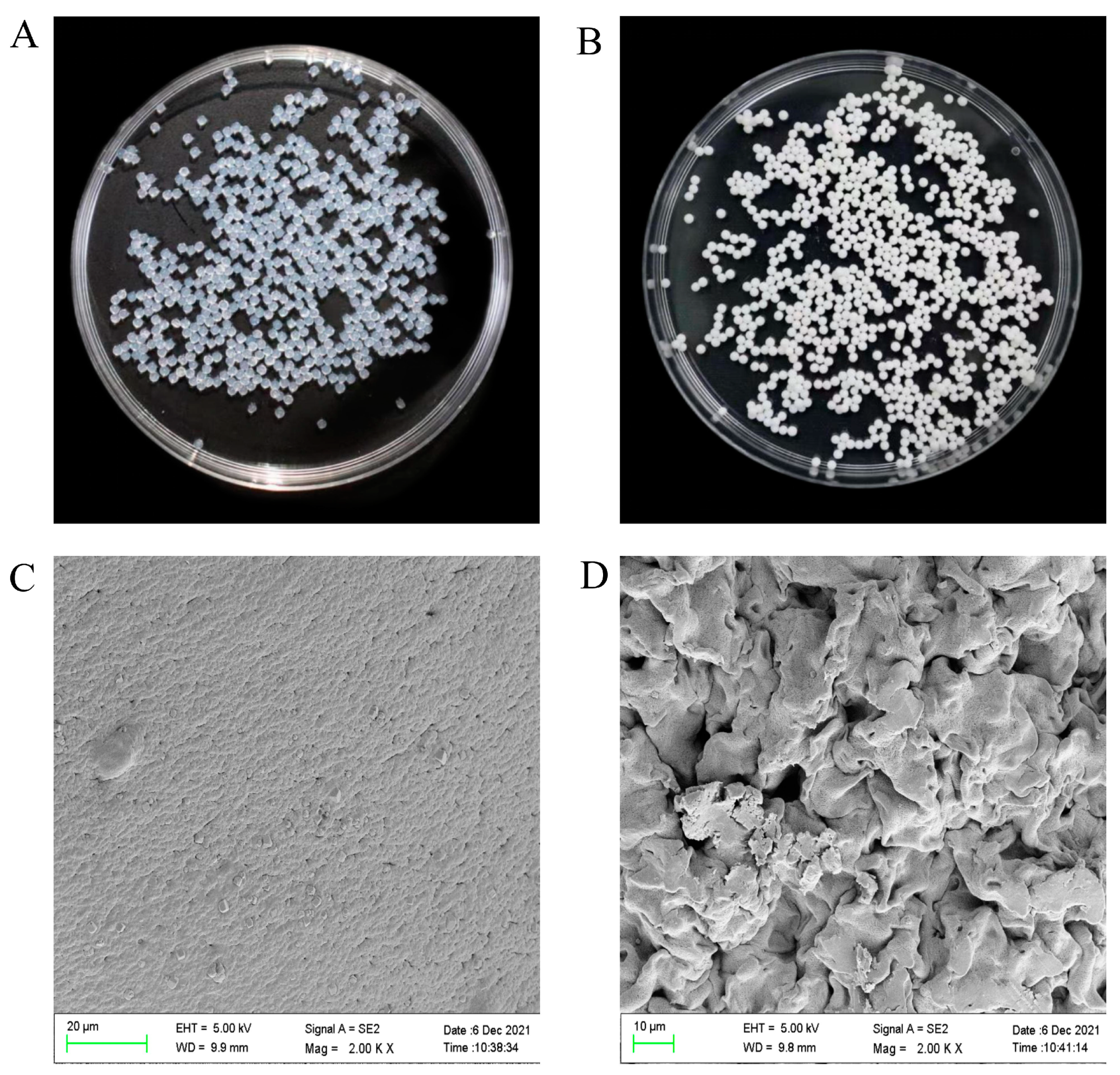
3.2.4. Morphology and Size of the Hydrogel
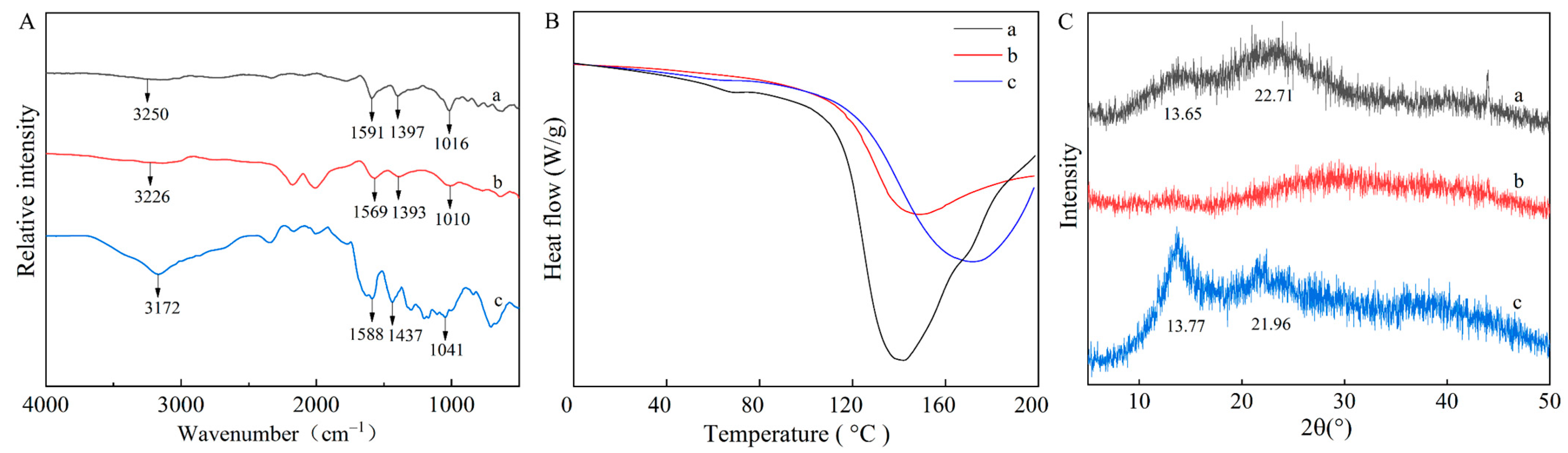
3.2.5. Fourier-Transform Infrared (FTIR) Spectroscopy
3.2.6. Differential Scanning Calorimetry (DSC) Analysis
3.2.7. X-ray Diffraction (XRD) Spectroscopy
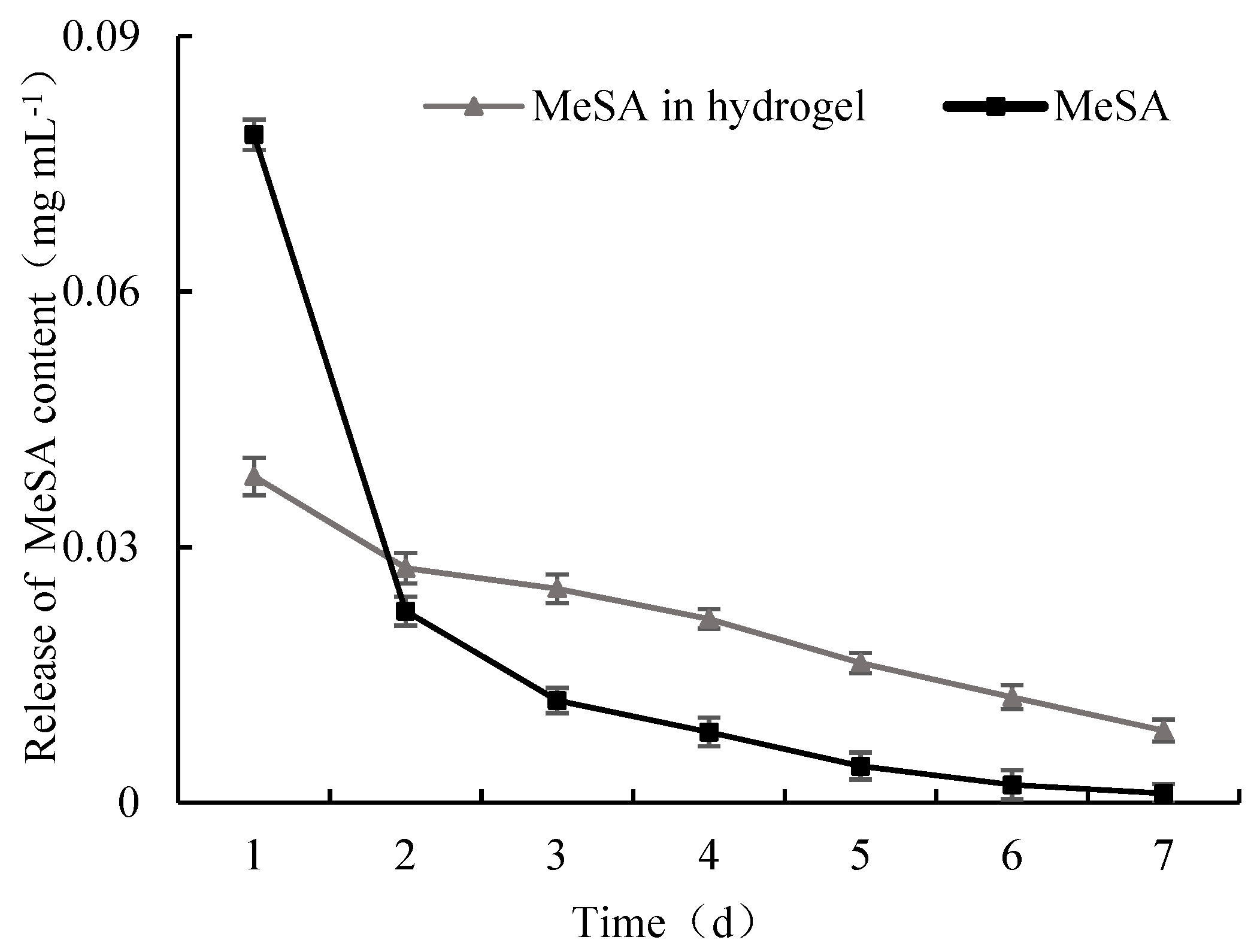
3.3. Release Properties of MeSA Hydrogel
3.4. Inhibition of MeSA Hydrogel on Potato Sprouting
4. Conclusions
5. Future Work
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sampaio, S.L.; Petropoulos, S.A.; Alexopoulos, A.; Heleno, S.A.; Santos-Buelga, C.; Barros, L.; Ferreira, I.C.F.R. Potato peels as sources of functional compounds for the food industry: A review. Trends Food Sci. Technol. 2020, 103, 118–129. [Google Scholar] [CrossRef]
- Alamar, M.C.; Tosetti, R.; Landahl, S.; Bermejo, A.; Terry, L.A. Assuring potato tuber quality during storage: A future perspective. Front. Plant Sci. 2017, 8, 2034. [Google Scholar] [CrossRef] [PubMed]
- Orawetz, T.; Malinova, I.; Orzechowski, S.; Fettke, J. Reduction of the plastidial phosphorylase in potato (Solanum tuberosum L.) reveals impact on storage starch structure during growth at low temperature. Plant Physiol. Biochem. 2016, 100, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, B.; Dhatt, A.S.; Sandhu, K.S.; Garg, A. Effect of CIPC (isopropyl-N (3-chlorophenyl) carbamate) on storage and processing quality of potato. J. Food Agric. Environ. 2008, 6, 34–38. [Google Scholar] [CrossRef]
- Son, N.A.; Ha, N.T.N.; Sang, N.T.M.; Duc, L.D.D.; Trieu, L.N. Effects of low energy (160 keV) X-ray on microbial inactivation, sprouting inhibition and genetic variation in potato. Food Biosci. 2022, 47, 101555. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, S.; Liu, J.; Ou, Y.; Song, B.; Zhang, C.; Lin, Y.; Li, X.; Xie, C. The potato protease inhibitor gene, St-Inh, plays roles in the cold-induced sweetening of potato tubers by modulating invertase activity. Postharvest Biol. Technol. 2013, 86, 265–271. [Google Scholar] [CrossRef]
