Low CO2 Levels Are Detrimental for In Vitro Plantlets through Disturbance of Photosynthetic Functionality and Accumulation of Reactive Oxygen Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Conditions
2.2. Arabidopsis Seedling Growth
2.3. Removal of CO2 from the Headspace
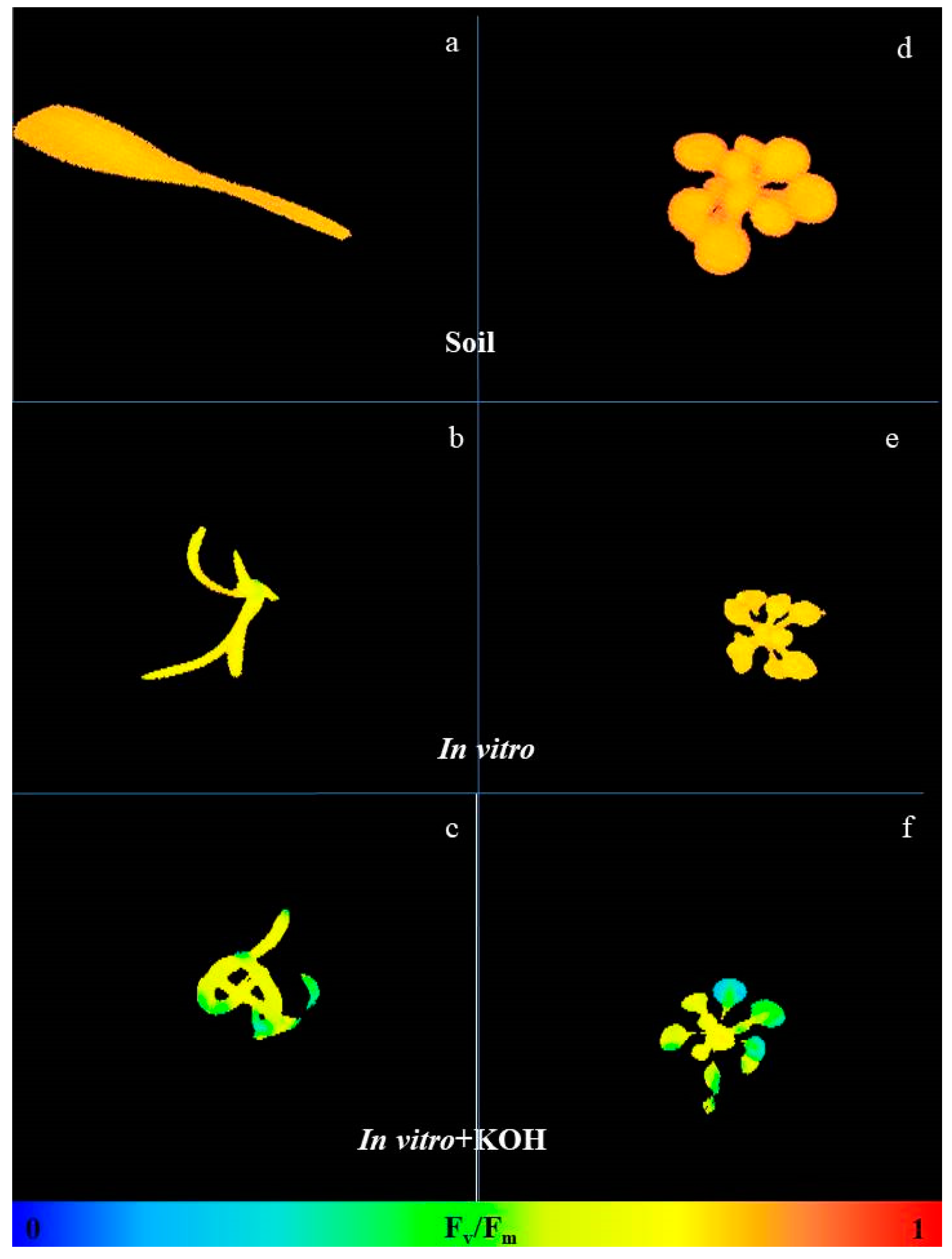
2.4. Mapping of Maximum Quantum Efficiency of Photosystem II Using Chlorophyll Fluorescence
2.5. Visualization of Superoxide Radicals
2.6. Statistical Analysis
3. Results
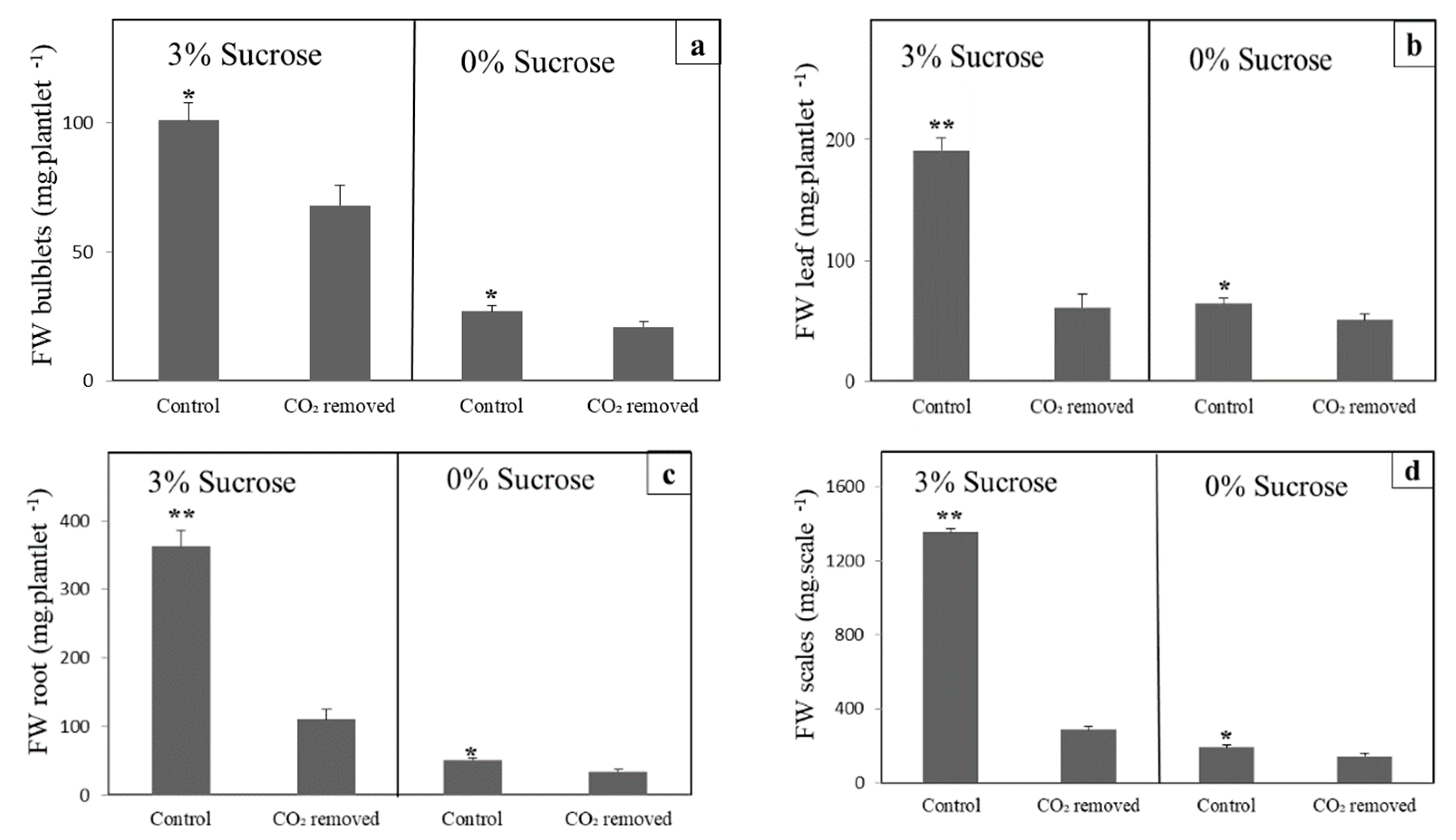
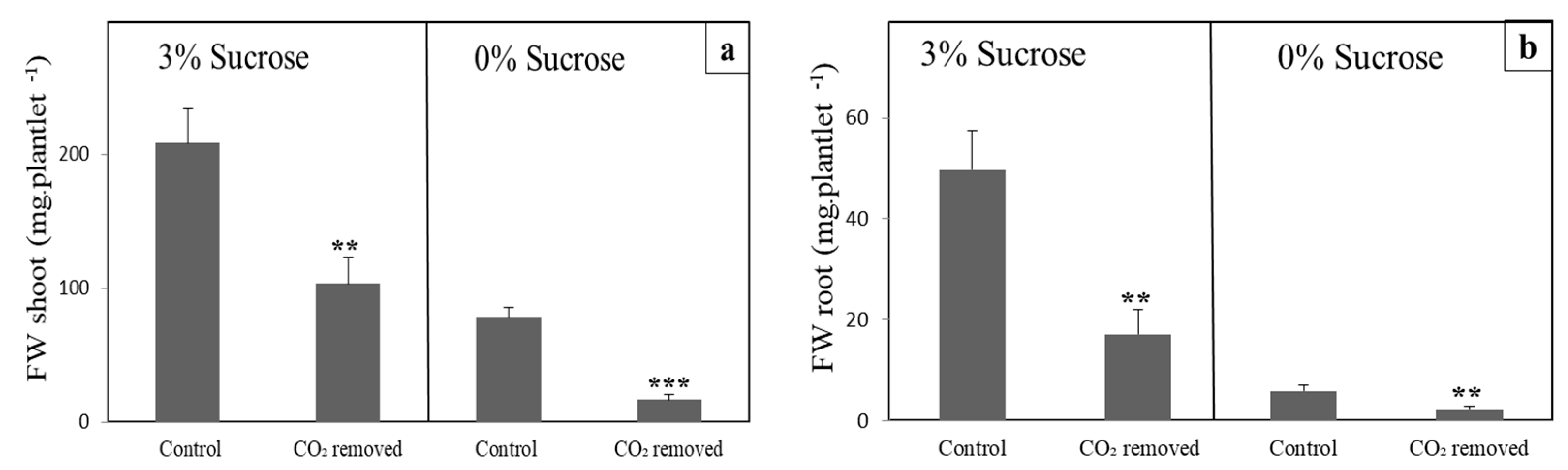
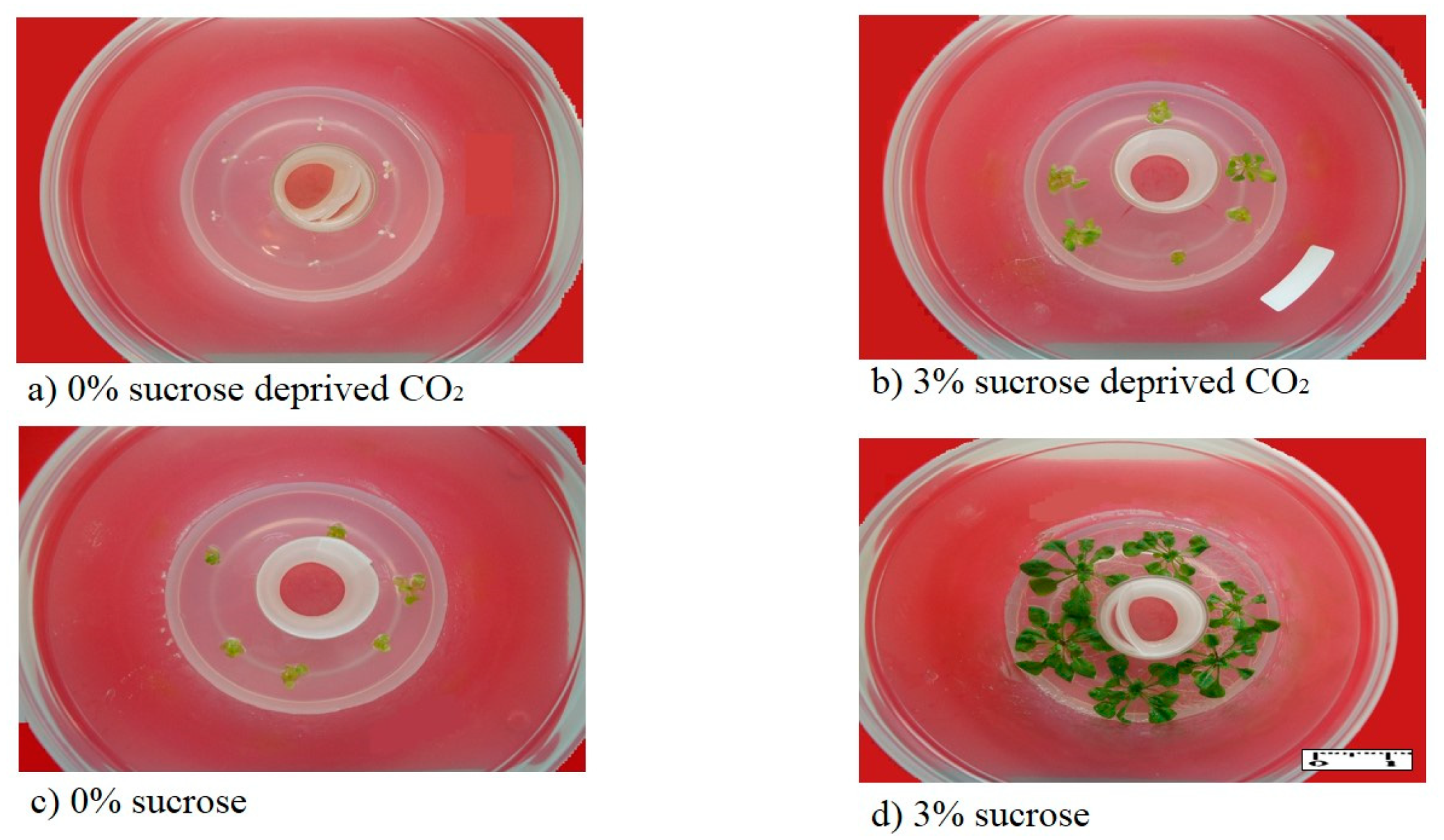
3.1. CO2 Removal Reduces Growth in Lily and Arabidopsis
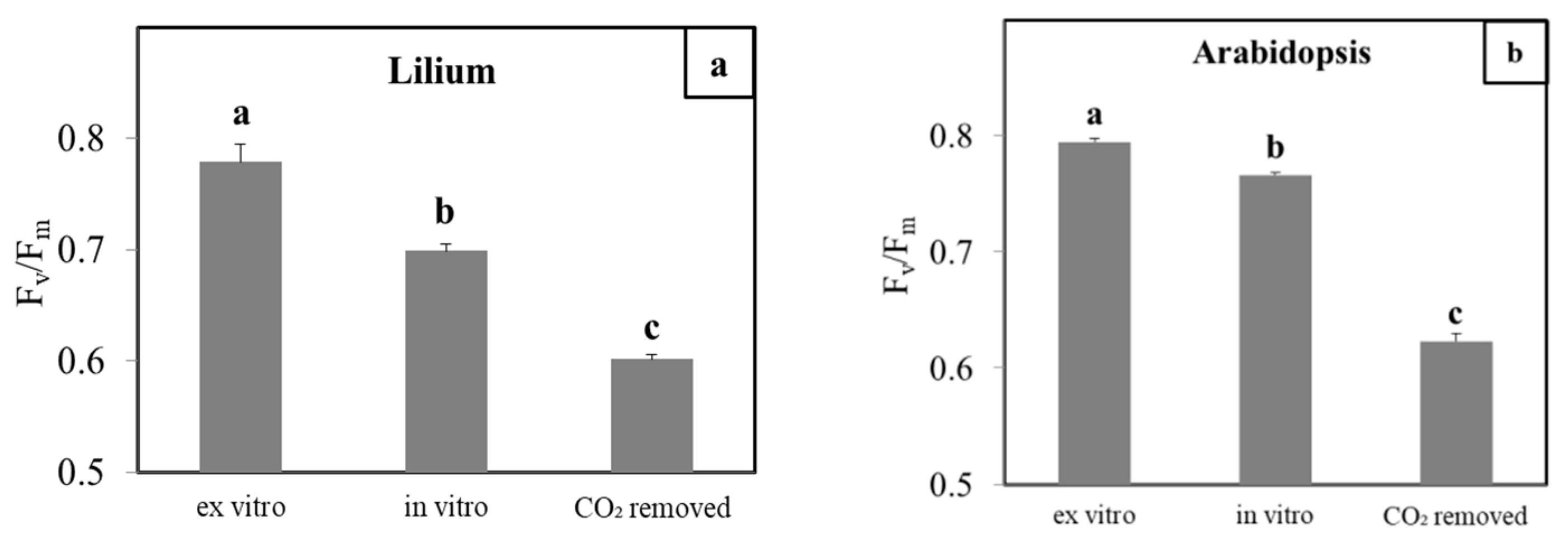
3.2. CO2-Low Conditions Disturbs the Physiology of the In Vitro Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neumann, K.-H.; Kumar, A.; Imani, J. Historical Developments of Cell and Tissue Culture Techniques. In Plant Cell and Tissue Culture—A Tool in Biotechnology; Springer: Cham, Germany, 2020; pp. 13–24. [Google Scholar]
- Maleki Asayesh, Z.; Vahdati, K.; Aliniaeifard, S. Investigation of physiological components involved in low water conservation capacity of in vitro Walnut plants. Sci. Hortic. 2017, 224, 1–7. [Google Scholar] [CrossRef]
