Interactive Effects of Intraspecific Competition and Drought on Stomatal Conductance and Hormone Concentrations in Different Tomato Genotypes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Design
2.3. Plant Measurements
2.4. Plant Hormone Analysis
2.5. Statistical Analysis
3. Results
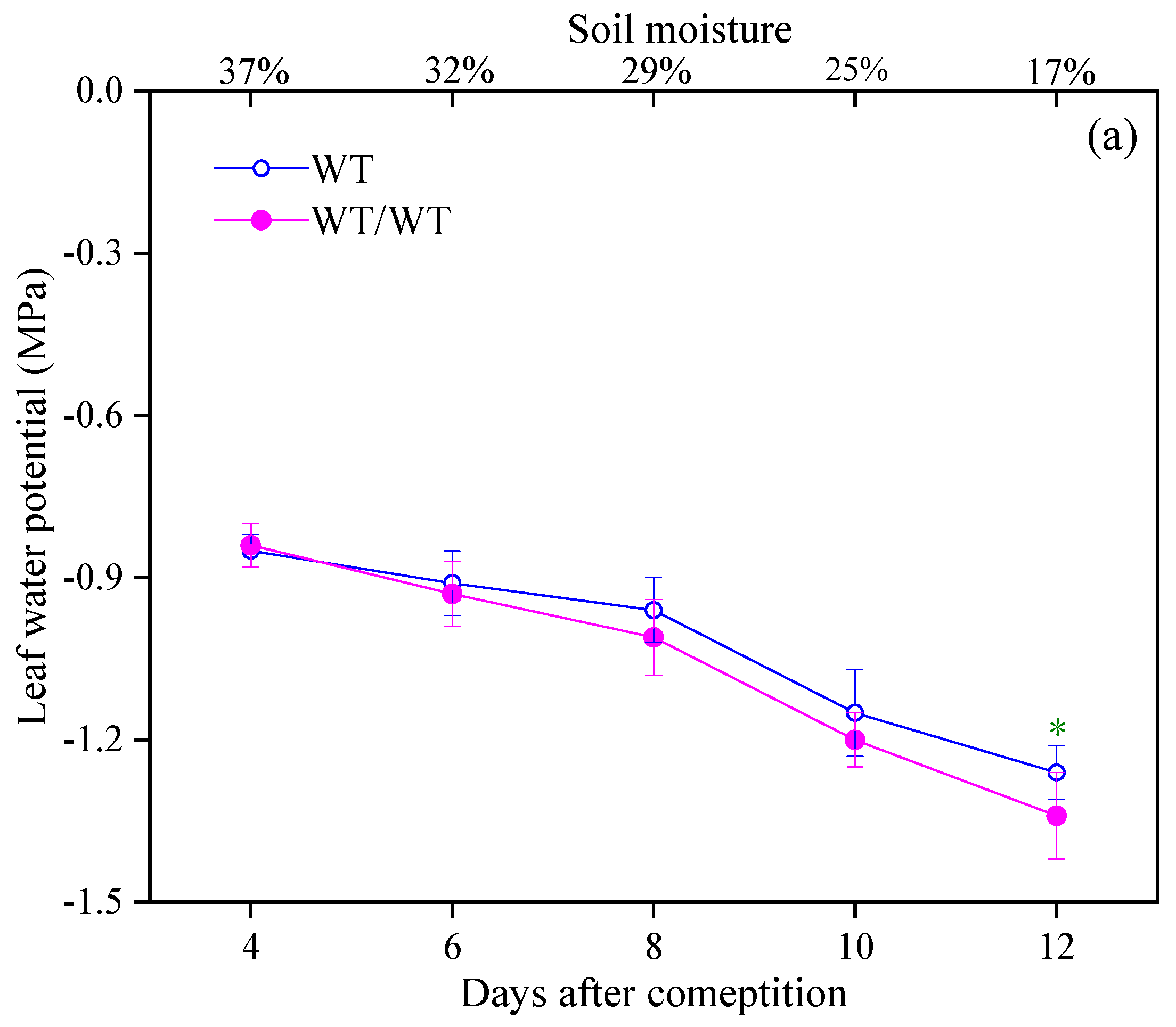
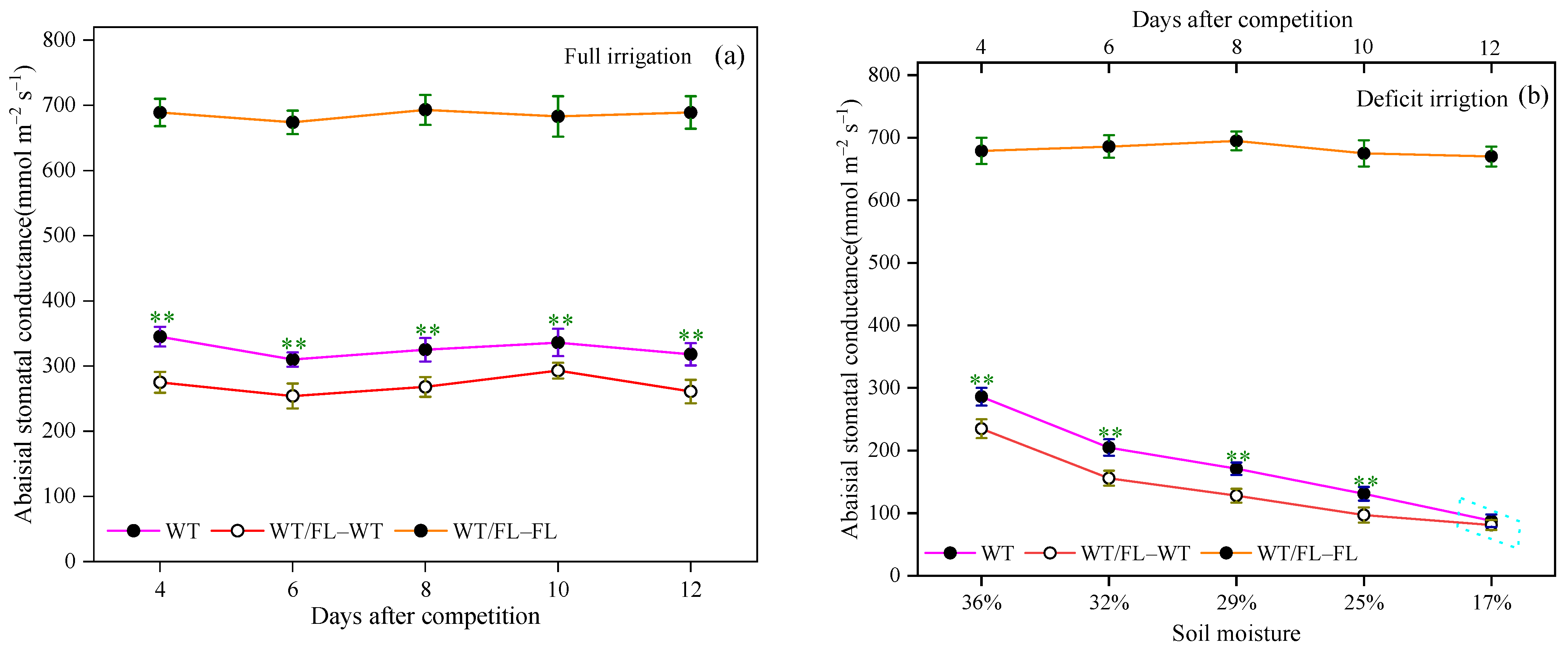
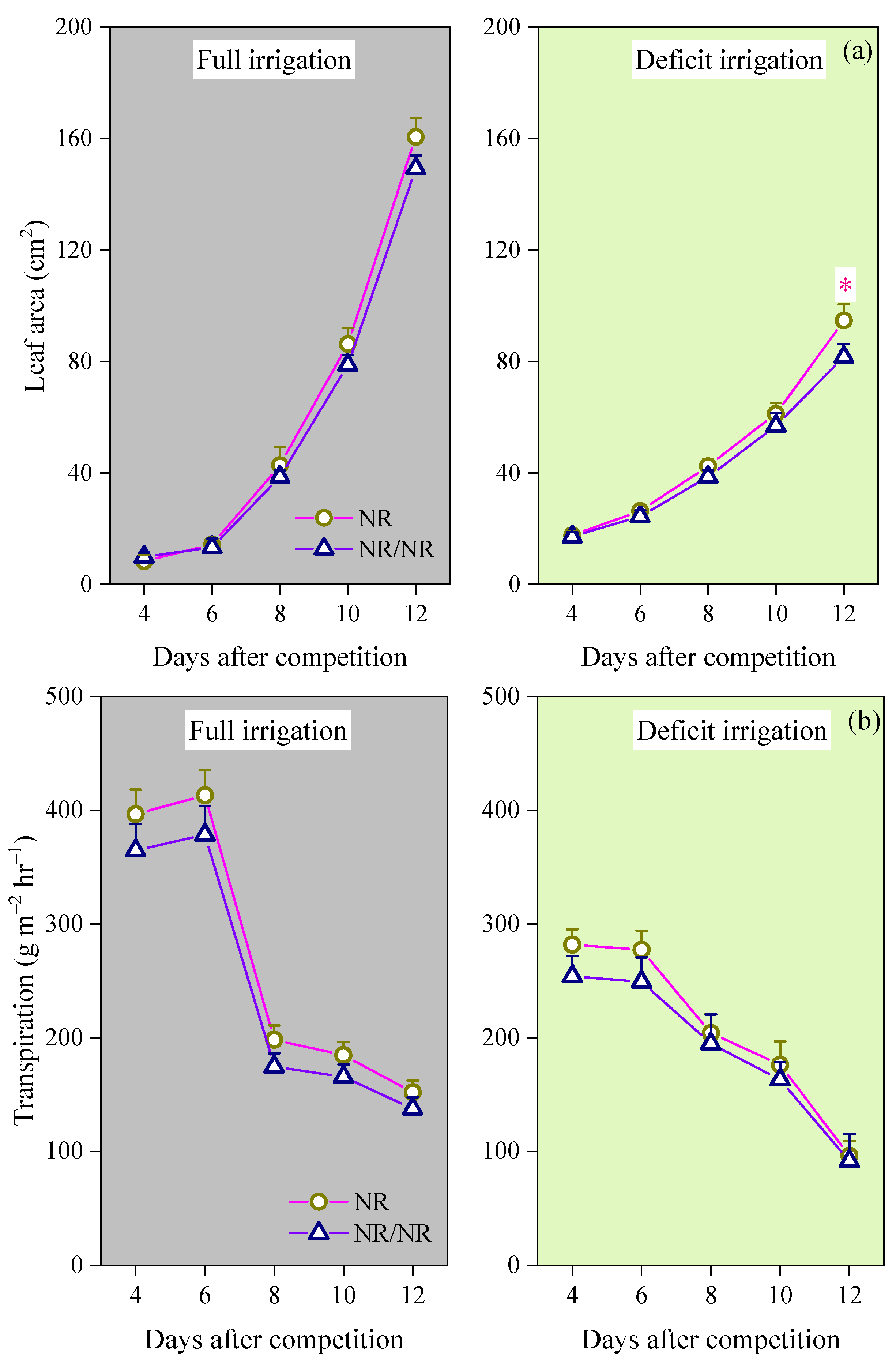
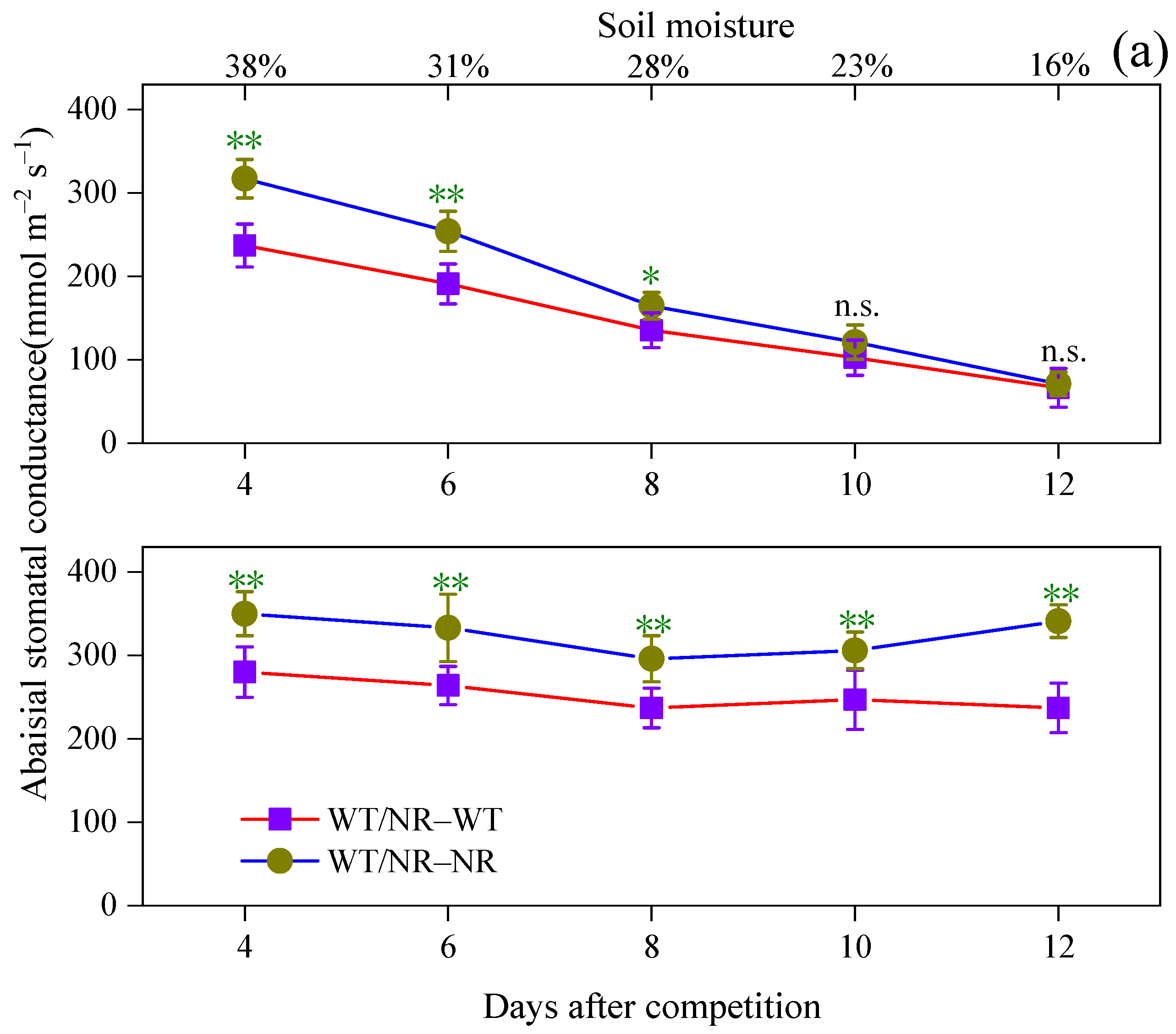
3.1. Effects of Intraspecific Competition on Plant Growth and Stomatal Opening of WT Tomato
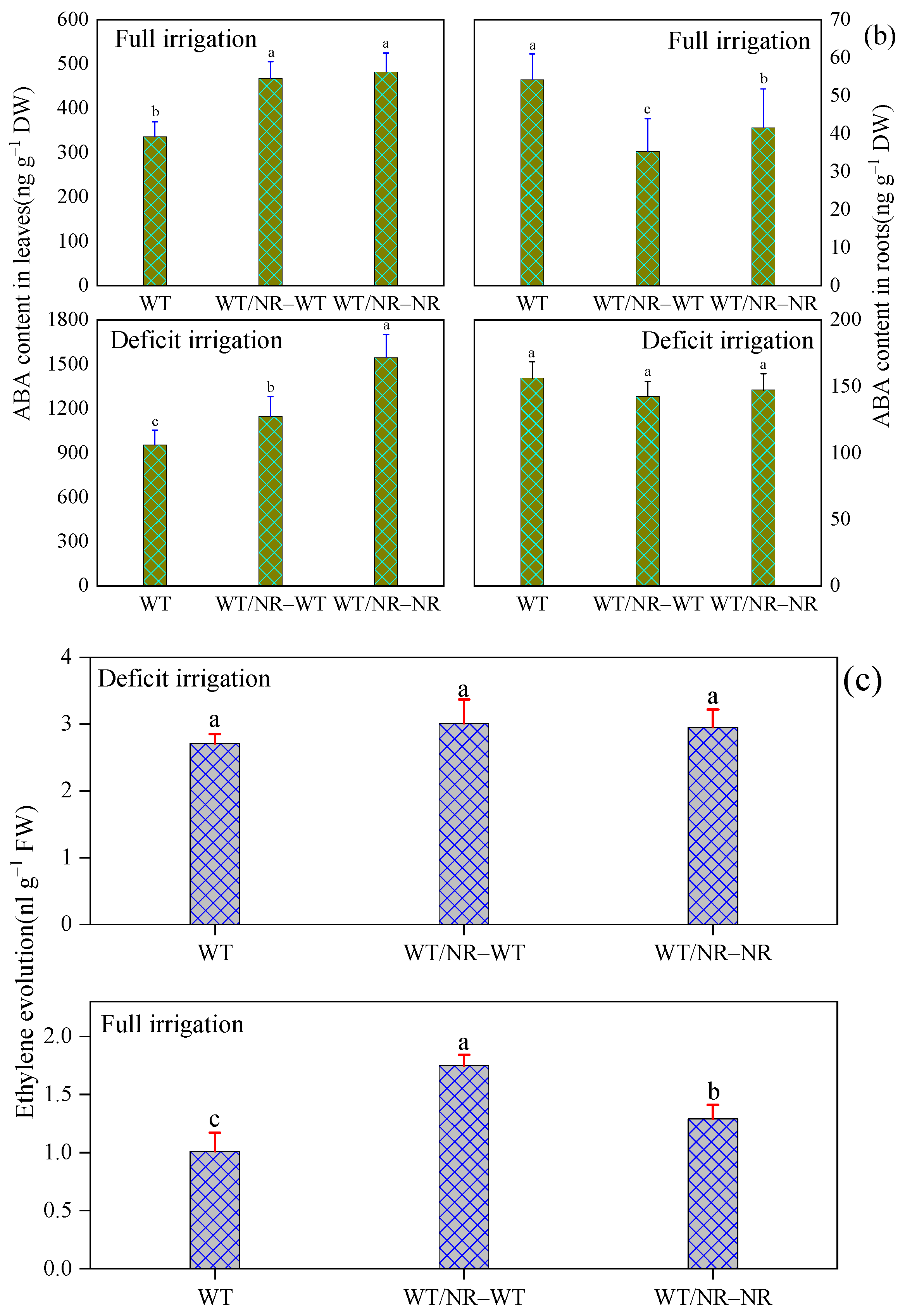
3.2. Involvement of ABA and Ethylene in Plant Response to Intraspecific Competition
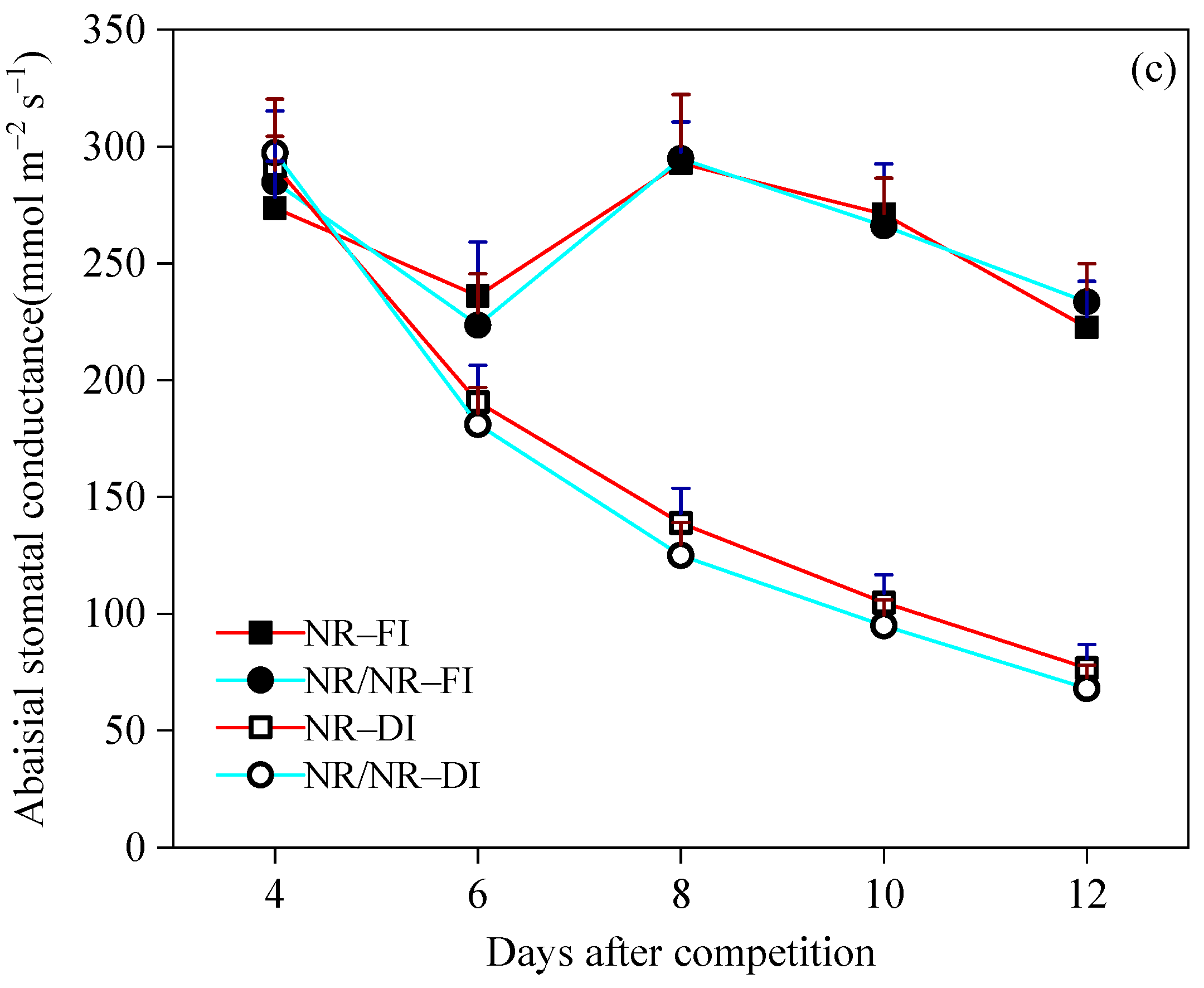
3.3. Effects of Above- and BelowGround Competition on Plant Response to Competition
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rejeb, I.B.; Pastor, V.; Mauch-Mani, B. Plant Responses to Simultaneous Biotic and Abiotic Stress: Molecular Mechanisms. Plants 2014, 3, 458–475. [Google Scholar] [CrossRef]
- Qin, F.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Achievements and Challenges in Understanding Plant Abiotic Stress Responses and Tolerance. Plant Cell Physiol. 2011, 52, 1569–1582. [Google Scholar] [CrossRef] [Green Version]
- Arkhipova, T.N.; Vysotskaya, L.B.; Martinenko, E.V.; Ivanov, I.I.; Kudoyarova, G.R. Participation of cytokinins in plant response to competitors. Russ. J. Plant Physiol. 2015, 62, 524–533. [Google Scholar] [CrossRef]
- Kazan, K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015, 20, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Peleg, Z.; Blumwald, E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vysotskaya, L.; Wilkinson, S.; Davies, W.J.; Arkhipova, T.; Kudoyarova, G. The effect of competition from neighbors on stomatal conductance in lettuce and tomato plants. Plant Cell Environ. 2011, 34, 729–737. [Google Scholar] [CrossRef]
