Biological Contribution of Ornamental Plants for Improving Slope Stability along Urban and Suburban Areas
Abstract
:1. Introduction
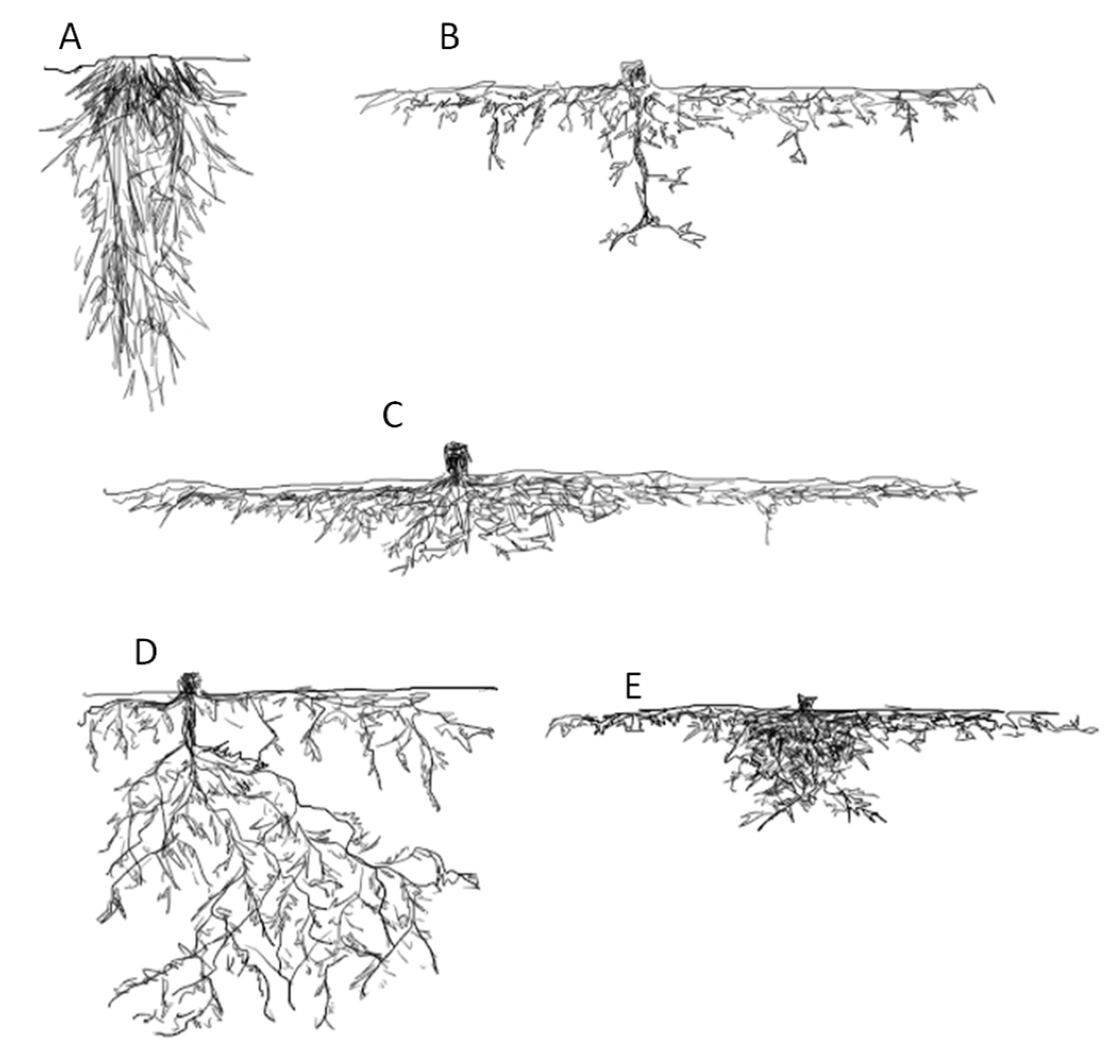
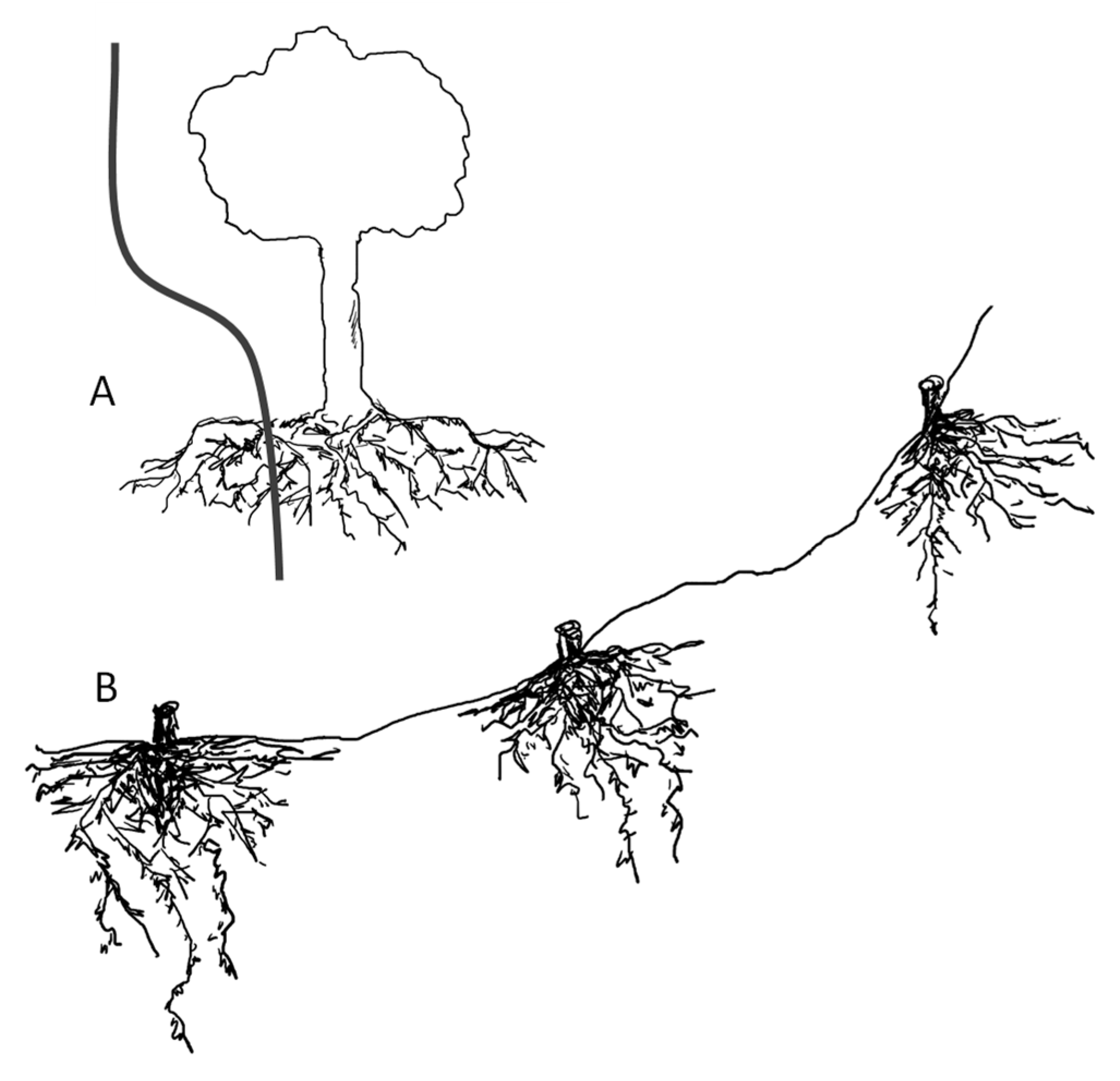
1.1. Root Growth under Slopes Increase
1.2. Root Morphology and Slope Stabilization
2. Root Physiology and Development under Abiotic Stress
3. Use of Plant Growth Regulators and Biostimulants for Increasing Root Biomass
4. Use of Ornamental Plants for Slope Greening
5. Limitations and Research Gaps in the Use of Ornamental Plants for Improving Slope Stability
- -
- Slow roots establishment and stability: the contribution of roots to stability increases with the roots’ development and establishment. The highest stability is achieved when the species used reach maturity and the roots network is well integrated with the soil. Therefore, the stability of the slope is not immediately obtained (a limitation to be considered);
- -
- Unpredictable environmental effects: plants development depends on environmental parameters and unpredicted events can reduce the efficacy of plants used in slope stabilization. These can also include soil-borne diseases and the limitation of the use of specific agrochemicals can represent an important limit in the maintenance of plants health.
- -
- High costs and regularity of maintenance: public urban areas are under municipality maintenance and the lack of funds or of regularity in the management can compromise the benefit of the plants on the stability of the slopes;
- -
- Minimum of soil for plants growth: the use of plants as slope stabilizers can be a solution if there is a minimum of soil for roots development and for a better anchorage that can harness the soil itself avoiding landslides.
6. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Bolund, P.; Hunhammar, S. Ecosystem services in urban areas. Ecol. Econ. 1999, 29, 293–301. [Google Scholar] [CrossRef]
- Lee, L.S.; Zhang, H.; Jim, C.Y. Serviceable tree volume: An alternative tool to assess ecosystem services provided by ornamental trees in urban forests. Urban For. Urban Green. 2021, 59, 127003. [Google Scholar] [CrossRef]
- Amoroso, G.; Frangi, P.; Piatti, R.; Fini, A.; Ferrini, F.; Faoro, M. Evaluation of shrubs for side slope greening and protection in urban landscape. HortTechnology 2011, 21, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Saifuddin, M.; Osman, N. Evaluation of hydro-mechanical properties and root architecture of plants for soil reinforcement. Curr. Sci. 2014, 107, 845–852. Available online: https://www.jstor.org/stable/24105589 (accessed on 14 May 2021).
- Ghestem, M.; Sidle, R.C.; Stokes, A. The influence of plant root systems on subsurface flow: Implications for slope stability. Bioscience 2011, 61, 869–879. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, X.; Wang, N.; Meng, M.; Jia, Z.; Wang, J.; Ma, S.; Tang, Y.; Li, C.; Zhai, L.; et al. Effects of vegetation type on soil shear strength in Fengyang Mountain Nature Reserve, China. Forests 2021, 12, 490. [Google Scholar] [CrossRef]
- Durán Zuazo, V.H.; Rodríguez Pleguezuelo, C.R. Soil-Erosion and Runoff Prevention by Plant Covers: A Review. In Sustainable Agriculture; Lichtfouse, E., Navarrete, M., Debaeke, P., Véronique, S., Alberola, C., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 785–811. [Google Scholar] [CrossRef] [Green Version]
- Pickup, G.; Marks, A. Identifying large-scale erosion and deposition processes from airborne gamma radiometrics and digital elevation models in a weathered landscape. Earth Surf. Process. Landf. 2000, 25, 535–557. [Google Scholar] [CrossRef]
- López-Vicente, M.; Kramer, H.; Keesstra, S. Effectiveness of soil erosion barriers to reduce sediment connectivity at small basin scale in a fire-affected forest. J. Environ. Manag. 2021, 278, 111510. [Google Scholar] [CrossRef] [PubMed]
- Hellmers, H.; Horton, J.S.; Juhren, G.; O’keefe, J. Root systems of some chaparral plants in southern California. Ecology 1955, 36, 667–678. Available online: https://www.jstor.org/stable/1931305 (accessed on 14 May 2021). [CrossRef]
- Ghestem, M.; Cao, K.; Ma, W.; Rowe, N.; Leclerc, R.; Gadenne, C.; Stokes, A. A framework for identifying plant species to be used as ‘ecological engineers’ for fixing soil on unstable slopes. PLoS ONE 2014, 9, e95876. [Google Scholar] [CrossRef]
- Durán Zuazo, V.D.; Rodríguez Pleguezuelo, C.R.; Martínez, J.F.; Rodríguez, B.C.; Raya, A.M.; Galindo, P.P. Harvest intensity of aromatic shrubs vs. soil erosion: An equilibrium for sustainable agriculture (SE Spain). Catena 2008, 73, 107–116. [Google Scholar] [CrossRef]
- Fernandes, J.P.; Guiomar, N. Simulating the stabilization effect of soil bioengineering interventions in Mediterranean environments using limit equilibrium stability models and combinations of plant species. Ecol. Eng. 2016, 88, 122–142. [Google Scholar] [CrossRef]
- Moresi, F.V.; Maesano, M.; Matteucci, G.; Romagnoli, M.; Sidle, R.C.; Scarascia Mugnozza, G. Root biomechanical traits in a montane Mediterranean forest watershed: Variations with species diversity and soil depth. Forests 2019, 10, 341. [Google Scholar] [CrossRef] [Green Version]
- Tsige, D.; Senadheera, S.; Talema, A. Stability analysis of plant-root-reinforced shallow slopes along mountainous road corridors based on numerical modeling. Geosciences 2020, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Ghafari, S.; Kaviani, B.; Sedaghathoor, S.; Allahyari, M.S. Ecological potentials of trees, shrubs and hedge species for urban green spaces by multi criteria decision making. Urban For. Urban Green. 2020, 55, 126824. [Google Scholar] [CrossRef]
- Reubens, B.; Poesen, J.; Danjon, F.; Geudens, G.; Muys, B. The role of fine and coarse roots in shallow slope stability and soil erosion control with a focus on root system architecture: A review. Trees 2007, 21, 385–402. [Google Scholar] [CrossRef]
