Inhibitory Effects of CaCl2 and Pectin Methylesterase on Fruit Softening of Raspberry during Cold Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Determination of Firmness and Weight Loss
2.3. Observation of Cell Wall Structure
2.4. Measurement of Cell Wall Enzymes
2.5. Pectin Determination
2.6. Observation of CSP by Atomic Force Microscopy (AFM)
2.7. Determination of Free Water
2.8. Data Analysis
3. Results
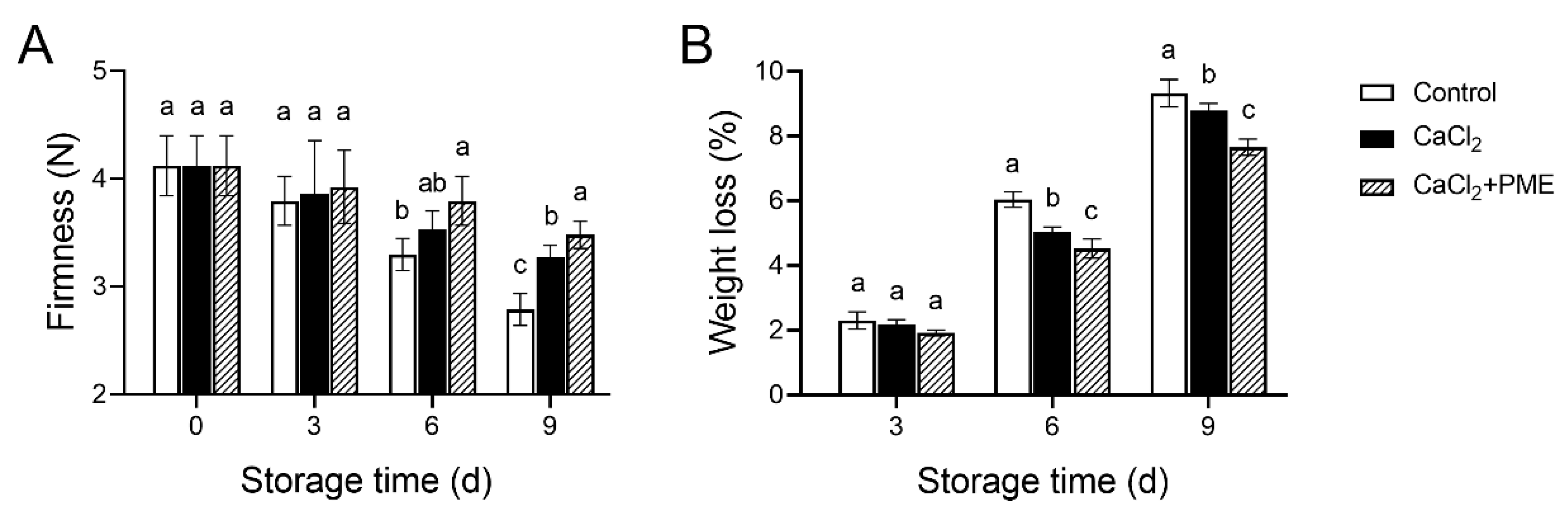
3.1. Effects of Different Treatments on Firmness and Weight Loss of Raspberry Fruit
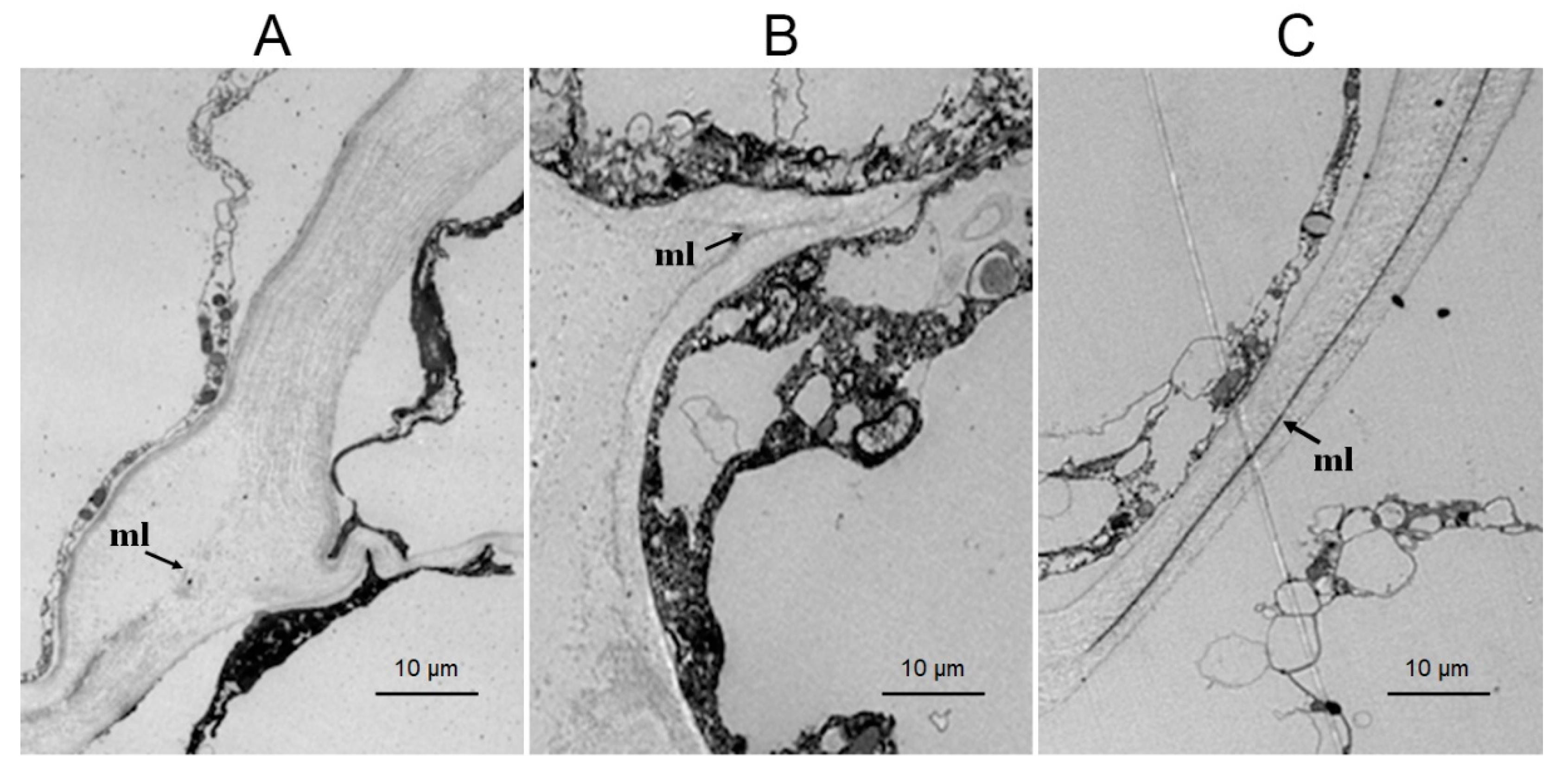
3.2. Effects of Different Treatments on the Cell Wall Structure of Raspberry Fruit
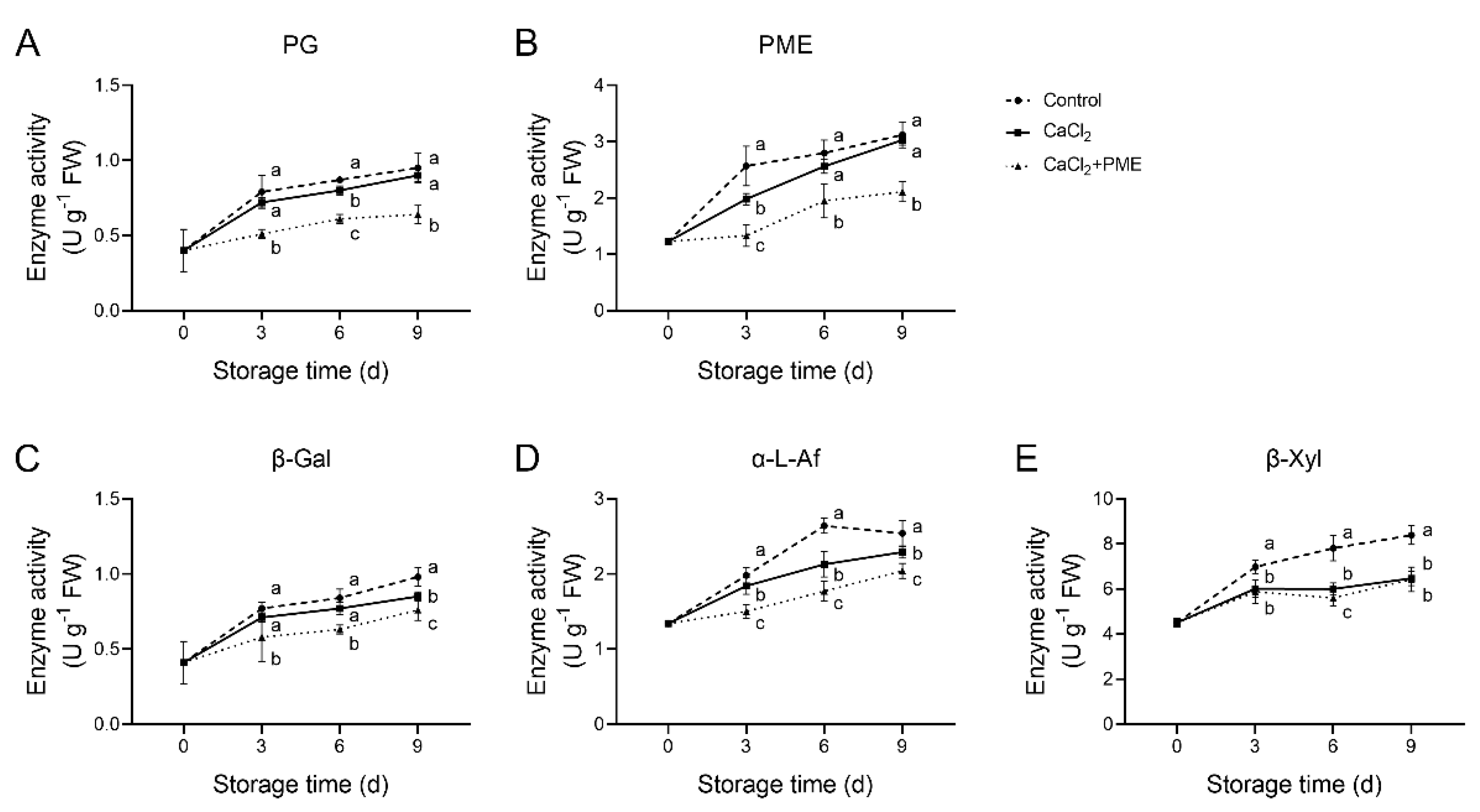
3.3. Effects of Different Treatments on Cell Wall-Degrading Enzyme Activity of Raspberry Fruit
3.4. Effects of Different Treatments on the CSP Content of Raspberry Fruit
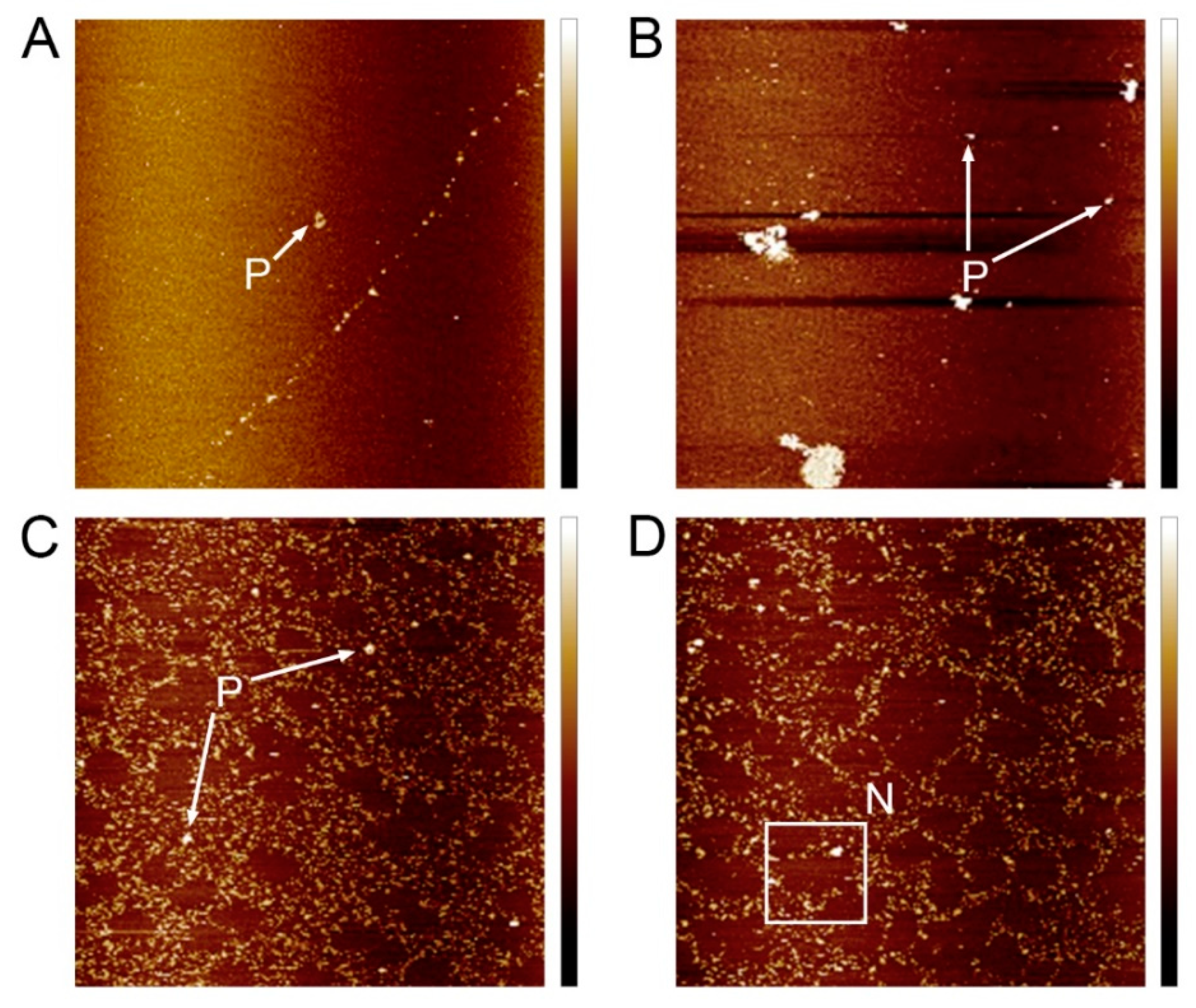
3.5. Effects of Different Treatments on the CSP Nanostructure of Raspberry Fruit
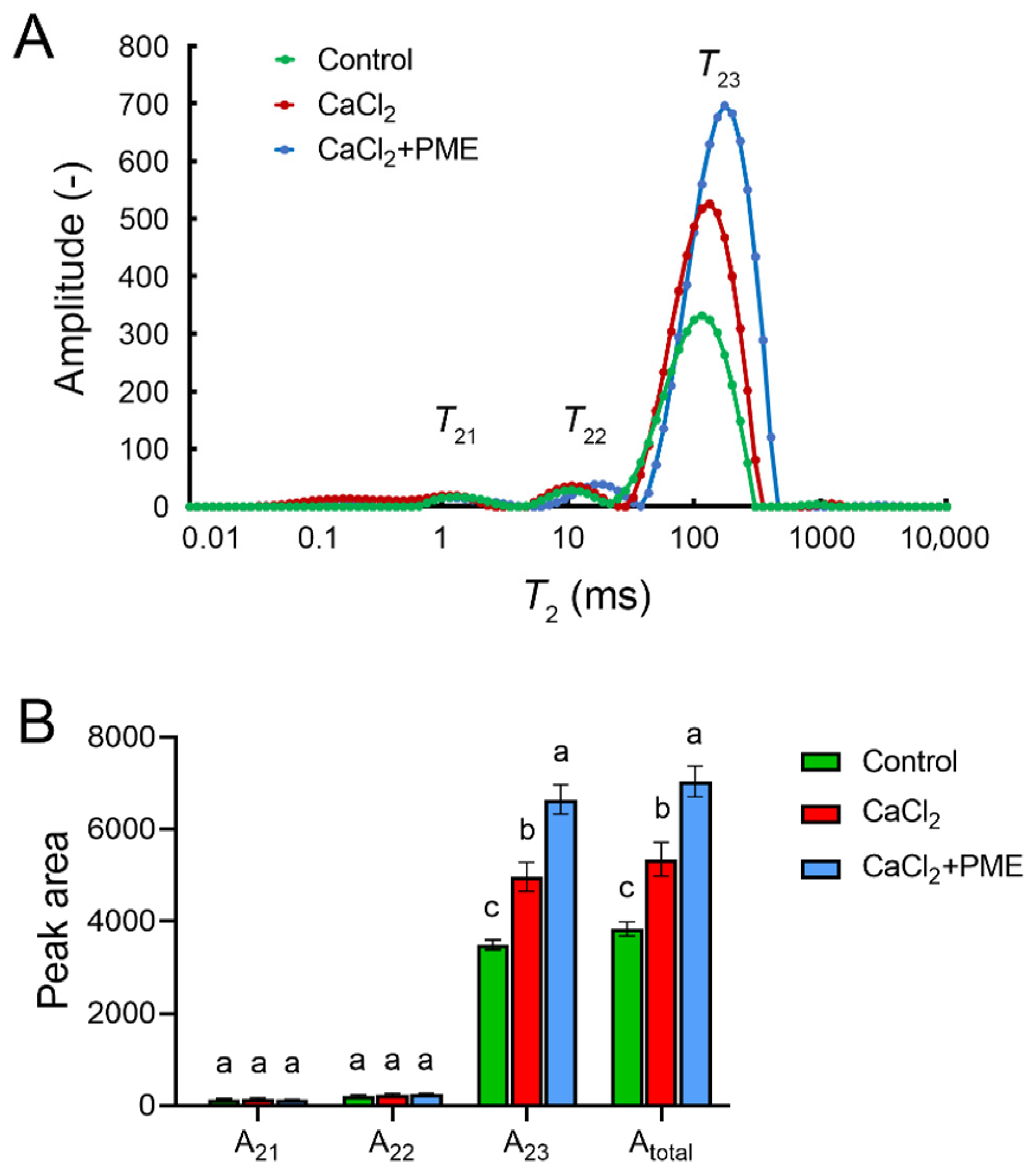
3.6. Effects of Different Treatments on the Free Water of Raspberry Fruit
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elias, M.I.; Madureira, J.; Santos, P.M.P.; Carolino, M.M.; Margaça, F.M.A.; Verde, S.C. Preservation treatment of fresh raspberries by e-beam irradiation. Innov. Food Sci. Emerg. 2020, 66, 102487. [Google Scholar] [CrossRef]
