Physiological and Qualitative Response of Cucurbita pepo L. to Salicylic Acid under Controlled Water Stress Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation, Crop Husbandry, and Experimental Design
2.2. Measured Traits
2.3. Antioxidant Enzymes Assay
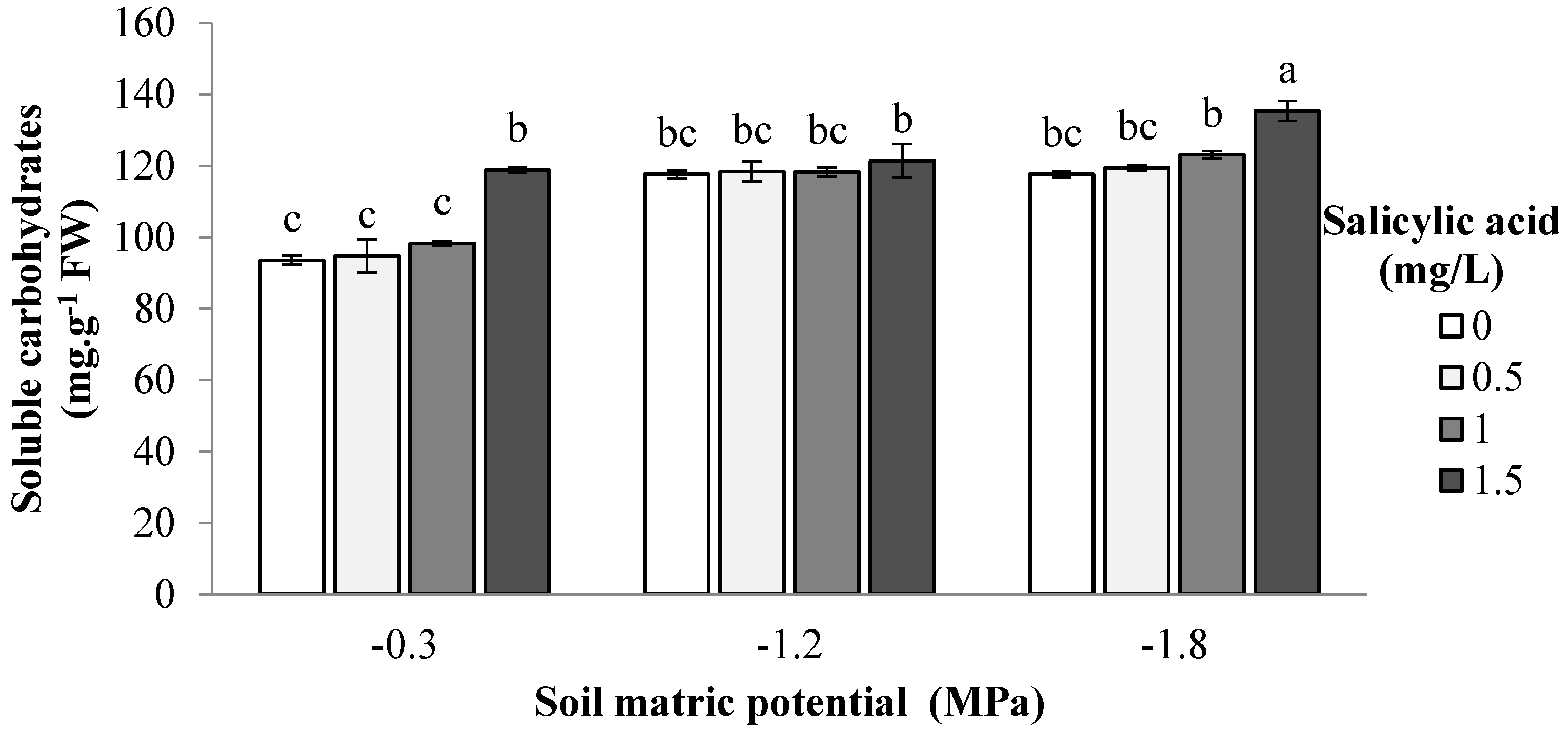
2.4. Quality Parameters
2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDA | Malondialdehyde |
| EL | Electrolytic leakage |
| CAT | Catalase |
| GR | Glutathione Reductase |
| POX | Peroxidase |
| APX | Ascorbate peroxidase |
| SOD | Superoxide Dismutase |
References
- Murkovic, M.; Piironen, V.; Lampi, A.M.; Kraushofer, T.; Sontag, G. Changes in chemical composition of pumpkin seeds during the roasting process for production of pumpkin seed oil (Part 1: Non-volatile compounds). Food Chem. 2004, 84, 359–365. [Google Scholar] [CrossRef]
- Medjakovic, S.; Hobiger, S.; Ardjomand-Woelkart, K.; Bucar, F.; Jungbauer, A. Pumpkin seed extract: Cell growth inhibition of hyperplastic and cancer cells, independent of steroid hormone receptors. Fitoterapia 2016, 110, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Richter, D.; Abarzua, S.; Chrobak, M.; Vrekoussis, T.; Weissenbacher, T.; Kühn, C.; Schulze, S.; Kupka, M.S.; Friese, K.; Briese, V.; et al. Effects of Phytoestrogen Extracts Isolated from Pumpkin Seeds on Estradiol Production and ER/PR Expression in Breast Cancer and Trophoblast Tumor Cells. Nutr. Cancer 2013, 65, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Abate, G.; Zhang, L.; Pucci, M.; Morbini, G.; Mac Sweeney, E.; Maccarinelli, G.; Ribaudo, G.; Gianoncelli, A.; Uberti, D.; Memo, M.; et al. Phytochemical Analysis and Anti-Inflammatory Activity of Different Ethanolic Phyto-Extracts of Artemisia annua L. Biomolecules 2021, 11, 975. [Google Scholar] [CrossRef]
- Gupta, A.K.; Dhua, S.; Sahu, P.P.; Abate, G.; Mishra, P.; Mastinu, A. Variation in Phytochemical, Antioxidant and Volatile Composition of Pomelo Fruit (Citrus grandis (L.) Osbeck) during Seasonal Growth and Development. Plants 2021, 10, 1941. [Google Scholar] [CrossRef]
- Gupta, A.K.; Rather, M.A.; Jha, A.K.; Shashank, A.; Singhal, S.; Sharma, M.; Pathak, U.; Sharma, D.; Mastinu, A. Artocarpus lakoocha Roxb. and Artocarpus heterophyllus Lam. Flowers: New Sources of Bioactive Compounds. Plants 2020, 9, 1329. [Google Scholar] [CrossRef] [PubMed]
- Karimmojeni, H.; Rahimian, H.; Alizadeh, H.; Yousefi, A.R.; Gonzalez-Andujar, J.L.; Mac Sweeney, E.; Mastinu, A. Competitive Ability Effects of Datura stramonium L. and Xanthium strumarium L. on the Development of Maize (Zea mays) Seeds. Plants 2021, 10, 1922. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Memo, M.; Mastinu, A. Plant behaviour: An evolutionary response to the environment? Plant Biol. 2020, 22, 961–970. [Google Scholar] [CrossRef]
- Kumar, A.; Premoli, M.; Aria, F.; Bonini, S.A.; Maccarinelli, G.; Gianoncelli, A.; Memo, M.; Mastinu, A. Cannabimimetic plants: Are they new cannabinoidergic modulators? Planta 2019, 249, 1681–1694. [Google Scholar] [CrossRef]
