Can the Caper (Capparis spinosa L.) Still Be Considered a Difficult-to-Propagate Crop?
Abstract
1. Introduction
2. Seed Propagation
3. Vegetative Propagation
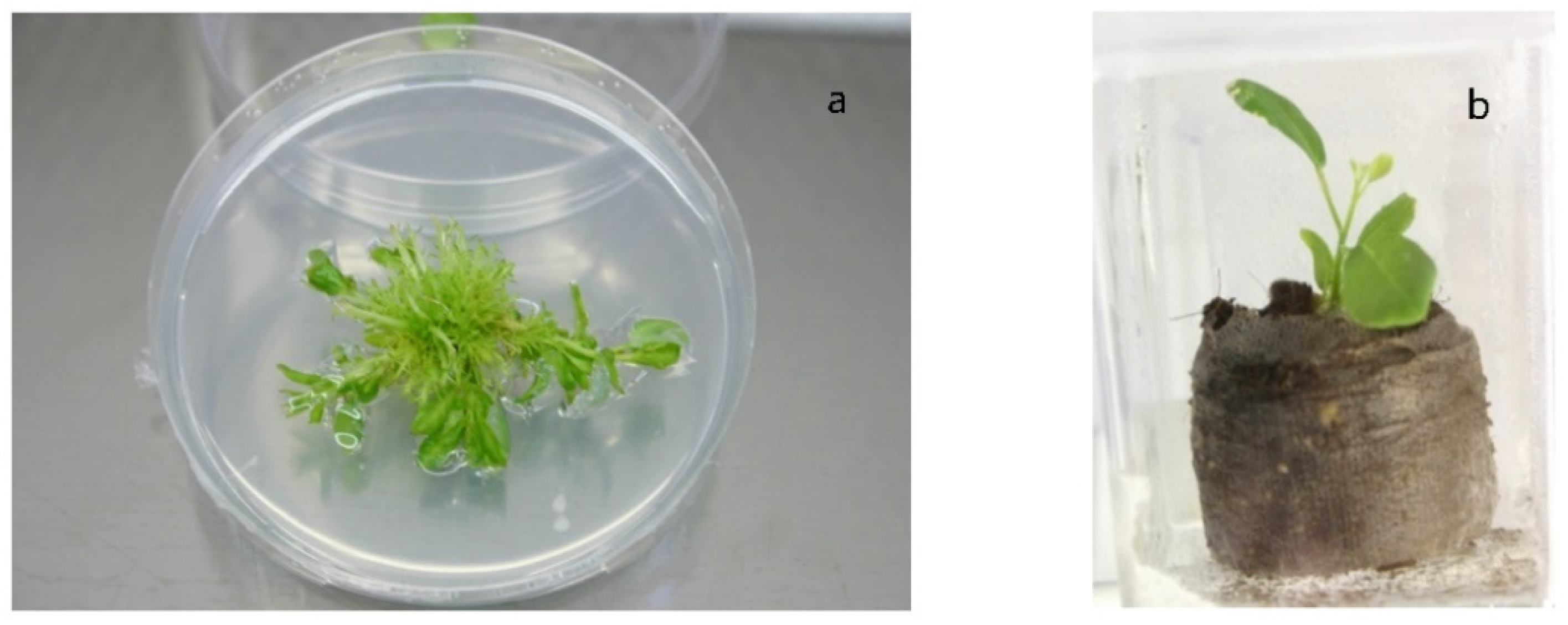
4. In Vitro Propagation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fici, S. Intraspecific variation and evolutionary trends in Capparis spinosa L. (Capparaceae). Plant Syst. Evol. 2001, 228, 123–141. [Google Scholar] [CrossRef]
- Ciftcioglu, G.C.; Ebedi, S.; Abak, K. Evaluation of the relationship between ornamental plants-based ecosystem services and human wellbeing: A case study from Lefke Region of North Cyprus. Ecol. Indic. 2019, 102, 278–288. [Google Scholar] [CrossRef]
- Gianguzzi, L.; Bazan, G. A phytosociological analysis of the Olea europaea L. var. sylvestris (Mill.) Lehr. forests in Sicily. Plant Biosyst.-Int. J. Deal. Asp. Plant Biol. 2020, 154, 705–725. [Google Scholar] [CrossRef]
- Distefano, G.; Caruso, M.; La Malfa, S.; Ferrante, T.; Del Signore, B.; Gentile, A.; Sottile, F. Genetic diversity and relationships among Italian and foreign almond germplasm as revealed by microsatellite markers. Sci. Hortic. 2013, 162, 305–312. [Google Scholar] [CrossRef]
- Fici, S. A taxonomic revision of the Capparis spinosa group (Capparaceae) from eastern Africa to Oceania. Phytotaxa 2015, 203, 24. [Google Scholar] [CrossRef]
- Rivera, D.; Inocencio, C.; Obon, C.; Carreno, E.; Reales, A.; Alcaraz, F. Archaeobotany of capers (Capparis) (Cappa-raceae). Veg. Hist. Archaeobot. 2002, 11, 295–313. [Google Scholar] [CrossRef]
- Bounous, G.; Barone, E. Prospettive di sviluppo di specie legnose per le zone aride e semi-aride del Meridione e nuovi criteri di utilizzo: Capero. Terra Sole 1989, 568, 733–735. [Google Scholar]
- Rivera, D.; Inocencio, C.; Obon, C.; Alcaraz, F. Review of Food and Medicinal Uses of Capparis L. Subgenus Capparis (Capparidaceae). Econ. Bot. 2003, 57, 515–534. [Google Scholar] [CrossRef]
- Gull, T.; Anwar, F.; Sultana, B.; Alcayde, M.A.C.; Nouman, W. Capparis species: A potential source of bioactives and high-value components: A review. Ind. Crops Prod. 2015, 67, 81–96. [Google Scholar] [CrossRef]
- Anwar, F.; Anwar, F.; Muhammad, G.; Ajaz Hussain, M.; Zengin, G.; Alkharfy, K.M.; Alkharfy, K.M.; Ashraf, M.; Gilani, A.H.; Gilani, A.H. Capparis spinosa L.: A plant with high potential for development of functional foods and nutraceu-ticals/pharmaceuticals. Int. J. Pharmacol. 2016, 12, 201–219. [Google Scholar] [CrossRef]