- Zhu, J.-K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Kuromori, T.; Seo, M.; Shinozaki, K. ABA Transport and Plant Water Stress Responses. Trends Plant Sci. 2018, 23, 513–522. [Google Scholar] [CrossRef]
- Pantin, F.; Monnet, F.; Jannaud, D.; Costa, J.M.; Renaud, J.; Muller, B.; Simonneau, T.; Genty, B. The dual effect of abscisic acid on stomata. New Phytol. 2012, 197, 65–72. [Google Scholar] [CrossRef]
- Iqbal, N.; Nazar, R.; Syeed, S.; Masood, A.; Khan, N.A. Exogenously-sourced ethylene increases stomatal conductance, photosynthesis, and growth under optimal and deficient nitrogen fertilization in mustard. J. Exp. Bot. 2011, 62, 4955–4963. [Google Scholar] [CrossRef] [Green Version]
- Pierik, R.; Tholen, D.; Poorter, H.; Visser, E.J.; Voesenek, L.A. The Janus face of ethylene: Growth inhibition and stimulation. Trends Plant Sci. 2006, 11, 176–183. [Google Scholar] [CrossRef]
- Fatma, M.; Iqbal, N.; Gautam, H.; Sehar, Z.; Sofo, A.; D’Ippolito, I.; Khan, N.A. Ethylene and sulfur coordinately modulate the antioxidante system and ABA accumulation in mustard plants under salt stress. Plants 2021, 10, 180. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene Role in Plant Growth, Development and Senescence: Interaction with Other Phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef] [Green Version]
