Light Spectrum Variably Affects the Acclimatization of Grafted Watermelon Seedlings While Maintaining Fruit Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Stage I Production
2.2. Grafting, Stage II Production, and Light Conditions
2.3. Grafted Seedlings’ Acclimatization in Stage III
2.4. Determinations after Acclimatization
2.5. Field Cultivation
2.6. Determinations after Field Cultivation
2.7. Statistical Analysis
3. Results and Discussion
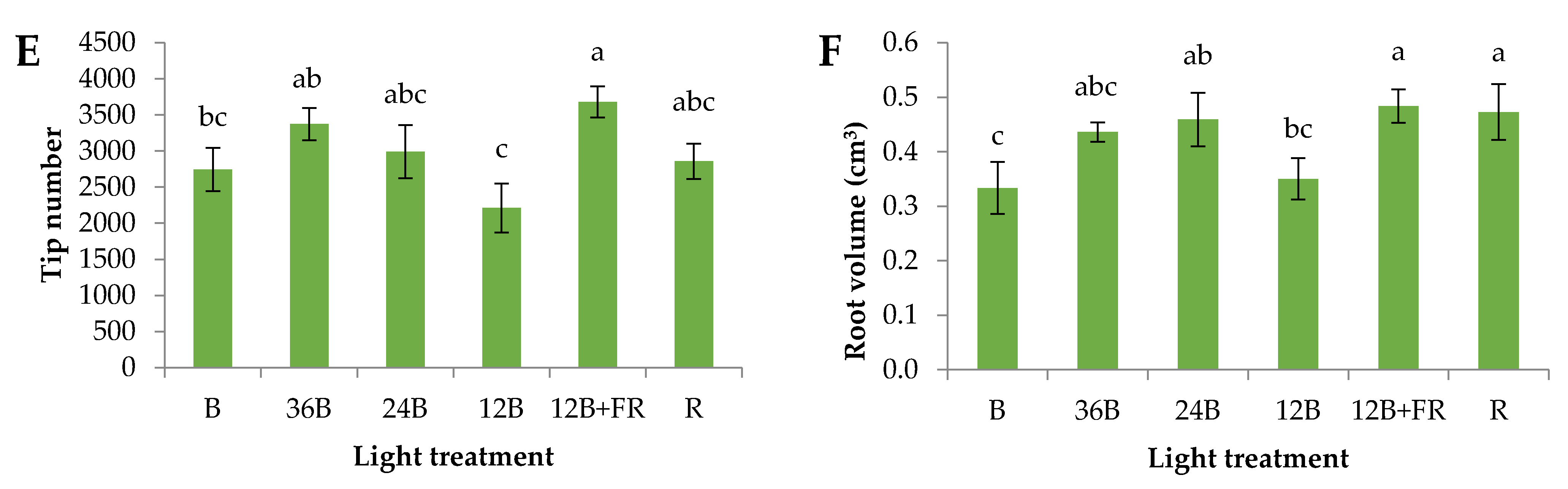