- Chen, R.; Rosen, E.; Masson, P.H. Gravitropism in higher plants. Plant Physiol. 1999, 120, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Danjon, F.; Reubens, B. Assessing and analyzing 3D architecture of woody root systems, a review of methods and applications in tree and soil stability, resource acquisition and allocation. Plant Soil 2008, 303, 1–34. [Google Scholar] [CrossRef]
- Lugo, A.E.; Gucinski, H. Function, effects, and management of forest roads. For. Ecol. Manag. 2000, 133, 249–262. [Google Scholar] [CrossRef]
- Stokes, A.; Atger, C.; Bengough, A.G.; Fourcaud, T.; Sidle, R.C. Desirable plant root traits for protecting natural and engineered slopes against landslides. Plant Soil 2009, 324, 1–30. [Google Scholar] [CrossRef]
- Chloupek, O.; Dostál, V.; Středa, T.; Psota, V.; Dvořáčková, O. Drought tolerance of barley varieties in relation to their root system size. Plant Breed. 2010, 129, 630–636. [Google Scholar] [CrossRef]
- Středa, T.; Dostál, V.; Horáková, V.; Chloupek, O. Effective use of water by wheat varieties with different root system sizes in rain-fed experiments in Central Europe. Agric. Water Manag. 2012, 104, 203–209. [Google Scholar] [CrossRef]
- Ghosh, D.; Xu, J. Abiotic stress responses in plant roots: A proteomics perspective. Front. Plant Sci. 2014, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Rahnama, A.; Munns, R.; Poustini, K.; Watt, M. A screening method to identify genetic variation in root growth responses to a salinity gradient. J. Exp. Bot. 2011, 62, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Singh, D.; Saksena, H.B.; Sharma, M.; Tiwari, A.; Awasthi, P.; Botta, H.K.; Shukla, B.N.; Laxmi, A. Understanding the intricate web of phytohormone signalling in modulating root system architecture. Int. J. Mol. Sci. 2021, 22, 5508. [Google Scholar] [CrossRef] [PubMed]
- Vives-Peris, V.; López-Climent, M.F.; Pérez-Clemente, R.M.; Gómez-Cadenas, A. Root involvement in plant responses to adverse environmental conditions. Agronomy 2020, 10, 942. [Google Scholar] [CrossRef]
- Cirillo, C.; Rouphael, Y.; Caputo, R.; Raimondi, G.; De Pascale, S. The influence of deficit irrigation on growth, ornamental quality, and water use efficiency of three potted Bougainvillea genotypes grown in two shapes. HortScience 2014, 49, 1284–1291. [Google Scholar] [CrossRef] [Green Version]
- Toscano, S.; Ferrante, A.; Romano, D. Response of Mediterranean ornamental plants to drought stress. Horticulturae 2019, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Ramamoorthy, P.; Lakshmanan, K.; Upadhyaya, H.D.; Vadez, V.; Varshney, R.K. Root traits confer grain yield advantages under terminal drought in chickpea (Cicer arietinum L.). Field Crop. Res. 2017, 201, 146–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zollinger, N.; Kjelgren, R.; Cerny-Koenig, T.; Kopp, K.; Koenig, R. Drought responses of six ornamental herbaceous perennials. Sci. Hortic. 2006, 109, 267–274. [Google Scholar] [CrossRef]
- Tahir, M.H.N.; Imran, M.; Hussain, M.K. Evaluation of sunflower (Helianthus annuus L.) inbred lines for drought tolerance. Int. J. Agric. Biol. 2002, 3, 398–400. [Google Scholar]
- Jaleel, C.A.; Gopi, R.; Sankar, B.; Gomathinayagam, M.; Panneerselvam, R. Differential responses in water use efficiency in two varieties of Catharanthus roseus under drought stress. Comptes Rendus Biol. 2008, 331, 42–47. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Lakshmanan, G.M.A.; Gomathinayagam, M.; Panneerselvam, R. Alterations in morphological parameters and photosynthetic pigment responses of Catharanthus roseus under soil water deficits. Colloids Surf. B Biointerfaces 2008, 61, 298–303. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Acosta-Motos, J.R.; Diaz-Vivancos, P.; Álvarez, S.; Fernández-García, N.; Sánchez-Blanco, M.J.; Hernández, J.A. NaCl-induced physiological and biochemical adaptive mechanisms in the ornamental Myrtus communis L. plants. J. Plant Physiol. 2015, 183, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Motos, J.R.; Díaz-Vivancos, P.; Álvarez, S.; Fernández-García, N.; Sánchez-Blanco, M.J.; Hernández, J.A. Physiological and biochemical mechanisms of the ornamental Eugenia myrtifolia L. plants for coping with NaCl stress and recovery. Planta 2015, 242, 829–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Fornes, F.; Belda, R.M.; Carrión, C.; Noguera, V.; García-Agustín, P.; Abad, M. Pre-conditioning ornamental plants to drought by mean of saline water irrigation as related to salinity tolerance. Sci. Hortic. 2007, 113, 52–59. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar]