- Paul, V.; Ezekiel, R.; Pandey, R. Sprout suppression on potato: Need to look beyond CIPC for more effective and safer alternatives. J. Food Sci. Technol. 2016, 53, 1–18. [Google Scholar] [CrossRef]
- Visse-Mansiaux, M.; Soyeurt, H.; Herrera, J.M.; Torche, J.; Vanderschuren, H.; Dupuis, B. Prediction of potato sprouting during storage. Field Crops Res. 2022, 278, 108396. [Google Scholar] [CrossRef]
- Cools, K.; Alamar, M.C.; Terry, L.A. Controlling sprouting in potato tubers using ultraviolet-C irradiance. Postharvest Biol. Technol. 2014, 98, 106–114. [Google Scholar] [CrossRef]
- Li, L.; Chen, J.; Li, Z.; Li, H.; Yang, S.; Ren, B.; Lu, Y.; Zheng, S.; Yu, L.; Wang, X.; et al. Proteomic analysis of garlic essential oil-treated potato reveals that StHSP26.5 as a vital gene involving in tuber sprouting. Postharvest Biol. Technol. 2022, 183, 111725. [Google Scholar] [CrossRef]
- Jia, B.; Xu, L.; Guan, W.; Lin, Q.; Brennan, C.; Yan, R.; Zhao, H. Effect of citronella essential oil fumigation on sprout suppression and quality of potato tubers during storage. Food Chem. 2019, 284, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Belay, D.W.; Asfaw, Z.; Lulekal, E.; Kassa, B.; Kifele, H. Effects of essential oils on potato tuber sprouting at room temperature storage in Ethiopia. Heliyon 2022, 8, e09090. [Google Scholar] [CrossRef]
- Fan, X.; Du, Z.; Cui, X.; Ji, W.; Ma, J.; Li, X.; Wang, X.; Zhao, H.; Liu, B.; Guo, F.; et al. Preharvest methyl salicylate treatment enhance the chilling tolerance and improve the postharvest quality of apricot during low temperature storage. Postharvest Biol. Technol. 2021, 177, 111535. [Google Scholar] [CrossRef]
- Mercer, N.H.; Bessin, R.T.; Obrycki, J.J. Impact of buckwheat and methyl salicylate lures on natural enemy abundance for early season management of Melanaphis sacchari (Hemiptera: Aphididae) in sweet sorghum. Crop Prot. 2020, 137, 105279. [Google Scholar] [CrossRef]
- Kalaivani, K.; Maruthi-Kalaiselvi, M.; Senthil-Nathan, S. Seed treatment and foliar application of methyl salicylate (MeSA) as a defense mechanism in rice plants against the pathogenic bacterium, Xanthomonas oryzae pv. Oryzae. Pestic. Biochem. Physiol. 2020, 171, 104718. [Google Scholar] [CrossRef]