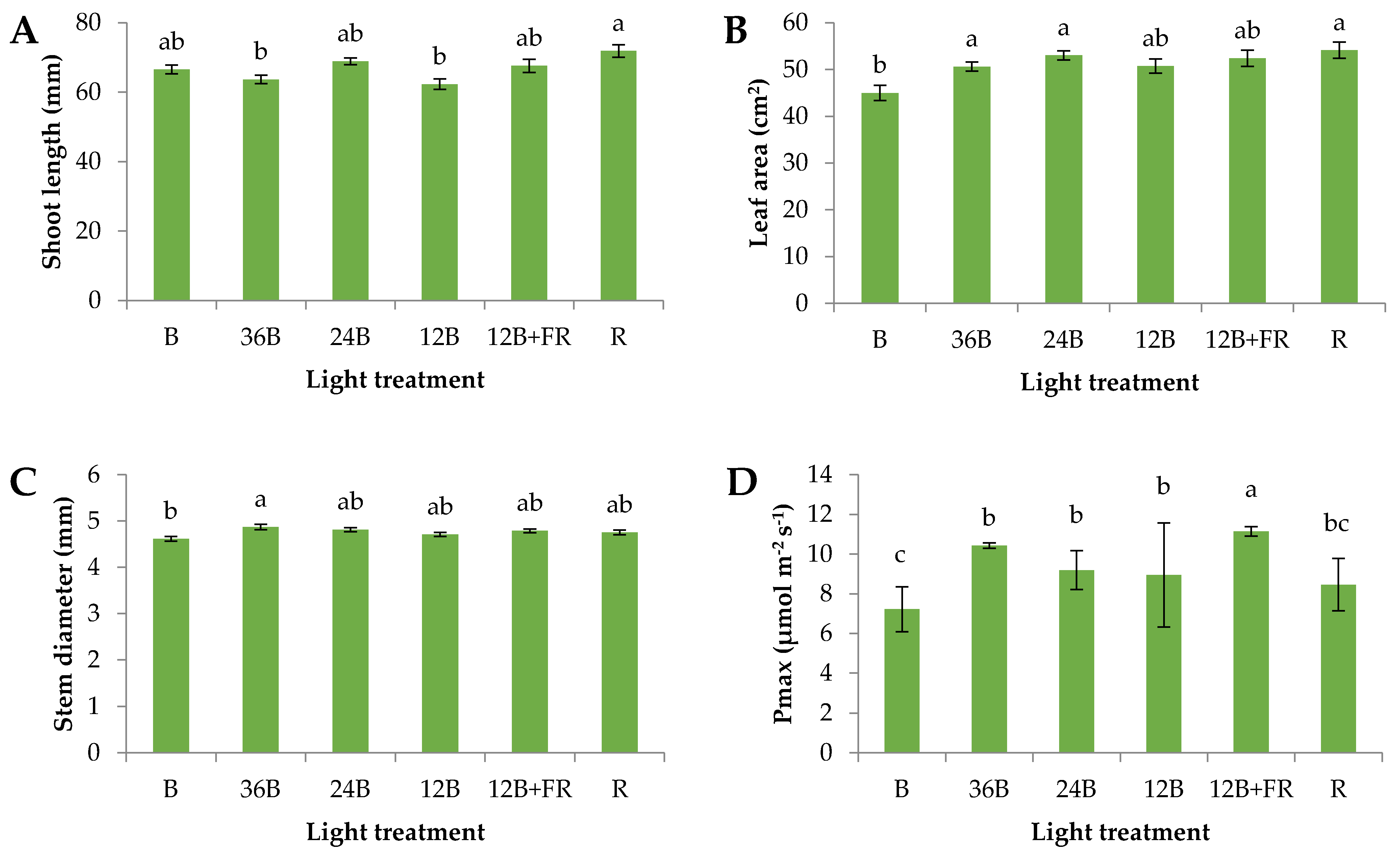
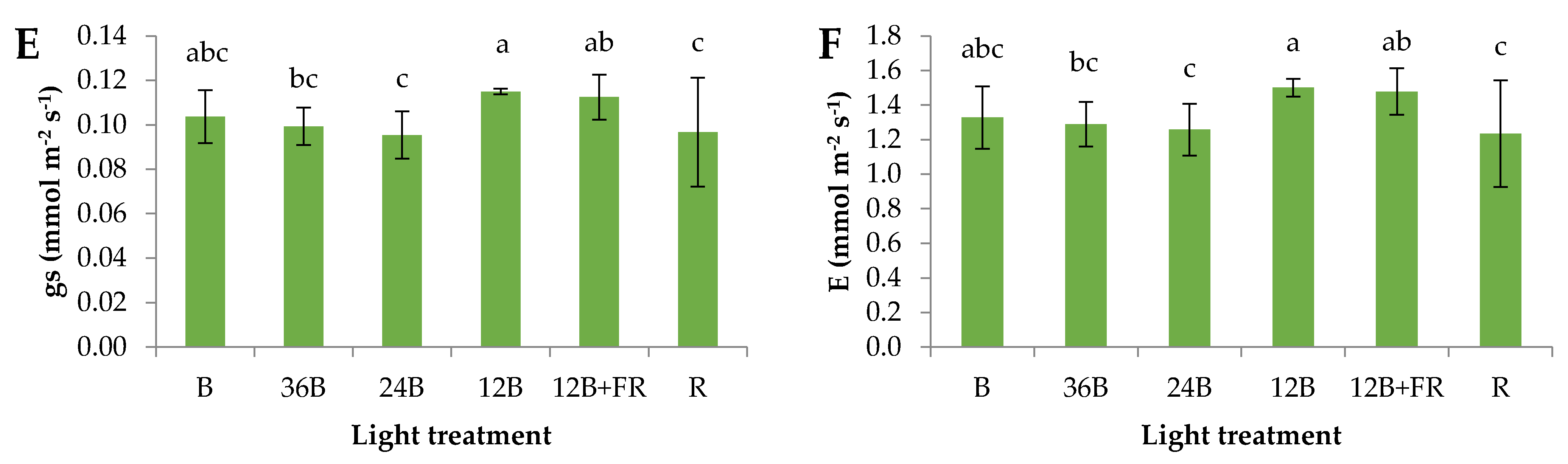
3.1. Grafted Seedlings’ Acclimatization
3.2. Field Cultivation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, J.-M.; Kubota, C.; Tsao, S.J.; Bie, Z.; Hoyos Echevarria, P.; Morra, L.; Odag, M. Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hortic. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. 2012 FAOSTAT Database. Available online: https://www.fao.org/faostat (accessed on 16 December 2021).