- Malamy, J.E. Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ. 2005, 28, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Spollen, W.G.; Sharp, R.E. Spatial distribution of turgor and root growth at low water potentials. Plant Physiol. 1991, 96, 438–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Zhou, G.; Shimizu, H. Plant responses to drought and rewatering. Plant Signal. Behav. 2010, 5, 649–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smirnoff, N. Plant resistance to environmental stress. Curr. Opin. Biotechnol. 1998, 9, 214–219. [Google Scholar] [CrossRef]
- Wu, F.; Bao, W.; Li, F.; Wu, N. Effects of drought stress and N supply on the growth, biomass partitioning and water-use efficiency of Sophora davidii seedlings. Environ. Exp. Bot. 2008, 63, 248–255. [Google Scholar] [CrossRef]
- Navarro, A.; Vicente, M.J.; Martínez-Sánchez, J.J.; Franco, J.A.; Fernández, J.A.; Bañón, S. Influence of deficit irrigation and paclobutrazol on plant growth and water status in Lonicera implexa seedlings. Acta Hortic. 2008, 782, 299–304. [Google Scholar] [CrossRef]
- Franco, J.A.; Bañón, S.; Fernández, J.A.; Leskovar, D.I. Effect of nursery regimes and establishment irrigation on root development of Lotus creticus seedlings following transplanting. J. Hortic. Sci. Biotechnol. 2001, 76, 174–179. [Google Scholar] [CrossRef]
- Bañón, S.; Fernández, J.A.; Franco, J.A.; Torrecillas, A.; Alarcón, J.J.; Sánchez-Blanco, M.J. Effects of water stress and night temperature pre-conditioning on water relations and morphological and anatomical changes of Lotus creticus plants. Sci. Hortic. 2004, 101, 333–342. [Google Scholar] [CrossRef]
- Niu, G.; Rodriguez, D.S.; Rodriguez, L.; Mackay, W. Effect of water stress on growth and flower yield of big bend bluebonnet. HortTechnology 2007, 17, 557–560. [Google Scholar]
- Bañón, S.; Ochoa, J.; Franco, J.A.; Alarcón, J.J.; Fernández, T.; Sánchez-Blanco, M.J. The influence of acclimation treatments on the morphology, water relations and survival of Myrtus communis L. plants. In Sustainable Use and Management of Soils in Arid and Semiarid Regions; Faz, A., Ortiz, R., Mermut, A.R., Eds.; Quaderna Editorial: Murcia, Spain, 2002; pp. 275–277. [Google Scholar]
- Bañón, S.; Ochoa, J.; Franco, J.; Alarcón, J.; Sánchez-Blanco, M.J. Hardening of oleander seedlings by deficit irrigation and low air humidity. Environ. Exp. Bot. 2006, 56, 36–43. [Google Scholar] [CrossRef]
- Niu, G.; Rodriguez, D.S.; Mackay, W. Growth and physiological responses to drought stress in four oleander clones. J. Am. Soc. Hortic. Sci. 2008, 133, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Snyman, H.A. Effects of various water application strategies on root development of Opuntia ficus-indica and Opuntia robusta under greenhouse growth conditions. J. Prof. Assoc. Cactus 2004, 6, 35–61. [Google Scholar]
- Bañón, S.; Ochoa, J.; Franco, J.A.; Sánchez-Blanco, M.J.; Alarcón, J.J. Influence of water deficit and low air humidity in the nursery on survival of Rhamnus alaternus seedlings following planting. J. Hortic. Sci. Biotechnol. 2003, 78, 518–522. [Google Scholar] [CrossRef]
- Sánchez-Blanco, M.J.; Ferrández, T.; Navarro, A.; Bañón, S.; Alarcón, J.J. Effects of irrigation and air humidity preconditioning on water relations, growth and survival of Rosmarinus officinalis plants during and after transplanting. J. Plant Physiol. 2004, 161, 1133–1142. [Google Scholar] [CrossRef]