- Maldini, M.; Foddai, M.; Natella, F.; Addis, R.; Chessa, M.; Petretto, G.L.; Tuberoso, C.I.G.; Pintore, G. Metabolomic study of wild and cultivated caper (Capparis spinosa L.) from different areas of Sardinia and their comparative evaluation. J. Mass Spectrom. 2016, 51, 716–728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, Z.F. Phytochemical and Pharmacological Properties of Capparis spinosa as a Medicinal Plant. Nutritions 2018, 10, 116. [Google Scholar] [CrossRef]
- Grimalt, M.; Hernández, F.; Legua, P.; Almansa, M.S.; Amorós, A. Physicochemical composition and antioxidant activ-ity of three Spanish caper (Capparis spinosa L.) fruit cultivars in three stages of development. Sci. Hortic. 2018, 240, 509–515. [Google Scholar] [CrossRef]
- Lo Bosco, F.; Guarrasi, V.; Moschetti, M.; Germanà, M.A.; Butera, D.; Corana, F.; Papetti, A. Nutraceutical Value of Pan-telleria Capers (Capparis spinosa L.). J. Food Sci. 2019, 84, 2337–2346. [Google Scholar] [CrossRef]
- Mollica, A.; Stefanucci, A.; Macedonio, G.; Locatelli, M.; Luisi, G.; Novellino, E.; Zengin, G. Chemical composition and biological activity of Capparis spinosa L. from Lipari Island. S. Afr. J. Bot. 2019, 120, 135–140. [Google Scholar] [CrossRef]
- Barbera, G.; Di Lorenzo, R.; Barone, E. Observations on Capparis populations cultivated in Sicily and on their vegetative and productive behaviour. Agric. Mediterr. 1991, 121, 32–39. [Google Scholar]
- Gristina, A.S.; Fici, S.; Siragusa, M.; Fontana, I.; Garfì, G.; Carimi, F. Hybridization in Capparis spinosa L.: Molecular and morphological evidence from a Mediterranean island complex. Flora-Morphol. Distrib. Funct. Ecol. Plants 2014, 209, 733–741. [Google Scholar] [CrossRef]
- Barbera, G.; Di Lorenzo, R. The Caper Culture in Italy. Acta Hortic. 1984, 144, 167–172. [Google Scholar] [CrossRef]
- Gruère, G.P.; Giuliani, A.; Smale, M. Marketing underutilized plant species for the poor: A conceptual framework. In Agrobiodiversity Conservation and Economic Development; Routledge: London, UK, 2008; pp. 62–81. [Google Scholar]
- Renna, M.; Montesano, F.F.; Serio, F.; Gonnella, M. The Mediterranean Diet between Traditional Foods and Human Health through Culinary Examples; Elsevier BV: Amsterdam, The Netherlands, 2021; pp. 75–99. [Google Scholar]
- Chedraoui, S.; Abi-Rizk, A.; El-Beyrouthy, M.; Chalak, L.; Ouaini, N.; Rajjou, L. Capparis spinosa L. in A systematic re-view: A xerophilous species of multi values and promising potentialities for agrosystems under the threat of global warming. Front. Plant Sci. 2017, 8, 1–18. [Google Scholar] [CrossRef]