- Si, X.; Chen, Q.; Bi, J.; Yi, J.; Zhou, L.; Wu, X. Infrared radiation and microwave vacuum combined drying kinetics and quality of raspberry. J. Food Process. Eng. 2016, 39, 377–390. [Google Scholar] [CrossRef]
- Ancos, B.D.; González, E.M.; Cano, M.P. Ellagic acid, vitamin c, and total phenolic contents and radical scavenging capacity affected by freezing and frozen storage in raspberry fruit. J. Agric. Food Chem. 2000, 48, 4565–4570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forney, C.F.; Jamieson, A.R.; Pennell, K.D.M.; Jordan, M.A.; Fillmore, S.A.E. Relationships between fruit composition and storage life in air or controlled atmosphere of red raspberry. Postharvest Biol. Technol. 2015, 110, 121–130. [Google Scholar] [CrossRef]
- Haffner, K.; Rosenfeld, H.J.; Skredeb, G.; Wang, L.X. Quality of red raspberry rubus idaeus l. cultivars after storage in controlled and normal atmospheres. Postharvest Biol. Technol. 2002, 24, 279–289. [Google Scholar] [CrossRef]
- Zhang, N.; Li, K.L.; Wang, W.S.; Yan, R.X. Application of ozone concentration precise control fumigation device improving quality of raspberries during cold storage. Trans. Chin. Soc. Agric. Eng. 2017, 33, 295–301. [Google Scholar]