- Mahdavi, A.; Moradi, P.; Mastinu, A. Variation in Terpene Profiles of Thymus vulgaris in Water Deficit Stress Response. Molecules 2020, 25, 1091. [Google Scholar] [CrossRef] [Green Version]
- Mastinu, A.; Ascrizzi, R.; Ribaudo, G.; Bonini, S.A.; Premoli, M.; Aria, F.; Maccarinelli, G.; Gianoncelli, A.; Flamini, G.; Pistelli, L.; et al. Prosocial Effects of Nonpsychotropic Cannabis sativa in Mice. Cannabis Cannabinoid Res. 2021. [Google Scholar] [CrossRef]
- Mastinu, A.; Bonini, S.A.; Premoli, M.; Maccarinelli, G.; Mac Sweeney, E.; Zhang, L.; Lucini, L.; Memo, M. Protective Effects of Gynostemma pentaphyllum (var. Ginpent) against Lipopolysaccharide-Induced Inflammation and Motor Alteration in Mice. Molecules 2021, 26, 570. [Google Scholar] [CrossRef]
- Ali, M.B.; Khatun, S.; Hahn, E.-J.; Paek, K.-Y. Enhancement of phenylpropanoid enzymes and lignin in Phalaenopsis orchid and their influence on plant acclimatisation at different levels of photosynthetic photon flux. Plant Growth Regul. 2006, 49, 137–146. [Google Scholar] [CrossRef]
- Pirbalouti, A.G.; Samani, M.R.; Hashemi, M.; Zeinali, H. Salicylic acid affects growth, essential oil and chemical compositions of thyme (Thymus daenensis Celak.) under reduced irrigation. Plant Growth Regul. 2013, 72, 289–301. [Google Scholar] [CrossRef]
- Noman, A.; Ali, Q.; Naseem, J.; Javed, M.T.; Kanwal, H.; Islam, W.; Aqeel, M.; Khalid, N.; Zafar, S.; Tayyeb, M.; et al. Sugar beet extract acts as a natural bio-stimulant for physio-biochemical attributes in water stressed wheat (Triticum aestivum L.). Acta Physiol. Plant. 2018, 40, 110. [Google Scholar] [CrossRef]
- Naservafaei, S.; Sohrabi, Y.; Moradi, P.; Mac Sweeney, E.; Mastinu, A. Biological Response of Lallemantia iberica to Brassinolide Treatment under Different Watering Conditions. Plants 2021, 10, 496. [Google Scholar] [CrossRef] [PubMed]
- Reza Yousefi, A.; Rashidi, S.; Moradi, P.; Mastinu, A. Germination and Seedling Growth Responses of Zygophyllum fabago, Salsola kali L. and Atriplex canescens to PEG-Induced Drought Stress. Environments 2020, 7, 107. [Google Scholar] [CrossRef]
- Noryan, M.; Hervan, I.M.; Sabouri, H.; Kojouri, F.D.; Mastinu, A. Drought Resistance Loci in Recombinant Lines of Iranian Oryza sativa L. in Germination Stage. BioTech 2021, 10, 26. [Google Scholar] [CrossRef]
- BBhusal, N.; Lee, M.; Han, A.R.; Han, A.; Kim, H.S. Responses to drought stress in Prunus sargentii and Larix kaempferi seedlings using morphological and physiological parameters. For. Ecol. Manag. 2020, 465, 118099. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Shahidan, N.; Othman, R.; Jaswir, I.; Hasyun-Hashim, Y. Effect of abiotic stress under light and dark conditions on carotenoid content in pumpkin (Cucurbita moshata) calluses. J. Fundam. Appl. Sci. 2018, 9, 861. [Google Scholar] [CrossRef] [Green Version]
- Langeroodi, A.R.S.; Campiglia, E.; Mancinelli, R.; Radicetti, E. Can biochar improve pumpkin productivity and its physiological characteristics under reduced irrigation regimes? Sci. Hortic. 2018, 247, 195–204. [Google Scholar] [CrossRef]