- Louws, F.J.; Rivard, C.L.; Kubota, C. Grafting fruiting vegetables to manage soilborne pathogens, foliar pathogens, arthropods and weeds. Sci. Hort. 2010, 127, 127–146. [Google Scholar] [CrossRef]
- Schwarz, D.; Rouphael, Y.; Colla, G.; Venema, J.H. Grafting as a tool to improve tolerance of vegetables to abiotic stresses: Thermal stress, water stress and organic pollutants. Sci. Hort. 2010, 127, 162–171. [Google Scholar] [CrossRef]
- Bourget, C.M. An introduction to light-emitting diodes. HortScience 2008, 43, 1944–1946. [Google Scholar] [CrossRef] [Green Version]
- Bantis, F.; Smirnakou, S.; Ouzounis, T.; Koukounaras, A.; Ntagkas, N.; Radoglou, K. Current status and recent achievements in the field of horticulture with the use of light-emitting diodes (LEDs). Sci. Hortic. 2018, 235, 437–451. [Google Scholar] [CrossRef]
- Lee, J.M.; Oda, M. Grafting of herbaceous vegetable and ornamental crops. In Horticultural Review; Janick, J., Ed.; John Wiley & Sons: New York, NY, USA, 2003; pp. 61–124. [Google Scholar]
- Bantis, F.; Panteris, E.; Dangitsis, C.; Carrera, E.; Koukounaras, A. Blue light promotes hormonal induced vascular reconnection, while red light boosts the physiological response and quality of grafted watermelon seedlings. Sci. Rep. 2021, 11, 21754. [Google Scholar] [CrossRef]
- Miceli, A.; Sabatino, L.; Moncada, A.; Vetrano, F.; D’ Anna, F. Nursery and field evaluation of eggplant grafted onto unrooted cuttings of Solanum torvum Sw. Sci. Hortic. 2014, 178, 203–210. [Google Scholar] [CrossRef]
- Sager, J.C.; Smith, W.O.; Edwards, J.L.; Cyr, K.L. Photosynthetic Efficiency and Phytochrome Photoequilibria Determination Using Spectral Data. Trans. ASAE 1988, 31, 1882–1889. [Google Scholar] [CrossRef]
- Pang, W.; Crow, W.T.; Luc, J.E.; McSorley, R.; Giblin-Davis, R.M. Comparison of water displacement and WinRHIZO software for plant root parameter assessment. APS Publ. 2011, 95, 1308–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saez-Plaza, P.; Navas, M.J.; Wybraniec, S.; Michalowski, T.; Asuero, A.G. An Overview of the Kjeldahl Method of Nitrogen Determination. Part II. Sample Preparation, Working Scale, Instrumental Finish, and Quality Control. Crit. Rev. Anal. Chem. 2013, 43, 224–272. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Metereol. 1972, 9, 191–216. [Google Scholar] [CrossRef]
- Salisbury, F.J.; Hall, A.; Grierson, C.S.; Halliday, K.J. Phytochrome coordinates Arabidopsis shoot and root development. Plant J. 2007, 50, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Rabara, R.C.; Behrman, G.; Timbol, T.; Rushton, P.J. Effect of spectral quality of monochromatic LED lights on the growth of artichoke seedlings. Front. Plant Sci. 2017, 8, 190. [Google Scholar] [CrossRef] [Green Version]
- Struve, D.K. Root Regeneration in Transplanted Deciduous Nursery Stock. HortScience 1990, 25, 266–270. [Google Scholar] [CrossRef]
- Bantis, F.; Koukounaras, A.; Siomos, A.; Menexes, G.; Dangitsis, C.; Kintzonidis, D. Assessing quantitative criteria for characterization of quality categories for grafted watermelon seedlings. Horticulturae 2019, 5, 16. [Google Scholar] [CrossRef] [Green Version]