- Morales, M.L.; Callejón, R.M.; Ubeda, C.; Guerreiroc, A.; Gago, G.; Miguel, M.G.; Antunes, M.D. Effect of storage time at low temperature on the volatile compound composition of Sevillana and Maravilla raspberries. Postharvest Biol. Technol. 2014, 96, 128–134. [Google Scholar] [CrossRef]
- Du, Y.M.; Yan, R.; Yang, X.Y.; Sun, F.; Han, C.; Liu, G.M.; Liu, Y.M.; Fu, M.R. Research progress on storage and preservation technology of postharvest raspberry fruit. Food Ferment. Ind. 2019, 45, 298–303. [Google Scholar]
- Zhang, L.F.; Wang, P.; Chen, F.S.; Lai, S.J.; Yu, H.G.; Yang, H.S. Effects of calcium and pectin methylesterase on quality attributes and pectin morphology of jujube fruit under vacuum impregnation during storage. Food Chem. 2019, 289, 40–48. [Google Scholar] [CrossRef]
- Hernández-Muñoz, P.; Almenar, E.; Valle, V.D.; Velez, D.; Gavara, R. Effect of chitosan coating combined with postharvest calcium treatment on strawberry (Fragaria × ananassa) quality during refrigerated storage. Food Chem. 2008, 110, 428–435. [Google Scholar] [CrossRef]
- Zhang, L.F.; Zhao, S.N.; Lai, S.J.; Chen, F.S.; Yang, H.S. Combined effects of ultrasound and calcium on the chelate-soluble pectin and quality of strawberries during storage. Carbohyd. Polym. 2018, 200, 427–435. [Google Scholar] [CrossRef]
- Gao, Q.; Tan, Q.; Song, Z.; Chen, W.; Li, X.; Zhu, X. Calcium chloride postharvest treatment delays the ripening and softening of papaya fruit. J. Food Process. Pres. 2020, 44, 14604. [Google Scholar] [CrossRef]