- Bajac, J.; Nikolovski, B.; Lončarević, I.; Petrović, J.; Bajac, B.; Đurović, S.; Petrović, L. Microencapsulation of juniper berry essential oil (Juniperus communis L.) by spray drying: Microcapsule characterization and release kinetics of the oil. Food Hydrocoll. 2022, 125, 107430. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, X.; Xia, Y. Preparation and properties of essential oil microspheres antibacterial alignate films. IOP Conf. Ser. Mater. Sci. Eng. 2018, 382, 022042. [Google Scholar] [CrossRef]
- Chuysinuan, P.; Chimnoi, N.; Reuk-Ngam, N.; Khlaychan, P.; Makarasen, A.; Wetprasit, N.; Dechtrirat, D.; Supaphol, P.; Techasakul, S. Development of gelatin hydrogel pads incorporated with Eupatorium adenophorum essential oil as antibacterial wound dressing. Polym. Bull. 2019, 76, 701–724. [Google Scholar] [CrossRef]
- Kesharwani, P.; Bisht, A.; Alexander, A.; Dave, V.; Sharma, S. Biomedical applications of hydrogels in drug delivery system: An update. J. Drug Deliv. Sci. Technol. 2021, 66, 102914. [Google Scholar] [CrossRef]
- Xue, X.; Hu, Y.; Wang, S.; Chen, X.; Jiang, Y.; Su, J. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bioact. Mater. 2022, 12, 327–339. [Google Scholar] [CrossRef]
- Shewan, H.M.; Stokes, J.R. Review of techniques to manufacture microhydrogel particles for the food industry and their applications. J. Food Eng. 2013, 119, 781–792. [Google Scholar] [CrossRef]
- Vazquez-Gonzalez, M.; Willner, I. Stimuli-responsive biomolecule-based hydrogels and their applications. Angew. Chem. Int. Ed. 2020, 59, 15342–15377. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Li, S.; Liu, Z.; Chen, H.; Liu, Y.; Li, W.; Wang, G. Preparation and characterization of starch-cellulose interpenetrating network hydrogels based on sequential Diels-Alder click reaction and photopolymerization. Int. J. Biol. Macromol. 2021, 194, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Ghauri, Z.H.; Islam, A.; Qadir, M.A.; Ghaffar, A.; Gull, N.; Azam, M.; Mehmood, A.; Ghauri, A.A.; Khan, R.U. Novel pH-responsive chitosan/sodium alginate/PEG based hydrogels for release of sodium ceftriaxone. Mater. Chem. Phys. 2022, 277, 125456. [Google Scholar] [CrossRef]