- Vahdati, K.; Maleki Asayesh, Z.; Aliniaeifard, S.; Leslie, C. Improvement of ex vitro desiccation through elevation of CO2 concentration in the atmosphere of culture vessels during in vitro growth. HortScience 2017, 52, 1006–1012. [Google Scholar] [CrossRef]
- Chen, C. In situ measurement of microclimate for the plantlets cultured in vitro. Biosyst. Eng. 2006, 95, 413–423. [Google Scholar] [CrossRef]
- Chen, C. Humidity in plant tissue culture vessels. Biosyst. Eng. 2004, 88, 231–241. [Google Scholar] [CrossRef]
- Kozai, T. Photoautotrophic micropropagation. Vitr. Cell. Dev. Biol. Plant. 1991, 27, 47–51. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; Maleki Asayesh, Z.; Driver, J.; Vahdati, K. Stomatal features and desiccation responses of Persian walnut leaf as caused by in vitro stimuli aimed at stomatal closure. Trees Struct. Funct. 2020, 34, 1219–1232. [Google Scholar] [CrossRef]
- Chen, L.; Lu, Y.; Hu, Y.; Xue, X. RNA-Seq reveals that sucrose-free medium improves the growth of potato (Solanum tuberosum L.) plantlets cultured in vitro. Plant Cell. Tissue Organ Cult. 2020, 140, 505–521. [Google Scholar] [CrossRef]
- Fuentes, G.; Talavera, C.; Desjardins, Y.; Santamaría, J.M. Low exogenous sucrose improves ex vitro growth and photosynthesis in coconut in vitro plantlets if grown in vitro under high light. Acta Hortic. 2007, 748, 151–155. [Google Scholar] [CrossRef]