- Niu, G.; Rodriguez, D.S. Growth and physiological responses of four rose rootstocks to drought stress. J. Am. Soc. Hortic. Sci. 2009, 134, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Feser, C.; St Hilaire, R.; Van Leeuwen, D. Development of in-ground container plants of Mexican elders exposed to drought. HortScience 2005, 40, 446–450. [Google Scholar] [CrossRef] [Green Version]
- Arreola, J.; Franco, J.A.; Vicente, M.J.; Martínez-Sánchez, J.J. Effect of nursery irrigation regimes on vegetative growth and root development of Silene vulgaris after transplantation into semiarid conditions. J. Hortic. Sci. Biotechnol. 2006, 81, 583–592. [Google Scholar] [CrossRef]
- Franco, J.A.; Arreola, J.; Vicente, M.J.; Martínez-Sánchez, J.J. Nursery irrigation regimes affect the seedling characteristics of Silene vulgaris as they relate to potential performance following transplanting into semi-arid conditions. J. Hortic. Sci. Biotechnol. 2008, 83, 15–22. [Google Scholar] [CrossRef]
- Toscano, S.; Scuderi, D.; Giuffrida, F.; Romano, D. Responses of Mediterranean ornamental shrubs to drought stress and recovery. HortScience 2014, 178, 145–153. [Google Scholar] [CrossRef]
- Tribulato, A.; Toscano, S.; Di Lorenzo, V.; Romano, D. Effects of water stress on gas exchange, water relations and leaf structure in two ornamental shrubs in the Mediterranean area. Agronomy 2019, 9, 381. [Google Scholar] [CrossRef] [Green Version]
- Davies, M.J.; Harrison-Murray, R.; Atkinson, C.J.; Grant, O.M. Application of deficit irrigation to container-grown hardy ornamental nursery stock via overhead irrigation, compared to drip irrigation. Agric. Water Manag. 2016, 163, 244–254. [Google Scholar] [CrossRef] [Green Version]
- Pollastrini, M.; Desotgiu, R.; Camin, F.; Ziller, L.; Marzuoli, R.; Gerosa, G.; Bussotti, F. Intra-annual pattern of photosynthesis, growth and stable isotope partitioning in a poplar clone subjected to ozone and water stress. Water Air Soil Pollut. 2013, 224, 1761. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, J.J.; Morales, M.A.; Ferrández, T.; Sánchez-Blanco, M.J. Effects of water and salt stresses on growth, water relations and gas exchange in Rosmarinus officinalis. J. Hortic. Sci. Biotechnol. 2006, 81, 845–853. [Google Scholar] [CrossRef]
- Álvarez, S.; Castillo, M.; Acosta, J.; Navarro, A.; Sánchez-Blanco, M. Photosynthetic response, biomass distribution and water status changes in Rhamnus alaternus plants during drought. Acta Hortic. 2012, 937, 853–860. [Google Scholar] [CrossRef]
- Franco, J.A.; Martínez-Sánchez, J.J.; Fernández, J.A.; Bañón, S. Selection and nursery production of ornamental plants for landscaping and xerogardening in semiarid environments. J. Hortic. Sci. Biotechnol. 2006, 81, 3–17. [Google Scholar] [CrossRef]
- Fernández, J.A.; Balenzategui, L.; Bañón, S.; Franco, J.A. Induction of drought tolerance by paclobutrazol and irrigation deficit in Phillyrea angustifolia during the nursery period. Sci. Hortic. 2006, 107, 277–283. [Google Scholar] [CrossRef]
- Gómez-Bellot, M.J.; Álvarez, S.; Castillo, M.; Bañón, S.; Ortuño, M.F.; Sánchez-Blanco, M.J. Water relations, nutrient content and developmental responses of Euonymus plants irrigated with water of different degrees of salinity and quality. J. Plant Res. 2013, 126, 567–576. [Google Scholar] [CrossRef]
- Álvarez, S.; Sánchez-Blanco, M.J. Long-term effect of salinity on plant quality, water relations, photosynthetic parameters and ion distribution in Callistemon citrinus. Plant Biol. 2014, 16, 757–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassaniti, C.; Romano, D.; Flowers, T.J. The Response of Ornamental Plants to Saline Irrigation Water, Irrigation—Water Management, Pollution and Alternative Strategies; Garcia-Garizabal, I., Ed.; InTech: London, UK, 2012; pp. 131–158. [Google Scholar]
- Cirillo, C.; Rouphael, Y.; Caputo, R.; Raimondi, G.; Sifola, M.I.; De Pascale, S. Effects of high salinity and the exogenous of an osmolyte on growth, photosynthesis and mineral composition in two ornamental shrubs. J. Hortic. Sci. Biotechnol. 2016, 91, 14–22. [Google Scholar] [CrossRef]
- Franco, J.A.; Cros, V.; Vicente, M.J.; Martínez-Sánchez, J.J. Effects of salinity on the germination, growth, and nitrate contents of purslane (Portulaca oleracea) cultivated under different climatic conditions. J. Hortic. Sci. Biotechnol. 2011, 86, 1–6. [Google Scholar] [CrossRef]