- Thao, N.P.; Khan, M.I.R.; Thu, N.B.A.; Hoang, X.L.T.; Asgher, M.; Khan, N.A.; Tran, L.-S.P. Role of Ethylene and Its Cross Talk with Other Signaling Molecules in Plant Responses to Heavy Metal Stress. Plant Physiol. 2015, 169, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierik, R.; Whitelam, G.C.; Voesenek, L.A.C.J.; de Kroon, H.; Visser, E.J.W. Canopy studies on ethylene-insensitive tobacco identify ethylene as a novel element in blue light and plant-plant signalling. Plant J. 2004, 38, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Semchenko, M.; Hutchings, M.J.; John, E. Challenging the tragedy of the commons in root competition: Confounding effects of neighbour presence and substrate volume. J. Ecol. 2007, 95, 252–260. [Google Scholar] [CrossRef]
- Vysotskaya, L.B.; Arkhipova, T.N.; Kudoyarova, G.R.; Veselov, S.Y. Dependence of growth inhibiting action of increased planting density on capacity of lettuce plants to synthesize ABA. J. Plant Physiol. 2018, 220, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Masclaux, F.G.; Bruessow, F.; Schweizer, F.; Gouhier-Darimont, C.; Keller, L.; Reymond, P. Transcriptome analysis of intra-specific competition in Arabidopsis thaliana reveals organ-specific signatures related to nutrient acquisition and general stress response pathways. BMC Plant Biol. 2012, 12, 227. [Google Scholar] [CrossRef] [Green Version]
- Kegge, W.; Pierik, R. Biogenic volatile organic compounds and plant competition. Trends Plant Sci. 2010, 15, 126–132. [Google Scholar] [CrossRef]
- Pierik, R.; Sasidharan, R.; Voesenek, L.A.C.J. Growth Control by Ethylene: Adjusting Phenotypes to the Environment. J. Plant Growth Regul. 2007, 26, 188–200. [Google Scholar] [CrossRef] [Green Version]
- Tholen, D.; Pons, T.L.; Voesenek, L.; Poorter, H. Ethylene Insensitivity Results in Down-Regulation of Rubisco Expression and Photosynthetic Capacity in Tobacco. Plant Physiol. 2007, 144, 1305–1315. [Google Scholar] [CrossRef] [Green Version]