- Sinu, P.A.; Pooja, A.; Aneha, K. Overhead sprinkler irrigation affects pollinators and pollination in pumpkin (Cucurbita maxima). Sci. Hortic. 2019, 258, 108803. [Google Scholar] [CrossRef]
- Seymen, M.; Yavuz, D.; Dursun, A.; Kurtar, E.S.; Türkmen, Ö. Identification of drought-tolerant pumpkin (Cucurbita pepo L.) genotypes associated with certain fruit characteristics, seed yield, and quality. Agric. Water Manag. 2019, 221, 150–159. [Google Scholar] [CrossRef]
- Szalai, G. Influence of salicylic acid on phytochelatin synthesis in maize during Cd stress. Turk. J. Bot. 2013. [Google Scholar] [CrossRef] [Green Version]
- Pál, M.; Kovács, V.; Vida, G.; Szalai, G.; Janda, T. Changes induced by powdery mildew in the salicylic acid and polyamine contents and the antioxidant enzyme activities of wheat lines. Eur. J. Plant Pathol. 2012, 135, 35–47. [Google Scholar] [CrossRef]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Khan, E.A. 24-Epibrassinolide application in plants: An implication for improving drought stress tolerance in plants. Plant Physiol. Biochem. 2018, 135, 295–303. [Google Scholar] [CrossRef]
- Santisree, P.; Jalli, L.C.L.; Bhatnagar-Mathur, P.; Sharma, K.K. Emerging Roles of Salicylic Acid and Jasmonates in Plant Abiotic Stress Responses. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress: Biochemical and Molecular Perspectives; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 342–373. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, H.; Ruan, W.; Deng, M.; Wang, F.; Peng, J.; Luo, J.; Chen, Z.; Yi, K. Abnormal inflorescence meristem1 functions in Salicylic Acid Biosynthesis to Maintain Proper Reactive Oxygen Species Levels for Root Meristem Activity in Rice. Plant Cell 2017, 29, 560–574. [Google Scholar] [CrossRef] [Green Version]
- Abbaspour, J.; Ehsanpour, A. The impact of salicylic acid on some physiological responses of Artemisia aucheri Boiss. under in vitro drought stress. Acta Agric. Slov. 2016, 107, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.H. Soil Sampling, Preparation, and Analysis, 2nd ed.; Taylor & Francis: Boca Raton, FL, USA, 2005; p. 623. [Google Scholar]
- Pratt, P.F. Salinity, Sodium, and Potassium in an Irrigated Soil Treated with Bovine Manure. Soil Sci. Soc. Am. J. 1984, 48, 823–828. [Google Scholar] [CrossRef]
- Noborio, K.; Horton, R.; Tan, C.S. Time Domain Reflectometry Probe for Simultaneous Measurement of Soil Matric Potential and Water Content. Soil Sci. Soc. Am. J. 1999, 63, 1500–1505. [Google Scholar] [CrossRef]
- Jha, Y. Effects of Salinity on Growth Physiology, Accumulation of Osmo-Protectant and Autophagy-Dependent Cell Death of Two Maize Variety. Russ. Agric. Sci. 2018, 44, 124–130. [Google Scholar] [CrossRef]
- Sairam, R.K.; Srivastava, G.C. Water Stress Tolerance of Wheat (Triticum aestivum L.): Variations in Hydrogen Peroxide Accumulation and Antioxidant Activity in Tolerant and Susceptible Genotypes. J. Agron. Crop Sci. 2001, 186, 63–70. [Google Scholar] [CrossRef]