- Singha, I.; Basu, A. Chitosan based injectable hydrogels for smart drug delivery applications. Sens. Int. 2022, 3, 100168. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Y.; Shu, F.; Zhou, R.; Bao, B.; Xiao, S.; Li, K.; Lin, Q.; Zhu, L.; Xia, Z. In situ-formed adhesive hyaluronic acid hydrogel with prolonged amnion-derived conditioned medium release for diabetic wound repair. Carbohydr. Polym. 2022, 276, 118752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, K.; Zhang, Z.; Xie, H.; Li, Z.; Lin, Z.; Mo, C.; Yang, W.; Wang, X.; Wei, J. Polypropylene non-woven supported calcium alginate hydrogel filtration membrane for efficient separation of dye/salt at low salt concentration. Desalination 2020, 500, 114845. [Google Scholar] [CrossRef]
- Song, X.; Guo, J.; Liu, Y.; Li, F.; Yang, Q.; Guan, F.; Di, C. Preparation and characterization of multi-network hydrogels based on sodium alginate/krill protein/polyacrylamide -Strength, shape memory, conductivity and biocompatibility. Intl. J. Biol. Macromol. 2022, 207, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Seo, S.; Park, I.; Hyun, J. Larvicidal composite alginate hydrogel com-bined with a Pickering emulsion of essential oil. Carbohydr. Polym. 2021, 254, 117381. [Google Scholar] [CrossRef]
- Rahiminezhad, Z.; Gahruie, H.H.; Esteghlal, S.; Mesbahi, G.R.; Golmakani, M.; Hosseini, S.M.H. Oxidative stability of linseed oil nano-emulsions filled in calcium al-ginate hydrogels. LWT Food Sci. Technol. 2020, 127, 109392. [Google Scholar] [CrossRef]
- Potiwiput, S.; Tan, H.; Yuan, G.; Li, S.; Zhou, T.; Li, J.; Jia, Y.; Xiong, D.; Hu, X.; Ling, Z.; et al. Dual-crosslinked alginate/carboxymethyl chitosan hydrogel contain-ing in situ synthesized calcium phosphate particles for drug delivery application. Mater. Chem. Phys. 2020, 241, 122354. [Google Scholar] [CrossRef]