- Nazareno, A.L.; Hernandez, B. A mathematical model of the interaction of abscisic acid, ethylene and methyl jasmonate on stomatal closure in plants. PLoS ONE 2017, 12, e0171065. [Google Scholar] [CrossRef]
- Wilkinson, S.; Davies, W.J. Ozone suppresses soil drying-and abscisic acid (ABA)-induced stomatal closure via an ethylene-dependent mechanism. Plant Cell Environ. 2009, 32, 949–959. [Google Scholar] [CrossRef]
- Vysotskaya, L.B.; Veselov, S.Y.; Kudoyarova, G.R. Effect of Competition and Treatment with Inhibitor of Ethylene Perception on Growth and Hormone Content of Lettuce Plants. J. Plant Growth Regul. 2017, 36, 450–459. [Google Scholar] [CrossRef]
- di Iorio, A.; Montagnoli, A.; Terzaghi, M.; Scippa, G.S.; Chiatante, D. Effect of tree density on root distribution in Fagus sylvatica stands: A semi-automatic digitising device approach to trench wall method. Trees 2013, 27, 1503–1513. [Google Scholar] [CrossRef] [Green Version]
- Contador, M.L.; Comas, L.H.; Metcalf, S.G.; Stewart, W.L.; Gomez, I.P.; Negron, C.; Lampinen, B.D. Root growth dynamics linked to above-ground growth in walnut (Juglans regia). Ann. Bot. 2015, 116, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Buckley, T.N. Stomatal responses to humidity: Has the ‘black box’ finally been opened? Plant Cell Environ. 2016, 39, 482–484. [Google Scholar] [CrossRef] [PubMed]
- Wolz, K.J.; Wertin, T.M.; Abordo, M.; Wang, D.; Leakey, A.D.B. Diversity in stomatal function is integral to modelling plant carbon and water fluxes. Nat. Ecol. Evol. 2017, 1, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Albert, R.; Acharya, B.R.; Jeon, B.W.; Zañudo, J.G.T.; Zhu, M.; Osman, K.; Assmann, S.M. A new discrete dynamic model of ABA-induced stomatal closure predicts key feedback loops. PLoS Biol. 2017, 