- Chen, J.; Song, L.; Dai, J.; Gan, N.; Liu, Z. Effects of microcystins on the growth and the activity of superoxide dismutase and peroxidase of rape (Brassica napus L.) and rice (Oryza sativa L.). Toxicon 2004, 43, 393–400. [Google Scholar] [CrossRef]
- Asada, K. Production and Scavenging of Reactive Oxygen Species in Chloroplasts and Their Functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Trawczyńska, I. New Method of Determining Kinetic Parameters for Decomposition of Hydrogen Peroxide by Catalase. Catalysts 2020, 10, 323. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.H.; Lee, C.B. Chilling stress-induced changes of antioxidant enzymes in the leaves of cucumber: In gel enzyme activity assays. Plant Sci. 2000, 159, 75–85. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Xu, B.; Li, J.; Huang, B. Exogenous Melatonin Suppresses Dark-Induced Leaf Senescence by Activating the Superoxide Dismutase-Catalase Antioxidant Pathway and Down-Regulating Chlorophyll Degradation in Excised Leaves of Perennial Ryegrass (Lolium perenne L.). Front. Plant Sci. 2016, 7, 1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, S.S. Food Analysis, 4th ed.; Springer: New York, NY, USA; Dordrecht, The Netherlands, 2010; pp. xiv, 602. [Google Scholar]
- Taufik, I.I.; Guntarti, A. Comparison of reduction sugar analysis method in cilembu sweet potato (Ipomoea batatas L.) using luff schoorl and anthrone method. J. Kedokt. Dan Kesehat. Indones. 2009, 7, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Karaosmanoğlu, H.; Üstün, N.Ş. Variations in fatty acid composition and oxidative stability of hazelnut (Corylus avellana L.) varieties stored by traditional method. Grasas Aceites 2019, 70, 288. [Google Scholar] [CrossRef] [Green Version]
- Weatherly, C.A.; Zhang, Y.; Smuts, J.P.; Fan, H.; Xu, C.; Schug, K.A.; Lang, J.C.; Armstrong, D.W. Analysis of Long-Chain Unsaturated Fatty Acids by Ionic Liquid Gas Chromatography. J. Agric. Food Chem. 2016, 64, 1422–1432. [Google Scholar] [CrossRef]
- Zhang, Y.; Luan, Q.; Jiang, J.; Li, Y. Prediction and Utilization of Malondialdehyde in Exotic Pine Under Drought Stress Using Near-Infrared Spectroscopy. Front. Plant Sci. 2021, 12, 735275. [Google Scholar] [CrossRef]
- Khaleghi, A.; Naderi, R.; Brunetti, C.; Maserti, B.; Salami, S.A.; Babalar, M. Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Sci. Rep. 2019, 9, 19250. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Sairam, R.K.; Srivastava, G.C.; Meena, R.C. Changes in antioxidant enzymes activity and oxidative stress by abscisic acid and salicylic acid in wheat genotypes. Biol. Plant. 2005, 49, 541–550. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fang, X.; Yuan, X.; Zhang, Y.; Li, H.; Zhou, Y.; Cui, X. Overexpression of Transcription Factor GmTGA15 Enhances Drought Tolerance in Transgenic Soybean Hairy Roots and Arabidopsis Plants. Agronomy 2021, 11, 170. [Google Scholar] [CrossRef]