- Hortensteiner, S. Chlorophyll degradation during senescence. Annu. Rev. Plant Biol. 2006, 57, 55–77. [Google Scholar] [CrossRef] [PubMed]
- Nanzin, M.T.; Lefsrud, M.; Gravel, V.; Azad, M.O.K. Blue light added with red LEDs enhance growth characteristics, pigments content, and antioxidant capacity in lettuce, spinach, kale, basil, and sweet pepper in a controlled environment. Plants 2019, 8, 93. [Google Scholar]
- Briggs, W.R.; Christie, J.M. Phototropins 1 and 2: Versatile plant blue-light receptors. Trends Plant Sci. 2002, 7, 204–210. [Google Scholar] [CrossRef]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origin. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.M.; Beaulieu, J.C.; Shewfelt, R. Color, flavor, texture, and nutritional quality of fresh-cut fruits and vegetables: Desirable levels, instrumental and sensory measurement, and the effects of processing. Crit. Rev. Food Sci. Nutr. 2010, 50, 369–389. [Google Scholar] [CrossRef] [PubMed]
- Madeira, A.C.; Ferreira, A.; De Varennes, A.; Vieira, M.I. SPAD meter versus tristimulus colorimeter to estimate chlorophyll content and leaf color in sweet pepper. Commun. Soil Sci. Plant Anal. 2003, 34, 2461–2470. [Google Scholar] [CrossRef]
- Casal, J.J. Photoreceptor signaling networks in plant responses to shade. Annu. Rev. Plant Biol. 2013, 64, 403–427. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Hernandez, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Li, Y.; Xin, G.; Wei, M.; Shi, Q.; Yang, F.; Wang, X. Carbohydrate accumulation and sucrose metabolism responses in tomato seedling leaves when subjected to different light qualities. Sci. Hortic. 2017, 225, 490–497. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Leperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Bantis, F.; Koukounaras, A.; Siomos, A.S.; Fotelli, M.N.; Kintzonidis, D. Bichromatic red and blue LEDs during healing enhance the vegetative growth and quality of grafted watermelon seedlings. Sci. Hortic. 2020, 261, 109000. [Google Scholar] [CrossRef]
- Whitelam, G.; Halliday, K. Light and Plant Development; Blackwell: Oxford, UK, 2007. [Google Scholar]
- Miao, Y.-X.; Wang, X.-Z.; Gao, L.-H.; Chen, Q.-Y.; Qu, M. Blue light is more essential than red light for maintaining the activities of photosystem II and I and photosynthetic electron transport capacity in cucumber leaves. J. Integrat. Agric. 2016, 15, 87–100. [Google Scholar] [CrossRef] [Green Version]
- Muneer, S.; Kim, E.J.; Park, J.S. Influence of green, red and blue light emitting diodes on multiprotein complex proteins and photosynthetic activity under different light intensities in lettuce leaves (Lactuca sativa L.). Int. J. Mol. Sci. 2014, 15, 4657–4670. [Google Scholar] [CrossRef] [Green Version]
- Mengel, K.; Kirkby, E.A.; Kosegarten, H.; Appel, T. Principles of Plant Nutrition; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001. [Google Scholar]