- Álvarez, S.; Navarro, A.; Nicolas, E.; Sánchez-Blanco, M.J. Transpiration, photosynthetic responses, tissue water relations and dry mass partitioning in Callistemon plants during drought conditions. Sci. Hortic. 2011, 129, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Fort, F.; Volaire, F.; Guilioni, L.; Barkaoui, K.; Navas, M.L.; Roumet, C. Root traits are related to plant water-use among rangeland Mediterranean species. Funct. Ecol. 2017, 31, 1700–1709. [Google Scholar] [CrossRef]
- Jaffe, M.; Takahashi, H.; Biro, R. A pea mutant for the study of hydrotropism in roots. Science 1985, 230, 445–447. [Google Scholar] [CrossRef]
- Takahashi, H. Hydrotropism: The current state of our knowledge. J. Plant Res. 1997, 110, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Yamazaki, Y.; Kobayashi, A.; Higashitani, A.; Takahashi, H. Hydrotropism interacts with gravitropism by degrading amyloplasts in seedling roots of Arabidopsis and radish. Plant Physiol. 2003, 132, 805–810. [Google Scholar] [CrossRef] [Green Version]
- Koike, T.; Kitao, M.; Quoreshi, A.M.; Matsuura, Y. Growth characteristics of root-shoot relations of three birch seedlings raised under different water regimes. Plant Soil 2003, 255, 303–310. [Google Scholar] [CrossRef]
- Croser, C.; Renault, S.; Franklin, J.; Zwiazek, J. The effect of salinity on the emergence and seedling growth of Picea mariana, Picea glauca, and Pinus banksiana. Environ. Pollut. 2001, 115, 9–16. [Google Scholar] [CrossRef]
- Cameron, R.W.F.; Harrison-Murray, R.S.; Atkinson, C.J.; Judd, H.L. Regulated deficit irrigation: A means to control growth in woody ornamentals. J. Hortic. Sci. Biotechnol. 2006, 81, 435–443. [Google Scholar] [CrossRef]
- Johnson, W.; Jackson, L.; Ochoa, O.; Van Wijk, R.; Peleman, J.; Clair, D.S.; Michelmore, R. Lettuce, a shallow-rooted crop, and Lactuca serriola, its wild progenitor, differ at QTL determining root architecture and deep soil water exploitation. Theor. Appl. Genet. 2000, 101, 1066–1073. [Google Scholar] [CrossRef]
- Price, A.H.; Steele, K.; Moore, B.; Jones, R. Upland rice grown in soil-filled chambers and exposed to contrasting water-deficit regimes: II. Mapping quantitative trait loci for root morphology and distribution. Field Crops Res. 2002, 76, 25–43. [Google Scholar] [CrossRef]
- Forster, B.; Thomas, W.; Chloupek, O. Genetic controls of barley root systems and their associations with plant performance. Aspect Appl. Biol. 2005, 73, 199–204. [Google Scholar]
- Hammer, G.L.; Dong, Z.; McLean, G.; Doherty, A.; Messina, C.; Schussler, J.; Zinselmeier, C.; Paszkiewicz, S.; Cooper, M. Can changes in canopy and/or root system architecture explain historical maize yield trends in the U.S. corn belt? Crop Sci. 2009, 49, 299–312. [Google Scholar] [CrossRef]
- Sankar, B.; Jaleel, C.A.; Manivannan, P.; Kishorekumar, A.; Somasundaram, R.; Panneerselvam, R. Drought induced biochemical modifications and proline metabolism in Abelmoschus esculentus (L.) Moench. Acta Bot. Croat. 2007, 66, 43–56. [Google Scholar]
- Sundaravalli, M.V.; Paliwal, K.; Ruckmani, A. Effect of water stress on photosynthesis, protein content and nitrate reductase activity of Albizzia seedlings. J. Plant Biol. 2005, 32, 13–17. [Google Scholar]
- Li, C.; Berninger, F.; Koskela, J.; Sonninen, E. Drought responses of Eucalyptus microtheca F. Muell. Provenances depend on seasonality of rainfall in their place of origin. Aust. J. Plant Physiol. 2000, 27, 231–238. [Google Scholar] [CrossRef]
- Chyliński, W.K.; Łukaszewska, A.J.; Kutnik, K. Drought response of two bedding plants. Acta Physiol. Plant. 2007, 29, 399–406. [Google Scholar] [CrossRef]
- Shober, A.L.; Moore, K.A.; Wiese, C.; Scheiber, S.M.; Gilman, E.F.; Paz, M.; Brennan, M.M.; Vyapari, S. Post-transplant irrigation frequency affects growth of container grown sweet viburnum in three hardiness zones. HortScience 2009, 44, 1683–1687. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Calderón, L.; Ibarra-Cortés, M.E.; Zepeda-Jazo, I. Root Development and Abiotic Stress Adaptation. In Abiotic Stress—Plant Responses and Applications in Agriculture; Vahdati, K., Leslie, C., Eds.; IntechOpen: London, UK, 2013; pp. 135–167. [Google Scholar]