- Naser, F.; Rabiei, V.; Razavi, F.; Khademi, O. Effect of calcium lactate in combination with hot water treatment on the nutritional quality of persimmon fruit during cold storage. Sci. Hortic-Amst. 2018, 233, 114–123. [Google Scholar] [CrossRef]
- Zhang, L.F.; Wang, P.; Sun, X.Y.; Chen, F.S.; Lai, S.J.; Yang, H.S. Calcium permeation property and firmness change of cherry tomatoes under ultrasound combined with calcium lactate treatment. Ultrason. Sonochem. 2019, 60, 104784. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, X.B.; Long, L.E. The effect of postharvest calcium application in hydro-cooling water on tissue calcium content, biochemical changes, and quality attributes of sweet cherry fruit. Food Chem. 2014, 160, 22–30. [Google Scholar] [CrossRef]
- Lv, J.Y.; Han, X.Z.; Bai, L.; Xu, D.L.; Ding, S.Y.; Ge, Y.H.; Li, C.Y.; Li, J.R. Effects of calcium chloride treatment on softening in red raspberry fruit during low-temperature storage. J. Food Biochem. 2020, 44, e13419. [Google Scholar] [CrossRef]
- Liu, H.; Chen, F.; Lai, S.; Tao, J.; Yang, H.; Jiao, Z. Effects of calcium treatment and low temperature storage on cell wall polysaccharide nanostructures and quality of postharvest apricot (Prunus armeniaca). Food Chem. 2017, 225, 87–97. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Gao, Z.F.; Zhang, X.X.; Bai, W.B.; Zhang, L.X.; Pei, H.B.; Zhang, Y.J. Control effects of Bacillus siamensis g-3 volatile compounds on raspberry postharvest diseases caused by Botrytis cinerea and Rhizopus stolonifer. Biol. Control 2020, 141, 104135. [Google Scholar] [CrossRef]
- Yang, H.; Wu, Q.; Ng, L.Y.; Wang, S. Effects of vacuum impregnation with calcium lactate and pectin methylesterase on quality attributes and chelate-soluble pectin morphology of fresh-cut papayas. Food Bioprocess Technol. 2017, 10, 901–913. [Google Scholar] [CrossRef]
- Silva, J.M.; Villar, H.P.; Pimentel, R. Structure of the cell wall of mango after application of ionizing radiation. Radiat. Phys. Chem. 2012, 81, 1770–1775. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, J.; Li, C.; Xue, W.; Lv, J. Trisodium phosphate delays softening of jujube fruit by inhibiting cell wall-degrading enzyme activities during ambient storage. Sci. Hortic-Amst. 2019, 262, 109059. [Google Scholar] [CrossRef]
- Tang, Q.; Li, C.; Ge, Y.; Li, X.; Cheng, Y.; Hou, J.B.; Li, J. Exogenous application of melatonin maintains storage quality of jujubes by enhancing anti-oxidative ability and suppressing the activity of cell wall-degrading enzymes. LWT Food Sci. Technol. 2020, 127, 109431. [Google Scholar] [CrossRef]