- Mosaleeyanon, K.; Cha-Um, S.; Kirdmanee, C. Enhanced growth and photosynthesis of rain tree (Samanea saman Merr.) plantlets in vitro under a CO 2-enriched condition with decreased sucrose concentrations in the medium. Sci. Hortic. 2004, 103, 51–63. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, Y.; Zhao, X.; Han, L.N.; Yang, S.J.; Li, W.F.; Mao, J.; Ma, Z.H.; Chen, B.H. Effects of CO2 on transplantation of grape plantlets cultured in vitro by promoting photosynthesis. Sci. Hortic. 2021, 287, 110286. [Google Scholar] [CrossRef]
- Park, J.E.; Park, Y.G.; Thi, L.T.; Soundararajan, P.; Jeong, B.R. Effect of sucrose concentration, photosynthetic photon flux density, and CO2 concentration on growth and development of micropropagated Mountain ash. Propag. Ornam. Plants 2018, 18, 58–63. [Google Scholar]
- Durchan, M.; Vácha, F.; Krieger-Liszkay, A. Effects of severe CO2 starvation on the photosynthetic electron transport chain in tobacco plants. Photosynth. Res. 2001, 68, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Habibi, N.; Purohit, S.D. Photosynthetic efficiency and in vitro growth of Celastrus paniculatus Willd. under varied concentrations of CO2. Int. J. Phytocosmet. Nat. Ingred. 2019, 6, 11. [Google Scholar] [CrossRef]