15, e2003451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, W.J.; Kudoyarova, G.; Hartung, W. Long-distance ABA Signaling and Its Relation to Other Signaling Pathways in the Detection of Soil Drying and the Mediation of the Plant’s Response to Drought. J. Plant Growth Regul. 2005, 24, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Munemasa, S.; Hauser, F.; Park, J.; Waadt, R.; Brandt, B.; Schroeder, J.I. Mechanisms of abscisic acid-mediated control of stomatal aperture. Curr. Opin. Plant Biol. 2015, 28, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Sarwat, M.; Tuteja, N. Hormonal signaling to control stomatal movement during drought stress. Plant Gene 2017, 11, 143–153. [Google Scholar] [CrossRef]
- Quarrie, S.; Whitford, P.N.; Appleford, N.E.J.; Wang, T.L.; Cook, S.K.; Henson, I.E.; Loveys, B.R. A monoclonal antibody to (S)-abscisic acid: Its characterisation and use in a radioimmunoassay for measuring abscisic acid in crude extracts of cereal and lupin leaves. Planta 1988, 173, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Kunstler, G.; Falster, D.; Coomes, D.A.; Hui, F.; Kooyman, R.; Laughlin, D.C.; Poorter, L.; Vanderwel, M.; Vieilledent, G.; Wright, S.J.; et al. Plant functional traits have globally consistent effects on competition. Nature 2016, 529, 204–207. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, S.; Davies, W.J. Drought, ozone, ABA and ethylene: New insights from cell to plant to community. Plant Cell Environ. 2010, 33, 510–525. [Google Scholar] [CrossRef]
- Kurepin, L.V.; Emery, R.J.N.; Pharis, R.P.; Reid, D.M. Uncoupling light quality from light irradiance effects in Helianthus annuus shoots: Putative roles for plant hormones in leaf and internode growth. J. Exp. Bot. 2007, 58, 2145–2157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schachtman, D.P.; Goodger, J. Chemical root to shoot signaling under drought. Trends Plant Sci. 2008, 13, 281–287. [Google Scholar] [CrossRef]