- Villarreal, N.M.; Bustamante, C.A.; Civello, P.M.; Martínez, G.A. Effect of ethylene and 1-MCP treatments on strawberry fruit ripening. J. Sci. Food Agric. 2010, 90, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Manganaris, G.A.; Vicente, A.R.; Crisosto, C.H.; Labavitch, J.M. Cell wall modifications in chilling-injured plum fruit (Prunus salicina). Postharvest Biol. Technol. 2008, 48, 77–83. [Google Scholar] [CrossRef]
- Fry, S.C. Ripening. In Encyclopedia of Applied Plant Sciences, 2nd ed.; Thomas, B., Murray, B.G., Murphy, D.J., Eds.; Academic Press: Amsterdam, The Netherlands, 2017; Volume 1, pp. 323–334. [Google Scholar]
- Michael Eskin, N.A.; Hoehn, E. Chapter 2–Fruits and Vegetables. In Biochemistry of Foods, 3rd ed.; Michael Eskin, N.A., Shahidi, F., Eds.; Academic Press: Amsterdam, The Netherlands, 2013; Volume 1, pp. 49–126. [Google Scholar]
- Husain, Q. β-Galactosidases and their potential applications: A review. Crit. Rev. Biotechnol. 2010, 30, 41–62. [Google Scholar] [CrossRef] [PubMed]
- Lagaert, S.; Pollet, A.; Courtin, C.M.; Volckaert, G. β-Xylosidases and α-l-arabinofuranosidases: Accessory enzymes for arabinoxylan degradation. Biotechnol. Adv. 2014, 32, 316–332. [Google Scholar] [CrossRef]
- Brummell, D.A. Cell wall disassembly in ripening fruit. Funct. Plant Biol. 2006, 33, 103–119. [Google Scholar] [CrossRef]