- Kozuleva, M.A.; Ivanov, B.N.; Vetoshkina, D.V.; Borisova-Mubarakshina, M.M. Minimizing an electron flow to molecular oxygen in photosynthetic electron transfer chain: An evolutionary view. Front. Plant Sci. 2020, 11, 211. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef]
- Askari, N.; de Klerk, G.J. Elimination of epidermal wax from explants increases growth in tissue culture of lily. Sci. Hortic. 2020, 274, 109637. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with Tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Solomon, M.E. Control of humidity with potassium hydroxide, sulphuric acid, or other solutions. Bull. Entomol. Res. 1951, 42, 543–554. [Google Scholar] [CrossRef]
- Maleki Asayesh, Z.; Vahdati, K.; Aliniaeifard, S.; Askari, N. Enhancement of ex vitro acclimation of Walnut plantlets through modification of stomatal characteristics in vitro. Sci. Hortic. 2017, 220, 114–121. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; Van Meeteren, U. Natural variation in stomatal response to closing stimuli among Arabidopsis thaliana accessions after exposure to low VPD as a tool to recognize the mechanism of disturbed stomatal functioning. J. Exp. Bot. 2014, 65, 6529–6542. [Google Scholar] [CrossRef]
- Van Den Dries, N.; Gianní, S.; Czerednik, A.; Krens, F.A.; de Klerk, G.J.M. Flooding of the apoplast is a key factor in the development of hyperhydricity. J. Exp. Bot. 2013, 64, 5221–5230. [Google Scholar] [CrossRef] [PubMed]