- Abid, M.; Tian, Z.; Ata-Ul-Karim, S.T.; Liu, Y.; Cui, Y.; Zahoor, R.; Jiang, D.; Dai, T. Improved tolerance to post-anthesis drought stress by pre-drought priming at vegetative stages in drought-tolerant and -sensitive wheat cultivars. Plant Physiol. Biochem. 2016, 106, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liang, D.; Li, C.; Hao, Y.; Ma, F.; Shu, H. Influence of drought stress on the cellular ultrastructure and antioxidant system in leaves of drought-tolerant and drought-sensitive apple rootstocks. Plant Physiol. Biochem. 2012, 51, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, K.A.A.; Rashed, S.H.; Hossain, A.; El Sabagh, A. Yield and quality of two sugar beet (Beta vulgaris L. ssp. vulgaris var. altissima Döll) cultivars are influenced by foliar application of salicylic acid, irrigation timing, and planting density. Acta Agric. Slov. 2020, 115, 273. [Google Scholar] [CrossRef]
- Aghajanlou, F.; Mirdavoudi, H.; Shojaee, M.; Mac Sweeney, E.; Mastinu, A.; Moradi, P. Rangeland Management and Ecological Adaptation Analysis Model for Astragalus curvirostris Boiss. Horticulturae 2021, 7, 67. [Google Scholar] [CrossRef]
- Moradi, P.; Aghajanloo, F.; Moosavi, A.; Monfared, H.H.; Khalafi, J.; Taghiloo, M.; Khoshzaman, T.; Shojaee, M.; Mastinu, A. Anthropic Effects on the Biodiversity of the Habitats of Ferula gummosa. Sustainability 2021, 13, 7874. [Google Scholar] [CrossRef]
- Zangani, E.; Afsahi, K.; Shekari, F.; Mac Sweeney, E.; Mastinu, A. Nitrogen and Phosphorus Addition to Soil Improves Seed Yield, Foliar Stomatal Conductance, and the Photosynthetic Response of Rapeseed (Brassica napus L.). Agriculture 2021, 11, 483. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Drought Induces Oxidative Stress and Enhances the Activities of Antioxidant Enzymes in Growing Rice Seedlings. Plant Growth Regul. 2005, 46, 209–221. [Google Scholar] [CrossRef]
- Qayyum, A.; Razzaq, A.; Bibi, Y.; Khan, S.U.; Abbasi, K.S.; Sher, A.; Mehmood, A.; Ahmed, W.; Mahmood, I.; Manaf, A.; et al. Water stress effects on biochemical traits and antioxidant activities of wheat (Triticum aestivum L.) under In vitro conditions. Acta Agric. Scand. Sect. B Soil Plant Sci. 2017, 68, 283–290. [Google Scholar] [CrossRef]
- Läuchli, A.; Grattan, S. Plant Growth and Development Under Salinity Stress. In Advances in Molecular Breeding Toward Drought and Salt Tolerant Crops; Metzler, J.B., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 1–32. [Google Scholar] [CrossRef]
- Roshdy, A.E.-D.; Alebidi, A.; Almutairi, K.; Al-Obeed, R.; Elsabagh, A. The Effect of Salicylic Acid on the Performances of Salt Stressed Strawberry Plants, Enzymes Activity, and Salt Tolerance Index. Agronomy 2021, 11, 775. [Google Scholar] [CrossRef]
- Miura, K.; Tada, Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geilfus, C.-M. The pH of the Apoplast: Dynamic Factor with Functional Impact Under Stress. Mol. Plant 2017, 10, 1371–1386. [Google Scholar] [CrossRef] [PubMed]
- Mccollum, T.G.; Huber, D.J.; Cantliffe, D.J. Soluble Sugar Accumulation and Activity of Related Enzymes during Muskmelon Fruit-Development. J. Am. Soc. Hortic. Sci. 1988, 113, 399–403. [Google Scholar]