- Ge, X.; Huang, Z.; Tian, J.; Xu, R.; Wu, X.; Tian, S. S-(+)-carvone/HPβCD inclu-sion complex: Preparation, characterization and its application as a new sprout sup-pressant during potato storage. Food Chem. Adv. 2022, 1, 100100. [Google Scholar] [CrossRef]
- Arnon-Rips, H.; Sabag, A.; Tepper-Bamnolker, P.; Chalupovich, D.; Levi-Kalisman, Y.; Eshel, D.; Porat, R.; Poverenov, E. Effective suppression of potato tuber sprouting using polysaccharide-based emulsified films for prolonged release of citral. Food Hydrocoll. 2020, 103, 105644. [Google Scholar] [CrossRef]
- Şanlı, A.; Karadoğan, T.; Tonguç, M.; Baydar, H. Effects of caraway (Carum carvi L.) seed on sprouting of potato (Solanum tuberosum L.) tubers under different temperature conditions. Turk. J. Field Crop. 2010, 15, 54–58. [Google Scholar] [CrossRef]
- Mokhtari, S.; Jafari, S.M.; Assadpour, E. Development of a nutraceutical nano-delivery system through emulsification/internal gelation of alginate. Food Chem. 2017, 229, 286–295. [Google Scholar] [CrossRef]
- Deng, X.; Chen, J.; Chen, W. Hydrogel particles as a controlled release delivery system for lavender essential oil using pH triggers. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125134. [Google Scholar] [CrossRef]
- Thiengkaew, P.; Thanitwatthanasak, S.; Srisala, S.; Jittorntrum, B.; Chunhabundit, R.; Chitprasert, P. Response surface optimization of microfluidic formulations of nanobilosomes for enhancement of aqueous solubility, digestive stability, and cellular antioxidant activity of mangiferin. Food Chem. 2021, 351, 129315. [Google Scholar] [CrossRef]
- Zhang, W.; Shu, C.; Chen, Q.; Cao, J.; Jiang, W. The multi-layer film system improved the release and retention properties of cinnamon essential oil and its application as coating in inhibition to penicillium expansion of apple fruit. Food Chem. 2019, 299, 125109. [Google Scholar] [CrossRef]
- Choi, C.H.; Jung, J.H.; Rhee, Y.W.; Kim, D.P.; Shim, S.E.; Lee, C.S. Generation of monodisperse alginate microbeads and in situ encapsulation of cell in microfluidic device. Biomed. Microdevices 2017, 9, 855–862. [Google Scholar] [CrossRef]