- Lara, I.; Garcia, P.; Vendrell, M. Modifications in cell wall composition after cold storage of calcium-treated strawberry (Fragaria × ananassa duch) fruit. Postharvest Biol. Technol. 2004, 34, 331–339. [Google Scholar] [CrossRef]
- Abu-Sarra, A.F.; Abu-Goukh, A.A. Changes in pectinesterase, polygalacturonase and cellulase activity during mango fruit ripening. J. Hortic. Sci. 1992, 67, 561–568. [Google Scholar] [CrossRef]
- Bennett, A.B.; Labavitch, J.M. Ethylene and ripening-regulated expression and function of fruit cell wall modifying proteins. Plant Sci. 2008, 175, 130–136. [Google Scholar] [CrossRef]
- Wang, D.D.; Yeats, T.H.; Uluisik, S.; Rose, J.K.C.; Seymour, G.B. Fruit softening: Revisiting the role of pectin. Trends Plant Sci. 2018, 23, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Zhang, L.; Chen, F.; Lai, S.; Yang, H. Effect of vacuum impregnation combined with calcium lactate on the firmness and polysaccharide morphology of Kyoho grapes (Vitis vinifera x V. labrusca). Food Bioprocess Technol. 2017, 10, 699–709. [Google Scholar] [CrossRef]
- Liu, H.; Chen, F.S.; Yang, H.S.; Yao, Y.Z.; Gong, X.Z.; Xin, Y.; Ding, C.H. Effect of calcium treatment on nanostructure of chelate-soluble pectin and physicochemical and textural properties of apricot fruits. Food Res. Int. 2009, 42, 1131–1140. [Google Scholar] [CrossRef]