- Ribas-Carbo, M.; Taylor, N.L.; Giles, L.; Busquets, S.; Finnegan, P.M.; Day, D.A.; Lambers, H.; Medrano, H.l.; Berry, J.A.; Flexas, J. Effects of Water Stress on Respiration in Soybean Leaves. Plant Physiol. 2005, 139, 466–473. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, H.N.; Mahmud, T.M.M.; Edaroyati, M.W.P. Deficit Irrigation for Improving the Postharvest Quality of Lowland Tomato Fruits. Pertanika J. Trop. Agr. 2018, 41, 741–757. [Google Scholar]
- Ghaderi, N.; Normohammadi, S.; Javadi, T. Morpho-physiological Responses of Strawberry (Fragariaxananassa) to Exogenous Salicylic Acid Application under Drought Stress. J. Agr. Sci. Technol. 2015, 17, 167–178. [Google Scholar]
- Demiralay, M.; Saglam, A.; Kadioglu, A. Salicylic acid delays leaf rolling by inducing antioxidant enzymes and modulating osmoprotectant content in Ctenanthe setosa under osmotic stress. Turk. J. Biol. 2013, 37, 49–59. [Google Scholar] [CrossRef]
- Sariñana-Aldaco, O.; Sánchez-Chávez, E.; Troyo-Diéguez, E.; Tapia-Vargas, L.M.; Díaz-Pérez, J.C.; Preciado-Rangel, P. Foliar Aspersion of Salicylic Acid Improves Nutraceutical Quality and Fruit Yield in Tomato. Agriculture 2020, 10, 482. [Google Scholar] [CrossRef]
- Jahangirlou, M.R.; Akbari, G.A.; Alahdadi, I.; Soufizadeh, S.; Parsons, D. Grain Quality of Maize Cultivars as a Function of Planting Dates, Irrigation and Nitrogen Stress: A Case Study from Semiarid Conditions of Iran. Agriculture 2020, 11, 11. [Google Scholar] [CrossRef]
- Sehgal, A.; Sita, K.; Siddique, K.H.M.; Kumar, R.; Bhogireddy, S.; Varshney, R.K.; HanumanthaRao, B.; Nair, R.M.; Prasad, P.V.V.; Nayyar, H. Drought or/and Heat-Stress Effects on Seed Filling in Food Crops: Impacts on Functional Biochemistry, Seed Yields, and Nutritional Quality. Front. Plant Sci. 2018, 9, 1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, M.; Kumar, R. Foliar application of plant growth regulators modulates the productivity and chemical profile of damask rose (Rosa damascena Mill.) under mid hill conditions of the western Himalaya. Ind. Crop. Prod. 2020, 158, 113024. [Google Scholar] [CrossRef]
- Gao, J.; Hao, X.; Thelen, K.D.; Robertson, G.P. Agronomic Management System and Precipitation Effects on Soybean Oil and Fatty Acid Profiles. Crop Sci. 2009, 49, 1049–1057. [Google Scholar] [CrossRef]
- Carrera, C.; Martínez, M.J.; Dardanelli, J.; Balzarini, M. Water Deficit Effect on the Relationship between Temperature during the Seed Fill Period and Soybean Seed Oil and Protein Concentrations. Crop Sci. 2009, 49, 990–998. [Google Scholar] [CrossRef]
- Ashrafi, E.; Razmjoo, K. Effect of Irrigation Regimes on Oil Content and Composition of Safflower (Carthamus tinctorius L.) Cultivars. J. Am. Oil Chem. Soc. 2010, 87, 499–506. [Google Scholar] [CrossRef]