- Olmez, Z.; Gokturk, A.; Gulcu, S. Effects of cold stratification on germination rate and percentage of caper (Capparis ovata Desf.) seeds. J. Environ. Biol. 2006, 27, 667–670. [Google Scholar] [PubMed]
- Gan, L.; Zhang, C.; Yin, Y.; Lin, Z.; Huang, Y.; Xiang, J.; Fu, C.; Li, M. Anatomical adaptations of the xerophilous me-dicinal plant, Capparis spinosa, to drought conditions. Hortic. Environ. Biotechnol. 2013, 54, 156–161. [Google Scholar] [CrossRef]
- Levizou, E.; Drilias, P.; Kyparissis, A. Exceptional Photosynthetic Performance of Capparis spinosa L. Under Adverse Conditions of Mediterranean summer. Photosynthetica 2004, 42, 229–235. [Google Scholar] [CrossRef]
- Pugnaire, F.I.; Esteban, E. Nutritional adaptations of caper shrub (Capparis Ovata Desf.) to environmental stress. J. Plant Nutr. 1991, 14, 151–161. [Google Scholar] [CrossRef]
- Sozzi, G.O. Caper bush: Botany and Horticulture. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons.: Hoboken, NJ, USA, 2001; Volume 27, pp. 125–188. ISBN 9780471387909. [Google Scholar]
- Puche, J.L. Ministerio de Agricultura, 1977th ed.; Hojas Divulgadoras del Ministerio de Agricultura: Madrid, Spain, 1977; ISBN 84-341-0143-2. [Google Scholar]
- Rhizopoulou, S.; Psaras, G.K. Development and structure of drought-tolerant leaves of the Mediterranean shrub Capparis spinosa L. Ann. Bot. 2003, 92, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Ramezani-Gask, M.; Bahrani, M.; Shekafandeh, A. A comparison of different propagation methods of common Caper-bush (Capparis spinosa L.) as a new horticultural crop. Int. J. Plant Dev. 2008, 2, 106–110. [Google Scholar]
- Sakcali, M.S.; Bahadir, H.; Ozturk, M. Eco-physiology of capparis spinosa L: A plant suitable for combating desertification. Pak. J. Bot. 2008, 40, 1481–1486. [Google Scholar]
- Neyisci, T. A study on the slow burning plant species suitable for controlling forest fires’ (summary in English). Turk. J. Agric. For. 1987, 11, 595–604. [Google Scholar]
- Ashraf, U.; Chaudhry, M.N.; Ahmad, S.R.; Ashraf, I.; Arslan, M.; Noor, H.; Jabbar, M. Impacts of climate change on Capparis spinosa L. based on ecological niche modeling. PeerJ 2018, 6, e5792. [Google Scholar] [CrossRef]
- Rankou, H.; M’Sou, S.; Diarra, A.; Ait Babahmad, R.A. Capparis Spinosa. The IUCN Red List of Threatened Species 2020: E.T137745831A139593491. 2020. Available online: https://www.iucnredlist.org/species/137745831/139593491 (accessed on 17 July 2021).