- Eisenhut, M.; Bräutigam, A.; Timm, S.; Florian, A.; Tohge, T.; Fernie, A.R.; Bauwe, H.; Weber, A.P.M. Photorespiration is crucial for dynamic response of photosynthetic metabolism and stomatal movement to altered CO2 availability. Mol. Plant 2017, 10, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Siemianowski, O.; Liu, M.; Lind, K.R.; Tian, X.; Nettleton, D.; Cademartiri, L. Stress response to CO2 deprivation by Arabidopsis thaliana in plant cultures. PLoS ONE 2019, 14, e0212462. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, Y.; Dubuc, J.F.; Badr, A. In vitro culture of plants: A stressful activity! Acta Hortic. 2009, 812, 29–50. [Google Scholar] [CrossRef]
- Martins, J.P.R.; de Almeida Rodrigues, L.C.; Santos, E.R.; Gontijo, A.B.P.L.; Falqueto, A.R. Impacts of photoautotrophic, photomixotrophic, and heterotrophic conditions on the anatomy and photosystem II of in vitro propagated Aechmea blanchetiana (Baker) L.B. Sm. (Bromeliaceae). Vitr. Cell. Dev. Biol. Plant 2020, 56, 350–361. [Google Scholar] [CrossRef]
- De Oliveira, T.; Balduino, M.C.M.; de Carvalho, A.A.; Bertolucci, S.K.V.; Cossa, M.C.; Coelho, A.D.; Leite, J.J.F.; Pinto, J.E.B.P. The effect of alternative membrane system, sucrose, and culture methods under photosynthetic photon flux on growth and volatile compounds of mint in vitro. Vitr. Cell. Dev. Biol. Plant 2021, 57, 529–540. [Google Scholar] [CrossRef]
- Sáez, P.L.; Bravo, L.A.; Sánchez-Olate, M.; Bravo, P.B.; Ríos, D.G. Effect of photon flux density and exogenous sucrose on the photosynthetic performance during in vitro culture of Castanea sativa. Am. J. Plant Sci. 2016, 7, 2087–2105. [Google Scholar] [CrossRef][Green Version]
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef]
- Dubuc, J.F.; Desjardins, Y. Stress gene expression modulation in in vitro tomato plantlets. Acta Hortic. 2009, 812, 507–514. [Google Scholar] [CrossRef]
- De Klerk, G.J.; Kim, K.; Schadewijk, M.; Gerrits, M. Growth of bulblets of Lilium speciosum in vitro and in soil. Acta Hortic. 1992, 513–520. [Google Scholar] [CrossRef]
- Wei, H.; Seidi, F.; Zhang, T.; Jin, Y.; Xiao, H. Ethylene scavengers for the preservation of fruits and vegetables: A review. Food Chem. 2021, 337, 127750. [Google Scholar] [CrossRef]
- Dong, J.G.; Fernandez-Maculet, J.C.; Yang, S.F. Purification and characterization of 1-aminocyclopropane-1-carboxylate oxidase from apple fruit. Proc. Natl. Acad. Sci. USA 1992, 89, 9789–9793. [Google Scholar] [CrossRef] [PubMed]