- Sevda, S.B.; Rodrigues, L. The making of pomegranate wine using yeast immobilized on sodium alginate. Afr. J. Food Sci. 2011, 5, 299–304. [Google Scholar] [CrossRef]
- Soliman, E.A.; El-Moghazy, A.Y.; El-Din, M.S.M.; Massoud, M. Microencapsulation of Essential Oils within Alginate: Formulation and In Vitro Evaluation of Antifungal Activity. J. Encapsulation Adsorpt. Sci. 2013, 3, 48–55. [Google Scholar] [CrossRef]
- Gholamian, S.; Nourani, M.; Bakhshi, N. Formation and characterization of calcium alginate hydrogel beads filled with cumin seeds essential oil. Food Chem. 2021, 338, 128143. [Google Scholar] [CrossRef]
- Shabkhiz, M.A.; Pirouzifard, M.K.; Pirsa, S.; Mahdavinia, G.R. Alginate hydrogel beads containing Thymus daenensis essential oils/Glycyrrhizic acid loaded in β-cyclodextrin. Investigation of structural, antioxidant/antimicrobial properties and release assessment. J. Mol. Liq. 2021, 344, 117738. [Google Scholar] [CrossRef]
- Faidi, A.; Lassoued, M.A.; Becheikh, M.E.H.; Touati, M.; Stumbéb, J.F.; Farhat, F. Application of sodium alginate extracted from a Tunisian brown algae Padina pavonica for essential oil encapsulation: Microspheres preparation, characterization and in vitro release study. Int. J. Biol. Macromol. 2019, 9, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Zeeb, B.; Saberi, A.H.; Weiss, J.; McClements, D.J. Formation and characterization of filled hydrogel beads based on calcium alginate: Factors influencing nanoemulsion retention and release. Food Hydrocoll. 2015, 50, 27–36. [Google Scholar] [CrossRef]

| Independent Variable | Coded Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A: Sodium alginate (%) | 1.0 | 1.75 | 2.5 |
| B: CaCl2 (%) | 1.5 | 2.0 | 2.5 |
| C: Core–wall ratio | 1:1 | 2:1 | 3:1 |
| Run | A | B | C | Y Encapsulation Efficiency/% |
|---|---|---|---|---|
| 1 | 1.75 | 2.0 | 2:1 | 83.21 |
| 2 | 1.75 | 2.0 | 2:1 | 82.25 |
| 3 | 1.00 | 2.0 | 1:1 | 71.68 |
| 4 | 1.75 | 2.0 | 2:1 | 82.54 |
| 5 | 1.75 | 1.5 | 1:1 | 78.74 |
| 6 | 2.50 | 1.5 | 2:1 | 75.26 |
| 7 | 1.75 | 2.5 | 1:1 | 79.67 |
| 8 | 1.75 | 2.5 | 3:1 | 80.51 |