- Paniagua, A.C.; East, A.R.; Hindmarsh, J.P.; Heyes, J.A. Moisture loss is the major cause of firmness change during postharvest storage of blueberry. Postharvest Biol. Technol. 2013, 79, 13–19. [Google Scholar] [CrossRef]
- Zhu, D.; Liang, J.; Liu, H.; Cao, X.; Ge, Y.; Li, J. Sweet cherry softening accompanied with moisture migration and loss during low-temperature storage. J. Sci. Food Agric. 2018, 98, 3651–3658. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, R.; Han, C.; Fu, M.; Jiao, W.; Wang, W. Inhibitory Effects of CaCl2 and Pectin Methylesterase on Fruit Softening of Raspberry during Cold Storage. Horticulturae 2022, 8, 1. https://doi.org/10.3390/horticulturae8010001
Yan R, Han C, Fu M, Jiao W, Wang W. Inhibitory Effects of CaCl2 and Pectin Methylesterase on Fruit Softening of Raspberry during Cold Storage. Horticulturae. 2022; 8(1):1. https://doi.org/10.3390/horticulturae8010001
Chicago/Turabian StyleYan, Ran, Cong Han, Maorun Fu, Wenxiao Jiao, and Weihao Wang. 2022. "Inhibitory Effects of CaCl2 and Pectin Methylesterase on Fruit Softening of Raspberry during Cold Storage" Horticulturae 8, no. 1: 1. https://doi.org/10.3390/horticulturae8010001
APA StyleYan, R., Han, C., Fu, M., Jiao, W., & Wang, W. (2022). Inhibitory Effects of CaCl2 and Pectin Methylesterase on Fruit Softening of Raspberry during Cold Storage. Horticulturae, 8(1), 1. https://doi.org/10.3390/horticulturae8010001