- Zunun-Pérez, A.Y.; Guevara-Figueroa, T.; Jimenez-Garcia, S.N.; Feregrino-Pérez, A.A.; Gautier, F.; Guevara-González, R.G. Effect of foliar application of salicylic acid, hydrogen peroxide and a xyloglucan oligosaccharide on capsiate content and gene expression associated with capsinoids synthesis in Capsicum annuum L. J. Biosci. 2017, 42, 245–250. [Google Scholar] [CrossRef]
- Ghasemi, M.; Modarresi, M.; Babaeian Jelodar, N.; Bagheri, N.; Jamali, A. The Evaluation of Exogenous Application of Salicylic Acid on Physiological Characteristics, Proline and Essential Oil Content of Chamomile (Matricaria chamomilla L.) under Normal and Heat Stress Conditions. Agriculture 2016, 6, 31. [Google Scholar] [CrossRef] [Green Version]
- Yousefzadi, M.; Sharifi, M.; Behmanesh, M.; Ghasempour, A.; Moyano, E.; Palazon, J. Salicylic acid improves podophyllotoxin production in cell cultures of Linum album by increasing the expression of genes related with its biosynthesis. Biotechnol. Lett. 2010, 32, 1739–1743. [Google Scholar] [CrossRef] [PubMed]
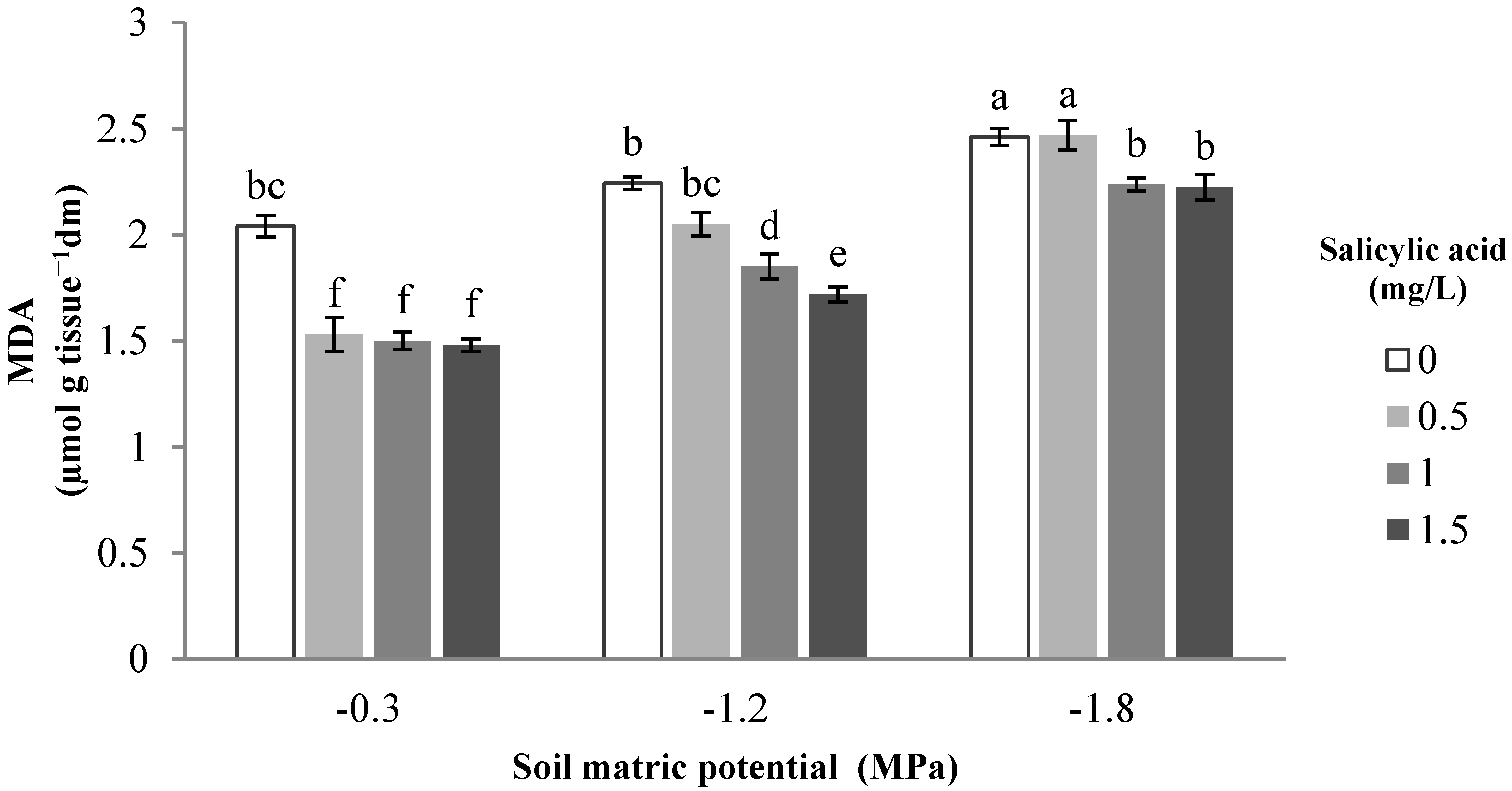
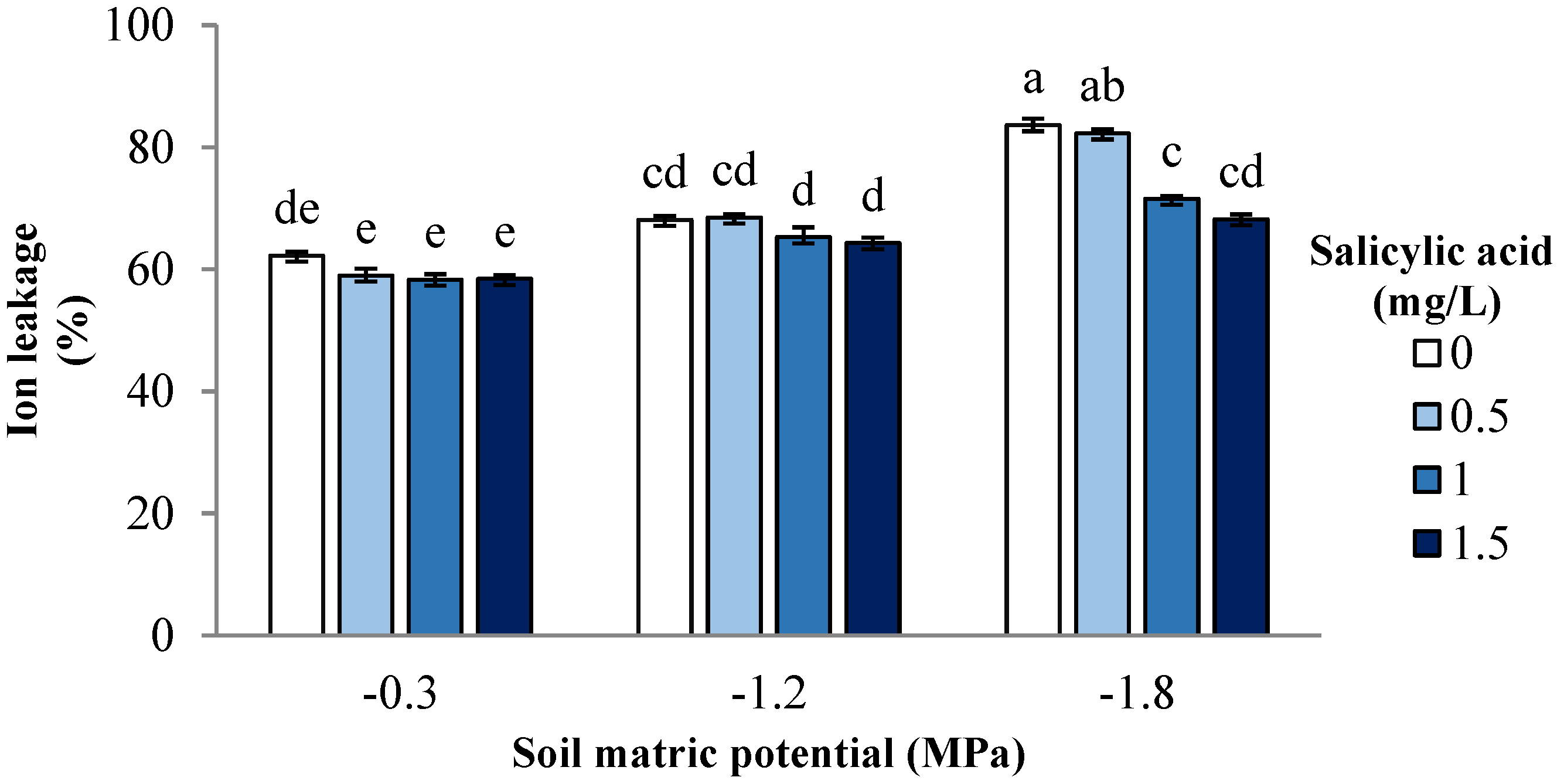
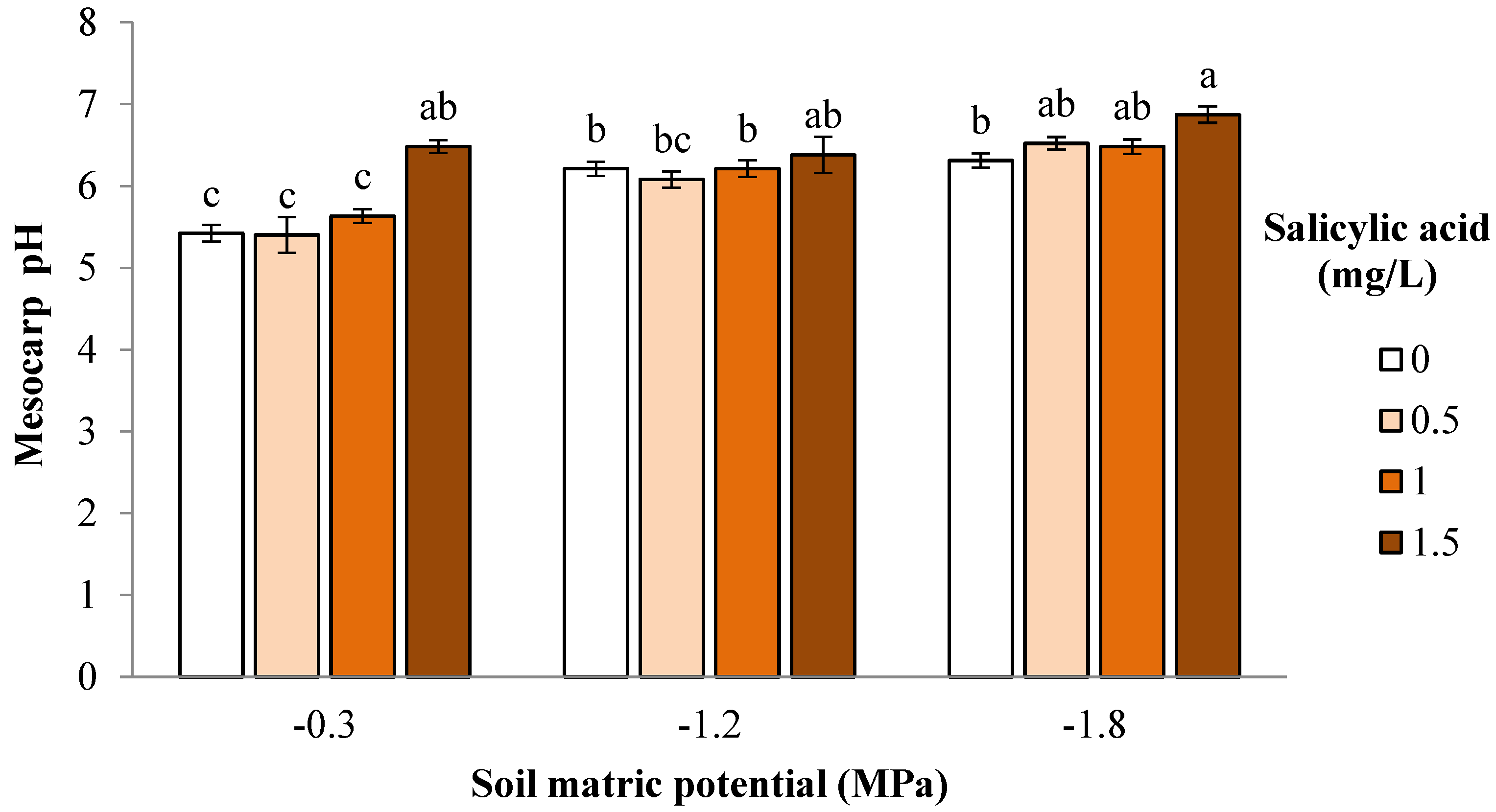
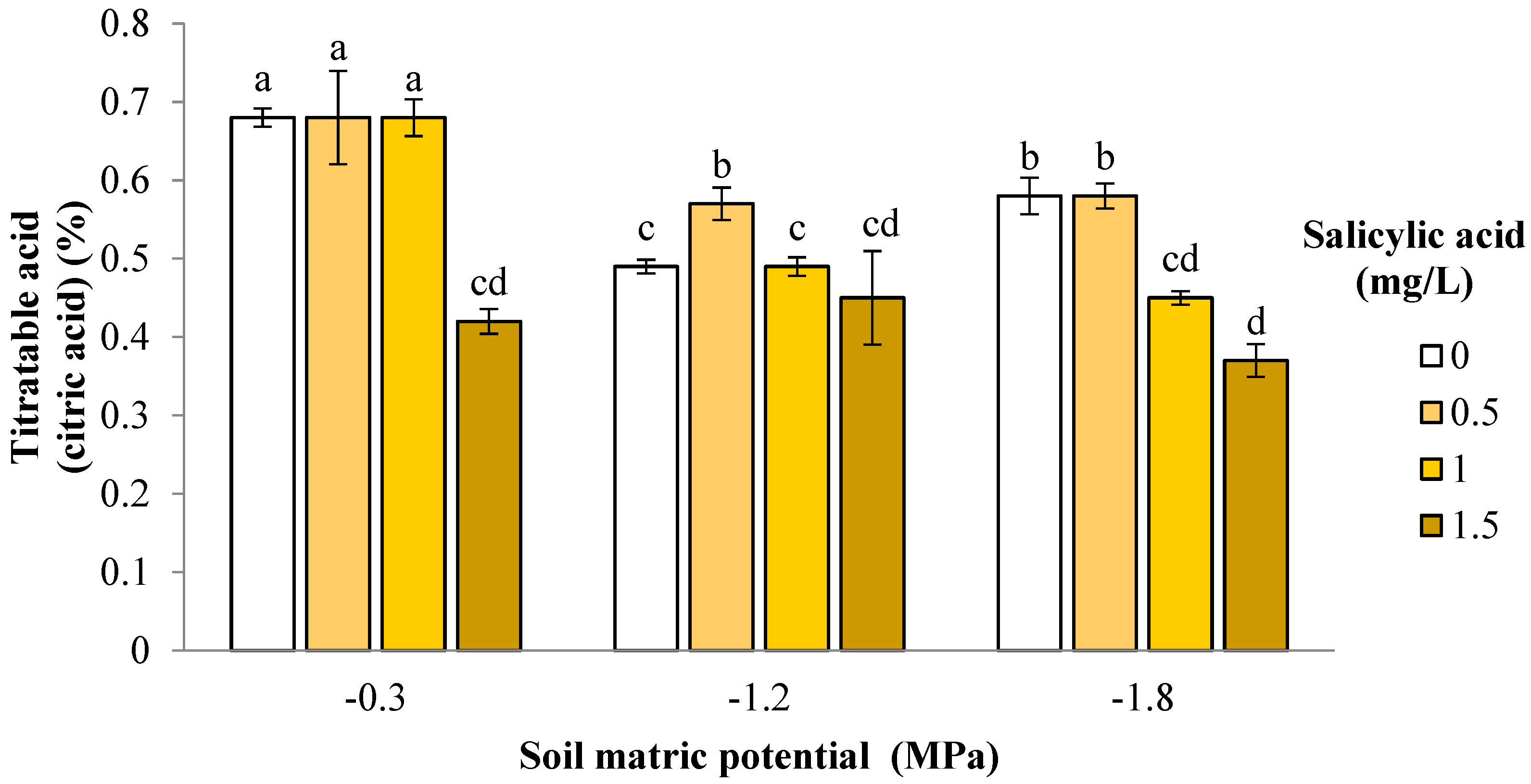
| Months | Mean of Precipitation (mm) | Mean of Temperature (°C) | Evaporation (mm) | |||
|---|---|---|---|---|---|---|
| 2016 | 2017 | 2016 | 2017 | 2016 | 2017 | |
| April | 2.6 | 2 | 10.7 | 9.5 | 3.1 | 2.9 |
| May | 0.2 | 0.9 | 16.65 | 16.5 | 4.2 | 3.8 |
| June | 0.01 | 0.5 | 22.3 | 19.8 | 7.1 | 6.5 |
| July | 0.03 | 0.05 | 26.55 | 24.6 | 11.1 | 10.2 |
| August | 0 | 0 | 25.9 | 26.85 | 10.4 | 11.2 |
| September | 0.09 | 0 | 21.45 | 22.55 | 8.9 | 9.7 |
| Type of Analysis | Values |
|---|---|
| Organic matter (%) | 1.21 |
| Electrical conductivity (%) | 0.83 |
| pH | 8.18 |
| Total lime (%) | 6.0 |
| Clay (%) | 31 |
| Silt (%) | 27 |
| Sand (%) | 42 |
| Texture | Clay Loam |
| Total nitrogen (%) | 0.07 |
| Phosphorus (mg/kg) | 14.2 |
| Potassium (mg/kg) | 266 |
| Calcium (meq/L) | 2.0 |
| Magnesium (meq/L) | 1.0 |
| S.O.V | DF | MDA | EL | CAT | GR | POX | APX | SOD | Mesocarp pH | Titratable Acid | Soluble Carbohydrates |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Year (Y) | 1 | 0.001 | 0.114 | 0.015 | 0.016 | 0.028 | 0.006 | 0.030 | 0.001 | 0.00001 | 0.335 |
| Error (Year × Rep) | 4 | 0.65 | 22.512 | 0.0001 | 62.141 ** | 0.005 ** | 0.101 ** | 64.645 | 0.1282 | 0.0012 | 8.519 |
| Water stress (A) | 2 | 0.940 ** | 248.643 ** | 1.748 ** | 1368.103 ** | 1.099 ** | 6.196 ** | 1.094 | 1.3684 ** | 0.1387 ** | 924.304 ** |
| Y × A | 2 | 0.0001 | 0.002 | 0.0001 | 0.0001 | 0.000 | 0.0001 | 0.0001 | 0.00002 | 0.00002 | 0.006 |
| Error | 8 | 0.055 | 5.925 | 0.002 | 0.176 | 0.001 | 0.013 | 0.455 | 0.0505 | 0.0022 | 3.684 |
| Salicylic Acid (B) | 3 | 0.038 ** | 1732.814 ** | 0.521 ** | 694.606 ** | 0.048 ** | 3.102 ** | 125.908 ** | 4.0306 ** | 0.1107 ** | 3354.688 ** |
| Y × SA | 3 | 0.0001 | 0.00003 | 0.0001 | 0.0001 | 0.000 | 0.0001 | 0.0001 | 0.0000007 | 0.00000025 | 0.002 |
| A × B | 6 | 0.021 ** | 76.563 ** | 0.043 ** | 11.243 ** | 0.081 ** | 0.092 ** | 2.351 * | 0.2859 ** | 0.0218 ** | 160.005 ** |
| Y × A × B | 6 | 0.0001 | 0.001 | 0.0001 | 0.0001 | 0.0001 | 0.0011 | 0.0001 | 0.000005 | 0.0000006 | 0.002 |