- Padulosi, S. Priority-setting for underutilized and neglected plant species of the Mediterranean region. In Proceedings of the Report of the IPGRI Conference, Aleppo, Syria, 9–11 February 1998; Padulosi, S., Ed.; IPGRI: Rome, Italy, 1999. [Google Scholar]
- Padulosi, S.; Hodgkin, T.; Williams, J.T.; Haq, N. Underutilized crops: Trends, challenges and opportunities in the 21st century. In Managing Plant Genetic Diversity, Proceedings of the International Conference, Kuala Lumpur, Malaysia, 12–16 June 2000; CABI: Wallingford, UK, 2002; pp. 323–338. [Google Scholar]
- Sozzi, G.; Peter, K.V.; Babu, K.N.; Divakaran, M. Capers and caperberries. In Handbook of Herbs and Spices; Elsevier: Amsterdam, The Netherlands, 2012; Volume 2, pp. 193–224. [Google Scholar]
- Luna Lorente, F.; Perez Vicente, M. La Tapenera o Alcaparra. Cultivo y Aprovechamiento; Publicaciones de Extension Agraria: Madrid, Spain, 1985; ISBN 84-341-0382-6. [Google Scholar]
- Barbera, G. Le caprier (Capparis spp.); EUR 13617; Commission des Communautés Européennes: Luxembourg, 1991; ISBN 9282629783. [Google Scholar]
- Fici, S.; Lo Valvo, F. Seed dispersal of Capparis spinosa L. (Capparaceae) by Mediterranean lizards. Nat. Sicil. 2004, 28, 1147–1154. [Google Scholar]
- La Mantia, T.; Rühl, J.; Massa, B.; Pipitone, S.; Lo Verde, G.; Bueno, R.S. Vertebrate-mediated seed rain and artificial perches contribute to overcome seed dispersal limitation in a Mediterranean old field. Restor. Ecol. 2019, 27, 1393–1400. [Google Scholar] [CrossRef]
- Vigni, I.L.; Melati, M.R. Examples of seed dispersal by entomochory. Acta Bot. Gallica 1999, 146, 145–156. [Google Scholar] [CrossRef][Green Version]
- Yang, Y.; Lin, Y.; Shi, L. The effect of lizards on the dispersal and germination of Capparis spinosa (Capparaceae). PLoS ONE 2021, 16, e0247585. [Google Scholar] [CrossRef]
- Orphanos, P.I. Germination of caper (Capparis spinosa L.) seeds. J. Hortic. Sci. 1983, 58, 267–270. [Google Scholar] [CrossRef]
- Sozzi, G.O.; Chiesa, A. Improvement of caper (Capparis spinosa L.) seed germination by breaking seed coat-induced dormancy. Sci. Hortic. 1995, 62, 255–261. [Google Scholar] [CrossRef]
- Pascual, B.; San Bautista, A.; López-Galarza, S.; Alagarda, J.; Maroto, J.V. Germination behaviour after storage of caper seeds. Seed Sci. Technol. 2006, 34, 151–159. [Google Scholar] [CrossRef]
- Ferrer, M.J. Estudio Para la Mejora de las Técnicas de Propagación de la Alcaparra (Capparis spinosa L.); Universitat Politecnica de Valencia: Valencia, Spain, 2018. [Google Scholar]
- Macchia, M.; Casano, S. La propagazione del capero (Capparis spinosa L). Sementi Elette 1993, 39, 37. [Google Scholar]
- Germanà, M.; Chiancone, B. In Vitro Germination and Seedling Development of Caper (Capparis spinosa L.) Mature Seeds. Acta Hortic. 2009, 839, 181–186. [Google Scholar] [CrossRef]
- Tafti, M.M.; Farhoudi, M.; Rastifar, M.; Asilan, K.S. Methods of breaking seed dormancy in Caper (Capparis spinosa L.) (English summary). Iran. J. Range Desert Res. 2012, 18, 569–577. [Google Scholar]
- Pascual, B.; San Bautista, A.; Ferreros, N.; López-Galarza, S.; Maroto, J.V. Analysis of germination of caper seeds as in-fluenced by the position of fruit on the mother plant, fruit maturation stage and fruit weight. J. Hortic. Sci. Biotechnol. 2003, 78, 73–78. [Google Scholar]
- Zohary, M. The species of Capparis in the Mediterranean and the Near Eastern Countries. Bull. Res. Counc. Isr. 1960, 8D, 49–64. [Google Scholar]