- Boyle, R.K.A.; McAinsh, M.; Dodd, I.C. Stomatal closure of Pelargonium × hortorum in response to soil water deficit is as-sociated with decreased leaf water potential only under rapid soil drying. Physiol. Plant. 2016, 156, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Salazar, C.; Hernández, C.; Pino, M.T. Plant water stress: Associations between ethylene and abscisic arid response. Chil. J. Agric. Res. 2015, 75 (Suppl. 1), 71–79. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, S.; Kudoyarova, G.R.; Veselov, D.S.; Arkhipova, T.N.; Davies, W.J. Plant hormone interactions: Innovative targets for crop breeding and management. J. Exp. Bot. 2012, 63, 3499–3509. [Google Scholar] [CrossRef]
- Goodger, J.Q.D.; Sharp, R.E.; Marsh, E.L.; Schachtman, D.P. Relationships between xylem sap constituents and leaf conductance of well-watered and water-stressed maize across three xylem sap sampling techniques. J. Exp. Bot. 2005, 56, 2389–2400. [Google Scholar] [CrossRef] [Green Version]
- Ballaré, C.L. Phytochrome Responses: Think Globally, Act Locally. Trends Plant Sci. 2017, 22, 909–911. [Google Scholar] [CrossRef]
- de Kroon, H. How do roots interact? Science 2007, 318, 1562. [Google Scholar] [CrossRef] [PubMed]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Sano, T.; Tamaoki, M.; Nakajima, N.; Kondo, N.; Hasezawa, S. Ethylene Inhibits Abscisic Acid-Induced Stomatal Closure in Arabidopsis. Plant Physiol. 2005, 138, 2337–2343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- She, X.; Song, X. Ethylene inhibits abscisic acid-induced stomatal closure in Vicia faba via reducing nitric oxide levels in guard cells. N. Z. J. Bot. 2012, 50, 203–216. [Google Scholar] [CrossRef] [Green Version]