- Buddendorf-Joosten, J.M.C.; Woltering, E.J. Components of the gaseous environment and their effects on plant growth and development in vitro. Plant Growth Regul. 1994, 15, 1–16. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Askari, N.; Aliniaeifard, S.; Visser, R.G.F. Low CO2 Levels Are Detrimental for In Vitro Plantlets through Disturbance of Photosynthetic Functionality and Accumulation of Reactive Oxygen Species. Horticulturae 2022, 8, 44. https://doi.org/10.3390/horticulturae8010044
Askari N, Aliniaeifard S, Visser RGF. Low CO2 Levels Are Detrimental for In Vitro Plantlets through Disturbance of Photosynthetic Functionality and Accumulation of Reactive Oxygen Species. Horticulturae. 2022; 8(1):44. https://doi.org/10.3390/horticulturae8010044
Chicago/Turabian StyleAskari, Naser, Sasan Aliniaeifard, and Richard G. F. Visser. 2022. "Low CO2 Levels Are Detrimental for In Vitro Plantlets through Disturbance of Photosynthetic Functionality and Accumulation of Reactive Oxygen Species" Horticulturae 8, no. 1: 44. https://doi.org/10.3390/horticulturae8010044
APA StyleAskari, N., Aliniaeifard, S., & Visser, R. G. F. (2022). Low CO2 Levels Are Detrimental for In Vitro Plantlets through Disturbance of Photosynthetic Functionality and Accumulation of Reactive Oxygen Species. Horticulturae, 8(1), 44. https://doi.org/10.3390/horticulturae8010044