- Fici, S.; Gianguzzi, L. Diversity and conservation in wild and cultivated Capparis in Sicily. Bocconea 1997, 7, 437–443. [Google Scholar]
- Inocencio, C.; Rivera, D.; Concepción Obón, M.; Alcaraz, F.; Barreña, J.-A. A Systematic Revision of Capparis Section Capparis (Capparaceae) 1, 2. Ann. Missouri Bot. Gard. 2006, 93, 122–149. [Google Scholar] [CrossRef]
- Hartmann, H.T.; Kester, D.E.; Davies, F.T.; Geneve, R.L. Plant Propagation Principles and Practices, 7th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2002. [Google Scholar]
- Rinaldelli, E. Effect of ultrasonic waves on seed germination of Capparis spinora L. as related to exposure time, temperature, and gibberellic acid. Adv. Hortic. Sci. 2000, 14, 182–188. [Google Scholar]
- Chalak, L.; Elbitar, A.; Cordahi, N.; Hage, C.; Chehade, A. In Vitro Propagation of Capparis spinosa L. Acta Hortic. 2003, 616, 335–338. [Google Scholar] [CrossRef]
- Pascual, B.; Bautista, A.S.; Imbernon, A.; López-Galarza, S.; Alagarda, J.; Maroto, J. Seed treatments for improved germination of caper (Capparis spinosa). Seed Sci. Technol. 2004, 32, 637–642. [Google Scholar] [CrossRef][Green Version]
- Bahrani, M.; Gask, M.R.; Shekafandeh, A.; Taghvaei, M. Seed germination of wild caper (Capparis spinosa L., var. parviflora) as affected by dormancy breaking treatments and salinity levels. Seed Sci. Technol. 2008, 36, 776–780. [Google Scholar] [CrossRef]
- Pascual, B.; San Bautista, A.; Pascual Seva, N.; García Molina, R.; López-Galarza, S.; Maroto, J.V. Effects of soaking period and gibberellic acid addition on caper seed germination. Seed Sci. Technol. 2009, 37, 33–41. [Google Scholar] [CrossRef]
- Bhoyar, M.S.; Mishra, G.P.; Singh, R.; Singh, S.B. Effects of various dormancy breaking treatments on the germination of wild caper (Capparis spinosa) seeds from the cold arid desert of trans-Himalayas. Indian J. Agric. Sci. 2010, 80, 621–625. [Google Scholar]
- Soyler, D.; Khawar, K.M. Seed Germination of Caper (Capparis ovata var. Herbacea) Using α Naphthalene Acetic Acid and Gibberellic Acid. Int. J. Agric. Biol. 2007, 9, 35–37. [Google Scholar]
- Suleiman, M.K.; Bhat, N.R.; Abdal, M.S.; Jacob, S.; Thomas, R.R.; Al-Dossery, S.; Bellen, R. Germination studies of Capparis spinosa L. Propag. Ornam. Plants 2009, 9, 35–38. [Google Scholar]
- Olmez, Z.; Olmez, S.A. Effects of Different Growing Media and Sowing Depths on Seed Germination of Caper (Capparis Ovata Desf.). Int. J. Ecosyst. Ecol. Sci. 2017, 7, 331–336. [Google Scholar]
- Pascual-Seva, N.; San Bautista, A.; López-Galarza, S.; Maroto, J.V.; Pascual, B. Effect of accelerated ageing on germination in caper (Capparis spinosa L.) seeds. Acta Hortic. 2011, 898, 69–74. [Google Scholar] [CrossRef]
- Al-Safadi, B.; Elias, R. Improvement of caper (Capparis spinosa L.) propagation using in vitro culture and gamma irradiation. Sci. Hortic. 2011, 127, 290–297. [Google Scholar] [CrossRef]
- Arefi, I.H.; Nejad, S.K.; Kafi, M. Roles of duration and concentration of priming agents on dormancy breaking and germination of caper (Capparis spinosa L.) for the protection of arid degraded areas. Pak. J. Bot. 2012, 44, 225–230. [Google Scholar]
- Labbafi, M.; Mehrafarin, A.; Badi, H.; Ghorbani, M.; Tavakoli, M. Improve germination of caper (Capparis spinosa L.) seeds by different induction treatments of seed dormancy breaking. Trakia J. Sci. 2018, 16, 70–74. [Google Scholar] [CrossRef]