| Irrigation Factor | Competition Factor | ||
|---|---|---|---|
| Full Irrigation | Single Plant | a 1.86 L | WT, NR, FL |
| a 0.94 L | WT, NR, FL | ||
| Competing Plants | With root and canopy competition | WT/WT, WT/NR, WT/FL | |
| Without root competition | WT/WT, WT/NR, WT/FL | ||
| Without canopy competition | WT/WT, WT/NR, WT/FL | ||
| Deficit Irrigation | Single Plant | a 1.86 L | WT, NR, FL |
| a 0.94 L | WT, NR, FL | ||
| Competing Plants | With root and canopy competition | WT/WT, WT/NR, WT/FL | |
| Without root competition | WT/WT, WT/NR, WT/FL | ||
| Without canopy competition | WT/WT, WT/NR, WT/FL | ||
| Treatment | Deficit Irrigation | Full Irrigation | ||
|---|---|---|---|---|
| Leaf | Root | Leaf | Root | |
| WT | 961.5 ± 52 b | 115.4 ± 4.5 c | 332.6 ± 23 c | 65.4 ± 3.4 b |
| WT/WT-WT | 1080.4 ± 75 a | 155.3 ± 3.2 b | 505.2 ± 17 a | 43.5 ± 2.1 a |
| WT/FL-WT | 1136.6 ± 61 a | 174.7 ± 4.1 a | 458.6 ± 21 ab | 47.7 ± 3.1 a |
| Days after Deficit Irrigation | Leaf Area (cm2) | Transpiration (g m−2 hr−1) | Foliar ABA (ng g−1 DW) | Ethylene Evolution (nL g−1 FW) | ||||
|---|---|---|---|---|---|---|---|---|
| 1.86 L | 0.94 L | 1.86 L | 0.94 L | 1.86 L | 0.94 L | 1.86 L | 0.94 L | |
| 10 | 53.95 ± 4.6 a | 48.54 ± 3.6 a | 261.83 ± 21.3 a | 243.52 ± 23.5 a | 872.45 ± 67.5 a | 983.12 ± 56.3 a | 1.37 ± 0.04 b | 2.02 ± 0.05 a |
| 12 | 92.39 ± 6.3 a | 78.56 ± 8.1 b | 170.78 ± 15.2 a | 136.43 ± 10.5 b | 1474.32 ± 112.3 b | 2043.23 ± 151.2 a | 2.56 ± 0.62 b | 3.42 ± 0.81 a |
| Treatment | Full Irrigation | Deficit Irrigation | ||||||
|---|---|---|---|---|---|---|---|---|