- White, P.J.; Brown, P.H. Plant nutrition for sustainable development and global health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopsell, D.A.; Sams, C.E.; Barickman, T.C.; Morrow, R.C. Sprouting broccoli accumulate higher concentrations of nutritionally important metabolites under narrow-band light emitting diode lighting. J. Amer. Soc. Hort. Sci. 2014, 139, 469–477. [Google Scholar] [CrossRef]
- Gerovac, J.R.; Craver, J.K.; Boldt, J.K.; Lopez, R.G. Light intensity and quality from sole-source light-emitting diodes impact growth, morphology, and nutrient content of Brassica microgreens. HortScience 2016, 51, 497–503. [Google Scholar] [CrossRef] [Green Version]
- Barber, N.J.; Barber, J. Lycopene and prostate cancer. Prostate Cancer Prostate Dis. 2002, 5, 6–12. [Google Scholar] [CrossRef]
- Kim, C.-H.; Park, M.-K.; Kim, S.-K.; Cho, Y.-H. Antioxidant capacity and anti-inflammatory activity of lycopene in watermelon. Int. J. Food Sci. Technol. 2014, 49, 2083–2091. [Google Scholar] [CrossRef]


| Waveband | Light Treatment | |||||
|---|---|---|---|---|---|---|
| B | 36B | 24B | 12B | 12B+FR | R | |
| UV %; 380–399 nm | 0 | 0 | 0 | 0 | 0 | 0 |
| Blue %; 400–499 nm | 100 | 36 | 24 | 12 | 12 | 0 |
| Green %; 500–599 nm | 0 | 0 | 0 | 0 | 0 | 0 |
| Red %; 600–699 nm | 0 | 64 | 76 | 88 | 83 | 100 |
| Far-red %; 700–780 nm | 0 | 0 | 0 | 0 | 5 | 0 |
| YPFD (μmol m−2 s−1) | 63.8 | 73.5 | 75.4 | 77.2 | 73.9 | 79.1 |
| PPS | 0.51 | 0.88 | 0.89 | 0.89 | 0.88 | 0.89 |
| Parameters | Light Treatments | |||||
|---|---|---|---|---|---|---|
| B | 36B | 24B | 12B | 12B+FR | R | |
| R/S ratio | 0.15 ± 0.01c | 0.17 ± 0.01b | 0.17 ± 0.01b | 0.16 ± 0.01bc | 0.21 ± 0.01a | 0.13 ± 0.01d |
| (DW/L) *1000 | 4.80 ± 0.14a | 5.01 ± 0.13a | 4.86 ± 0.10a | 4.93 ± 0.12a | 4.78 ± 0.12a | 4.55 ± 0.14b |
| Chl. content | 32.45 ± 1.22a | 31.09 ± 1.09a | 29.18 ± 1.31ab | 29.16 ± 1.18ab | 28.07 ± 1.24ab | 26.16 ± 1.01b |
| Fv/Fm | 0.822 ± 0.002a | 0.823 ± 0.002a | 0.823 ± 0.002a | 0.825 ± 0.002a | 0.823 ± 0.002a | 0.822 ± 0.003a |
| Lightness | 40.33 ± 0.34a | 40.40 ± 0.43a | 39.72 ± 0.28a | 39.43 ± 0.31a | 40.53 ± 0.34a | 40.31 ± 0.27a |
| Chroma | 21.94 ± 0.39a | 23.75 ± 0.59a | 23.25 ± 0.43a | 23.41 ± 0.44a | 23.88 ± 0.57a | 23.37 ± 0.36a |
| Hue angle | 129.58 ± 0.23a | 129.18 ± 0.32a | 129.26 ± 0.21a | 129.31 ± 0.22a | 129.18 ± 0.30a | 128.85 ± 0.19a |
| Parameters | Light Treatments | |||||
|---|---|---|---|---|---|---|
| B | 36B | 24B | 12B | 12B+FR | R | |
| Total N % | 3.56 ± 0.24a | 3.12 ± 0.05b | 3.33 ± 0.03ab | 3.28 ± 0.10ab | 3.39 ± 0.01ab | 3.55 ± 0.12a |
| P % | 2.45 ± 0.04b | 2.84 ± 0.04a | 2.82 ± 0.03a | 2.83 ± 0.06a | 2.59 ± 0.06b | 2.48 ± 0.14b |
| K % | 5.29 ± 0.10bc | 5.80 ± 0.15a | 5.64 ± 0.13ab | 5.87 ± 0.04a | 5.32 ± 0.12bc | 4.96 ± 0.25c |
| Ca % | 2.72 ± 0.02ab | 2.84 ± 0.12a | 2.65 ± 0.09ab | 2.83 ± 0.10a | 2.77 ± 0.08ab | 2.50 ± 0.11b |
| Mg % | 0.95 ± 0.02b | 1.12 ± 0.08a | 1.01 ± 0.06ab | 1.01 ± 0.05ab | 1.03 ± 0.02ab | 1.00 ± 0.01ab |
| Na % | 0.05 ± 0.01ab | 0.06 ± 0.01a | 0.06 ± 0.01a | 0.06 ± 0.01ab | 0.05 ± 0.01ab | 0.05 ± 0.01b |