- Heydariyan, M.; Basirani, N.; Sharifi-Rad, M.; Khmmari, I.; Rafat Poor, S. Effect of Seed Priming on Germination and Seedling Growth of the Caper (Capparis Spinosa) Under Drought Stress. Int. J. Adv. Biol. Biomed. Res. 2014, 2, 2381–2389. [Google Scholar]
- Juan, M.; Pascual-Seva, N.; Iranzo, D.; Pascual, B. Improvement of seed germination of caper (Capparis spinosa L.) through magnetic fields. Acta Hortic. 2020, 1273, 433–440. [Google Scholar] [CrossRef]
- Foschi, M.L.; Juan, M.; Pascual, B.; Pascual-Seva, N. Water Uptake and Germination of Caper (Capparis spinosa L.) Seeds. Agronomy 2020, 10, 838. [Google Scholar] [CrossRef]
- Loreti, F.; Morini, S.; Fiorino, P. Propagation. In Principles of Modern Fruit Science; Sansavini, S., Ed.; ISHS: Leuven, Belgium, 2019; pp. 191–219. ISBN 978-94-6261-204-4. [Google Scholar]
- Sozzi, G.O.; Vicente, A.R. Capers and caperberries. In Handbook of Herbs and Spices; Peter, K.V., Ed.; CRC Press, Inc.: Washington, DC, USA, 2006; Volume 3, pp. 1689–1699. ISBN 978-0-8493-9155-2. [Google Scholar]
- San Bautista, A.; Pascual, B.; Sospedra, S.; Lopez-Galarza, S.; Laza, P.; Maroto, J.V. Influencia de la fecha y de la sección de las ramas en el enraizamiento y la brotación de las yemas en estaquillas de alcaparra. Actas Hortic. 2006, 46, 41–45. [Google Scholar]
- Pascual, B.; San Bautista, A.; Pascual-Seva, N.; Garcia-Molina, R.; Lopez Galarza, S.; Maroto, J.V. Estudio del enrai-zamiento de estaquillas de madera suave en alcaparra (Capparis spinosa L.). Actas Hortic. 2008, 50, 89–93. [Google Scholar]
- Pilone, N. Variazione del potenziale rizogeno naturale nel capero. L’Inform. Agrar. 1990, 13, 69–70. [Google Scholar]
- Pilone, N. Effetti dell’IBA sulla radicazione delle talee di Capparis spinosa in cassone riscaldato. L’Inform. Agrar. 1990, 40, 81–82. [Google Scholar]
- Salem, A.B.; Zemni, H.; Ghorbel, A. Propagation caper (Capparis spinosa L.) by herbaceous cuttings and in vitro culture. Agric. Mediterr. 2001, 131, 42–48. [Google Scholar]
- Abel, J.I. Estudio Para la Mejora de la Propagación de la Alcaparra Mediante Estaquillas. Ph.D. Thesis, Universitat Politècnica de València, València, Spain, 2015. [Google Scholar]
- George, E.F.; Hall, M.A.; De Klerk, G.J. Plant Propagation by Tissue Culture, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 1, ISBN 9781402050053. [Google Scholar]
- Rodriguez, R.; Rey, M.; Cuozzo, L.; Ancora, G. In vitro propagation of caper (Capparis spinosa L.). Vitr. Cell. Dev. Biol.-Anim. 1990, 26, 531–536. [Google Scholar] [CrossRef]
- Caglar, G.; Caglar, S.; Ergin, O.; Yarim, M. The influence of growth regulators on shoot proliferation and rooting of in vitro propagated caper. J. Environ. Biol. 2005, 26, 479–485. [Google Scholar] [PubMed]
- Musallam, I.; Duwayri, M.; Shibli, R.A. Micropropagation of Caper (Capparis spinosa L.) from Wild Plants. Funct. Plant Sci. Biotechnol. 2011, 5, 17–21. [Google Scholar]
- Deora, N.S.; Shekhawat, N.S. Micropropagation of Capparis decidua (Forsk.) Edgew—A tree of arid horticulture. Plant Cell Rep. 1995, 15, 278–281. [Google Scholar] [CrossRef]
- Tyagi, P.; Kothari, S.L. Micropropagation of Capparis decidua through In Vitro Shoot Proliferation on Nodal Explants of Mature Tree and Seedling Explants. J. Plant Biochem. Biotechnol. 1997, 6, 19–23. [Google Scholar] [CrossRef]
- Carra, A.; Del Signore, M.B.; Sottile, F.; Ricci, A.; Carimi, F. Potential use of new diphenylurea derivatives in micro-propagation of Capparis spinosa L. Plant Growth Regul. 2012, 66, 229–237. [Google Scholar] [CrossRef]