| gs | Foliar ABA | Root ABA | Ethylene | gs | Foliar ABA | Root ABA | Ethylene | |
| WT/WT | 238.62 ± 21.34 b | 601.34 ± 49.32 a | 49.34 ± 4.39 a | 1.23 ± 0.07 a | 144.17 ± 11.14 a | 1643.14 ± 137.92 a | 145.44 ± 14.33 b | 2.11 ± 0.15 b |
| WT/WT-NC | 298.83 ± 22.31 a | 561.83 ± 38.51 b | 48.43 ± 3.67 a | 0.91 ± 0.04 b | 143.36 ± 11.31 a | 1701.36 ± 132.47 a | 178.37 ± 13.07 a | 1.91 ± 0.14 b |
| WT/WT-NR | 247.31 ± 19.45 b | 611.23 ± 51.11 a | 51.23 ± 5.34 a | 1.27 ± 0.06 a | 157.31 ± 14.15 a | 1681.35 ± 138.13 a | 131.31 ± 15.43 b | 2.47 ± 0.16 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Liang, Y.; Fu, Y.; Si, Z.; Hamani, A.K.M. Interactive Effects of Intraspecific Competition and Drought on Stomatal Conductance and Hormone Concentrations in Different Tomato Genotypes. Horticulturae 2022, 8, 45. https://doi.org/10.3390/horticulturae8010045
Gao Y, Liang Y, Fu Y, Si Z, Hamani AKM. Interactive Effects of Intraspecific Competition and Drought on Stomatal Conductance and Hormone Concentrations in Different Tomato Genotypes. Horticulturae. 2022; 8(1):45. https://doi.org/10.3390/horticulturae8010045
Chicago/Turabian StyleGao, Yang, Yueping Liang, Yuanyuan Fu, Zhuanyun Si, and Abdoul Kader Mounkaila Hamani. 2022. "Interactive Effects of Intraspecific Competition and Drought on Stomatal Conductance and Hormone Concentrations in Different Tomato Genotypes" Horticulturae 8, no. 1: 45. https://doi.org/10.3390/horticulturae8010045
APA StyleGao, Y., Liang, Y., Fu, Y., Si, Z., & Hamani, A. K. M. (2022). Interactive Effects of Intraspecific Competition and Drought on Stomatal Conductance and Hormone Concentrations in Different Tomato Genotypes. Horticulturae, 8(1), 45. https://doi.org/10.3390/horticulturae8010045