| B ppm | 51.86 ± 1.44a | 53.44 ± 1.45a | 53.59 ± 1.78a | 52.88 ± 0.92a | 51.77 ± 1.24a | 51.01 ± 1.11a |
| Mn ppm | 46.47 ± 1.17ab | 47.90 ± 2.62ab | 48.11 ± 2.10ab | 47.63 ± 2.18ab | 50.94 ± 2.57a | 44.50 ± 1.17b |
| Zn ppm | 74.74 ± 1.50c | 78.93 ± 2.16bc | 80.18 ± 0.63bc | 81.39 ± 1.00b | 89.99 ± 0.36a | 80.68 ± 3.47b |
| Fe ppm | 108.63 ± 1.41c | 115.37 ± 1.01b | 114.63 ± 1.44b | 115.47 ± 2.16b | 120.60 ± 1.81a | 109.43 ± 1.28c |
| Cu ppm | 15.45 ± 0.75c | 17.00 ± 0.51abc | 17.38 ± 0.23ab | 17.44 ± 0.44a | 15.73 ± 0.38bc | 15.28 ± 0.81c |
| Parameters | Light Treatments | |||||
|---|---|---|---|---|---|---|
| B | 36B | 24B | 12B | 12B+FR | R | |
| Length (cm) | 29.63 ± 2.51a | 32.00 ± 1.61a | 31.97 ± 1.73a | 30.97 ± 2.29a | 33.30 ± 0.85a | 31.83 ± 0.68a |
| Width (cm) | 21.33 ± 1.17a | 22.17 ± 0.34a | 21.77 ± 0.90a | 22.13 ± 0.66a | 23.37 ± 0.13a | 22.43 ± 0.23a |
| Rind thick. (cm) | 1.82 ± 0.12a | 1.58 ± 0.12a | 1.85 ± 0.21a | 1.78 ± 0.13a | 1.85 ± 0.07a | 1.65 ± 0.17a |
| TSS (°Brix) | 10.73 ± 1.07a | 10.17 ± 0.57a | 10.07 ± 0.20a | 10.03 ± 0.24a | 10.80 ± 0.44a | 9.93 ± 0.28a |
| TPC (mg/g) | 0.13 ± 0.01a | 0.14 ± 0.02a | 0.12 ± 0.01a | 0.14 ± 0.01a | 0.14 ± 0.01a | 0.15 ± 0.02a |
| TCC (μg/g) | 35.09 ± 1.44a | 36.67 ± 5.23a | 36.79 ± 1.83a | 42.63 ± 5.22a | 42.42 ± 2.52a | 45.89 ± 8.56a |
| LC (μg/g) | 33.81 ± 1.45a | 35.31 ± 5.15a | 35.60 ± 1.87a | 41.15 ± 4.99a | 40.92 ± 2.14a | 44.06 ± 7.98a |
| FRAP (μg/g) | 35.52 ± 3.20a | 38.82 ± 6.20a | 35.18 ± 2.59a | 37.04 ± 4.22a | 40.36 ± 0.93a | 35.63 ± 2.79a |
| Lightness | 40.84 ± 2.67a | 40.76 ± 2.49a | 41.55 ± 0.43a | 41.03 ± 3.10a | 39.10 ± 3.47a | 42.00 ± 1.12a |
| Chroma | 27.48 ± 0.18a | 30.25 ± 0.51a | 30.90 ± 1.32a | 31.46 ± 1.26a | 33.46 ± 1.64a | 31.02 ± 3.16a |
| Hue angle | 34.82 ± 1.51a | 33.00 ± 1.29a | 33.22 ± 0.12a | 32.73 ± 1.17a | 32.84 ± 0.42a | 33.51 ± 0.92a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bantis, F.; Dangitsis, C.; Siomos, A.S.; Koukounaras, A. Light Spectrum Variably Affects the Acclimatization of Grafted Watermelon Seedlings While Maintaining Fruit Quality. Horticulturae 2022, 8, 10. https://doi.org/10.3390/horticulturae8010010
Bantis F, Dangitsis C, Siomos AS, Koukounaras A. Light Spectrum Variably Affects the Acclimatization of Grafted Watermelon Seedlings While Maintaining Fruit Quality. Horticulturae. 2022; 8(1):10. https://doi.org/10.3390/horticulturae8010010
Chicago/Turabian StyleBantis, Filippos, Christodoulos Dangitsis, Anastasios S. Siomos, and Athanasios Koukounaras. 2022. "Light Spectrum Variably Affects the Acclimatization of Grafted Watermelon Seedlings While Maintaining Fruit Quality" Horticulturae 8, no. 1: 10. https://doi.org/10.3390/horticulturae8010010
APA StyleBantis, F., Dangitsis, C., Siomos, A. S., & Koukounaras, A. (2022). Light Spectrum Variably Affects the Acclimatization of Grafted Watermelon Seedlings While Maintaining Fruit Quality. Horticulturae, 8(1), 10. https://doi.org/10.3390/horticulturae8010010