- Hegazi, G.A.; Eid, S.R.; El, A.; Sharaf, M.M. Micropropagation for conservation of two rare Capparis species from Egypt 1. Catrina 2011, 6, 29–39. [Google Scholar]
- Kereša, S.; Stanković, D.; Lodeta, K.B.; Jerčić, I.H.; Bolarić, S.; Barić, M.; Mihovilović, A.B. Efficient Protocol for the In Vitro Plantlet Production of Caper (Capparis orientalis Veill.) from the East Adriatic Coast. Agronomy 2019, 9, 303. [Google Scholar] [CrossRef]
- Chalak, L.; Elbitar, A. Micropropagation of Capparis spinosa L. subsp. Rupestris Sibth. & Sm. by nodal cuttings. Indian J. Biotechnol. 2006, 5, 555–558. [Google Scholar]
- Tian, X.-X.; Jiang, C.-Q.; Chen, X.-Y.; Qu, S.; Yang, W.-S. Studies on the tissue culture of axillary bud and rapid propa-gation in the Capparis spinosa (Abstract). For. Res. 2009, 22, 521–525. [Google Scholar]
- Al-Mahmood, H.J.; Shatnawi, M.A.; Shibli, R.A.; Makhadmeh, I.M.; Abubaker, S.M.; Shadiadeh, A.N. Clonal propaga-tion and medium-term conservation of Capparis spinosa: A medicinal plant. J. Med. Plants Res. 2012, 6, 3826–3836. [Google Scholar]
- Attia, A.; Dessoky, E.D.S.; Al-Sodany, Y.; Ismail, I.A. Ex situ preservation for some endemic and rare medicinal plants in Taif, KSA. Biotechnol. Biotechnol. Equip. 2017, 31, 912–920. [Google Scholar] [CrossRef]
- Carra, A.; Sajeva, M.; Abbate, L.; Siragusa, M.; Sottile, F.; Carimi, F. In vitro plant regeneration of caper (Capparis spinosa L.) from floral explants and genetic stability of regenerants. Plant Cell Tissue Organ Cult. 2011, 109, 373–381. [Google Scholar] [CrossRef]
- Sottile, F.; Giuggioli, N.R.; Marinoni, D.T.; Peano, C.; Del Signore, M.B. Selection and micropropagation of valuable caper genotypes. Hortic. Sci. 2020, 47, 110–116. [Google Scholar] [CrossRef]
- Gianguzzi, V.; Inglese, P.; Barone, E.; Sottile, F. In Vitro Regeneration of Capparis spinosa L. by Using a Temporary Immersion System. Plants 2019, 8, 177. [Google Scholar] [CrossRef]
- Krishna, H.; Alizadeh, M.; Singh, D.; Singh, U.; Chauhan, N.; Eftekhari, M.; Sadh, R.K. Somaclonal variations and their applications in horticultural crops improvement. Biotech 2016, 6, 1–18. [Google Scholar] [CrossRef]
- Gianguzzi, V.; Barone, E.; Sottile, A.F. In Vitro Rooting of Capparis spinosa L. as Affected by Genotype and by the Proliferation Method Adopted During the Multiplication Phase. Plants 2020, 9, 398. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sottile, F.; Caltagirone, C.; Peano, C.; Del Signore, M.B.; Barone, E. Can the Caper (Capparis spinosa L.) Still Be Considered a Difficult-to-Propagate Crop? Horticulturae 2021, 7, 316. https://doi.org/10.3390/horticulturae7090316
Sottile F, Caltagirone C, Peano C, Del Signore MB, Barone E. Can the Caper (Capparis spinosa L.) Still Be Considered a Difficult-to-Propagate Crop? Horticulturae. 2021; 7(9):316. https://doi.org/10.3390/horticulturae7090316
Chicago/Turabian StyleSottile, Francesco, Chiara Caltagirone, Cristiana Peano, Maria Beatrice Del Signore, and Ettore Barone. 2021. "Can the Caper (Capparis spinosa L.) Still Be Considered a Difficult-to-Propagate Crop?" Horticulturae 7, no. 9: 316. https://doi.org/10.3390/horticulturae7090316
APA StyleSottile, F., Caltagirone, C., Peano, C., Del Signore, M. B., & Barone, E. (2021). Can the Caper (Capparis spinosa L.) Still Be Considered a Difficult-to-Propagate Crop? Horticulturae, 7(9), 316. https://doi.org/10.3390/horticulturae7090316