| 9 | 1.75 | 2.0 | 2:1 | 82.97 |
| 10 | 1.75 | 1.5 | 3:1 | 75.75 |
| 11 | 2.50 | 2.0 | 3:1 | 74.69 |
| 12 | 2.50 | 2.5 | 2:1 | 79.21 |
| 13 | 1.00 | 1.5 | 2:1 | 69.82 |
| 14 | 1.00 | 2.5 | 2:1 | 74.24 |
| 15 | 1.00 | 2.0 | 3:1 | 73.27 |
| 16 | 1.75 | 2.0 | 2:1 | 82.04 |
| 17 | 2.50 | 2.0 | 1:1 | 78.92 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 283.33 | 9 | 31.48 | 97.54 | <0.0001 |
| A: Sodium alginate (%) | 45.46 | 1 | 45.46 | 140.84 | <0.0001 |
| B: CaCl2 (%) | 24.71 | 1 | 24.71 | 76.56 | <0.0001 |
| C: Core–wall ratio | 2.87 | 1 | 2.87 | 8.89 | 0.0205 |
| AB | 0.0552 | 1 | 0.0552 | 0.1711 | 0.6915 |
| AC | 8.47 | 1 | 8.47 | 26.24 | 0.0014 |
| BC | 3.67 | 1 | 3.67 | 11.36 | 0.0119 |
| A2 | 151.50 | 1 | 151.50 | 469.40 | <0.0001 |
| B2 | 16.36 | 1 | 16.36 | 50.68 | 0.0002 |
| C2 | 16.23 | 1 | 16.23 | 50.29 | 0.0002 |
| Residual | 2.26 | 7 | 0.3228 | ||
| Lack of fit | 1.31 | 3 | 0.4369 | 1.84 | 0.2799 |
| Pure error | 0.9487 | 4 | 0.2372 | ||
| Cor Total | 285.59 | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, L.; Wang, J.; Guan, Z.; Yue, F.; Wang, S.; Chen, Q.; Fu, M. Optimized Preparation of Methyl Salicylate Hydrogel and Its Inhibition Effect on Potato Tuber Sprouting. Horticulturae 2022, 8, 866. https://doi.org/10.3390/horticulturae8100866
Yuan L, Wang J, Guan Z, Yue F, Wang S, Chen Q, Fu M. Optimized Preparation of Methyl Salicylate Hydrogel and Its Inhibition Effect on Potato Tuber Sprouting. Horticulturae. 2022; 8(10):866. https://doi.org/10.3390/horticulturae8100866
Chicago/Turabian StyleYuan, Lixue, Jun Wang, Zhongliang Guan, Fengli Yue, Shufen Wang, Qingmin Chen, and Maorun Fu. 2022. "Optimized Preparation of Methyl Salicylate Hydrogel and Its Inhibition Effect on Potato Tuber Sprouting" Horticulturae 8, no. 10: 866. https://doi.org/10.3390/horticulturae8100866
APA StyleYuan, L., Wang, J., Guan, Z., Yue, F., Wang, S., Chen, Q., & Fu, M. (2022). Optimized Preparation of Methyl Salicylate Hydrogel and Its Inhibition Effect on Potato Tuber Sprouting. Horticulturae, 8(10), 866. https://doi.org/10.3390/horticulturae8100866