| Error | 36 | 0.005 | 3.68 | 0.001 | 1.113 | 0.0001 | 0.002 | 0.729 | 0.0402 | 0.0007 | 5.99 |
| CV | - | 3.25 | 2.83 | 2.98 | 2.97 | 2.32 | 2.01 | 1.42 | 3.25 | 4.97 | 2.13 |
| Soil Matric Potential (M Pa) | Salicylic Acid (mg/L) | Catalase (mg−1 Protein) | GR (mg/min FW) | POX (mg−1 Protein) | APX (mg/min FW) | SOD (mg/min FW) |
|---|---|---|---|---|---|---|
| −0.3 | 0.0 | 1.061 g | 35.89 ef | 0.263 k | 2.27 e | 57.01 e |
| 0.5 | 0.963 h | 28.78 h | 0.364 j | 1.96 g | 58.91 d | |
| 1.0 | 0.814 i | 23.91 i | 0.455 g | 1.58 h | 62.02 bc | |
| 1.5 | 0.775 j | 21.42 j | 0.551 e | 1.27 i | 63.01 ab | |
| −1.2 | 0.0 | 1.386 b | 43.69 b | 0.427 h | 2.88 a | 56.83 e |
| 0.5 | 1.205 e | 36.54 e | 0.395 i | 2.58 bc | 58.66 d | |
| 1.0 | 1.118 f | 34.24 f | 0.445 g | 2.29 e | 60.85 c | |
| 1.5 | 1.116 f | 32.21 g | 0.491 f | 2.11 f | 63.69 a | |
| −1.8 | 0.0 | 1.821 a | 52.99 a | 0.618 d | 2.46 c | 58.00 de |
| 0.5 | 1.389 b | 41.62 c | 0.678 c | 2.7 b | 58.91 d | |
| 1.0 | 1.301 c | 38.99 d | 0.737 b | 2.52 bc | 62.31 a–c | |
| 1.5 | 1.256 d | 36.29 e | 0.77 a | 2.44 e | 62.52 ab |
| Soil Matric Potential (MPa) | Salicylic Acid (mg/L) | 16:0 Palmitic Acid | 18:0 Stearic Acid | 18:1 Oleic Acid | 18:2 Linoleic Acid |
|---|---|---|---|---|---|
| −0.3 | 0.0 | 9.6 | 5.3 | 26.4 | 38.1 |
| 0.5 | 9.5 | 5.8 | 26.5 | 38.2 | |
| 1.0 | 8.6 | 4.7 | 28.4 | 40.5 | |
| 1.5 | 7.7 | 4.3 | 28.3 | 44.8 | |
| −1.2 | 0.0 | 12.8 | 6.9 | 31.3 | 31.4 |
| 0.5 | 12.4 | 6.8 | 32.1 | 31.0 | |
| 1.0 | 11.6 | 6.4 | 32.4 | 32.5 | |
| 1.5 | 11.2 | 6.5 | 34.1 | 33.6 | |
| −1.8 | 0.0 | 14.6 | 7.3 | 33.8 | 28.0 |
| 0.5 | 15.2 | 5.4 | 34.4 | 28.1 | |
| 1.0 | 13.5 | 5.8 | 35.4 | 31.8 | |
| 1.5 | 13.6 | 6.3 | 35.5 | 32.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biareh, V.; Shekari, F.; Sayfzadeh, S.; Zakerin, H.; Hadidi, E.; Beltrão, J.G.T.; Mastinu, A. Physiological and Qualitative Response of Cucurbita pepo L. to Salicylic Acid under Controlled Water Stress Conditions. Horticulturae 2022, 8, 79. https://doi.org/10.3390/horticulturae8010079
Biareh V, Shekari F, Sayfzadeh S, Zakerin H, Hadidi E, Beltrão JGT, Mastinu A. Physiological and Qualitative Response of Cucurbita pepo L. to Salicylic Acid under Controlled Water Stress Conditions. Horticulturae. 2022; 8(1):79. https://doi.org/10.3390/horticulturae8010079
Chicago/Turabian StyleBiareh, Vahideh, Farid Shekari, Saeed Sayfzadeh, Hamidreza Zakerin, Esmaeil Hadidi, José Gil Teixeira Beltrão, and Andrea Mastinu. 2022. "Physiological and Qualitative Response of Cucurbita pepo L. to Salicylic Acid under Controlled Water Stress Conditions" Horticulturae 8, no. 1: 79. https://doi.org/10.3390/horticulturae8010079
APA StyleBiareh, V., Shekari, F., Sayfzadeh, S., Zakerin, H., Hadidi, E., Beltrão, J. G. T., & Mastinu, A. (2022). Physiological and Qualitative Response of Cucurbita pepo L. to Salicylic Acid under Controlled Water Stress Conditions. Horticulturae, 8(1), 79. https://doi.org/10.3390/horticulturae8010079