- He, X.; Xu, L.; Pan, C.; Gong, C.; Wang, Y.; Liu, X.; Yu, Y. Drought resistance of Camellia oleifera under drought stress: Changes in physiology and growth characteristics. PLoS ONE 2020, 15, e0235795. [Google Scholar] [CrossRef] [PubMed]
- Shukla, N.; Awasthi, R.P.; Rawat, L.; Kumar, J. Biochemical and physiological responses of rice (Oryza sativa L.) as influenced by Trichoderma harzianum under drought stress. Plant Physiol. Biochem. 2012, 54, 78–88. [Google Scholar] [CrossRef]
- Shan, L.; Yang, C.; Li, Y.; Duan, Y.; Geng, D.; Li, Z.; Zhang, R.; Duan, G.; Bacильeвич, Ж.A. Effects of drought stress on root physiological traits and root biomass allocation of Reaumuria soongorica. Acta Ecol. Sin. 2015, 35, 155–159. [Google Scholar] [CrossRef]
- Franco, J.A.; Cros, V.; Bañón, S.; Martínez-Sánchez, J.J. Nursery irrigation regimes and establishment irrigation affect the postplanting growth of Limonium cossonianum in semiarid conditions. Israel J. Plant Sci. 2002, 50, 25–32. [Google Scholar] [CrossRef]
- Kirk, G.J.D.; Solivas, J.L.; Alberto, M.C. Effects of flooding and redox conditions on solute diffusion in soil. Eur. J. Soil Sci. 2003, 54, 617–624. [Google Scholar] [CrossRef]
- Dat, J.; Capelli, N.; Folzer, H.; Bourgeade, P.; Badot, P.-M. Sensing and signaling during plant flooding. Plant Physiol. Biochem. 2004, 42, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Das, K.K.; Panda, D.; Sarkar, R.K.; Reddy, J.N.; Ismail, A.M. Submergence tolerance in relation to variable floodwater conditions in rice. Environ. Exp. Bot. 2009, 66, 425–434. [Google Scholar] [CrossRef]
- Shiono, K.; Takahashi, H.; Colmer, T.D.; Nakazono, M. Role of ethylene in acclimations to promote oxygen transport in roots of plants in waterlogged soils. Plant Sci. 2008, 175, 52–58. [Google Scholar] [CrossRef]
- Vartapetian, B.B.; Jackson, M.B. Plant adaptation to anaerobic stress. Ann. Bot. 1997, 79, 3–20. [Google Scholar] [CrossRef]
- Yin, D.; Chen, S.; Chen, F.; Guan, Z.; Fang, W. Morphological and physiological responses of two chrysanthemum cultivars differing in their tolerance to waterlogging. Environ. Exp. Bot. 2009, 67, 87–93. [Google Scholar] [CrossRef]
- Bannoud, F.; Bellini, C. Adventitious rooting in Populus species: Update and perspectives. Front. Plant Sci. 2021, 12, 668837. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, S.R. Wetland plant responses to soil flooding. Environ. Exp. Bot. 2001, 46, 299–312. [Google Scholar] [CrossRef]
- Suralta, R.R.; Yamauchi, A. Root growth, aerenchyma development, and oxygen transport in rice genotypes subjected to drought and waterlogging. Environ. Exp. Bot. 2008, 64, 5–82. [Google Scholar] [CrossRef]
- Jackson, M.B.; Drew, M.C. Effects of flooding on growth and metabolism of herbaceous plants. In Flooding and Plant Growth; Kozlowski, T.T., Ed.; Academic Press: Orlando, FL, USA, 1984; pp. 47–128. [Google Scholar]
- Finlayson, C.M. Plant ecology of Australia’s tropical floodplain wetlands: A review. Ann. Bot. 2005, 96, 541–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalcanti, F.R.; Lima, J.P.M.S.; Ferreira-Silva, S.L.; Viégas, R.A.; Silveira, J.A.G. Roots and leaves display contrasting oxidative response during salt stress and recovery in cowpea. J. Plant Physiol. 2007, 164, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Toscano, S.; Romano, D.; Massa, D.; Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulant applications in low input horticultural cultivation systems. Italus Hortus 2018, 25, 27–36. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Ferrini, F.; Nicese, F.P. Response of English oak (Quercus robur L.) trees to biostimulants application in the urban environment. Arboric. J. 2002, 28, 70–75. [Google Scholar]
- Wise, K.; Gill, H.; Selby-Pham, J. Willow bark extract and the biostimulant complex Root Nectar® increase propagation efficiency in chrysanthemum and lavender cuttings. Sci. Hortic. 2020, 263, 109108. [Google Scholar] [CrossRef]
- Ruzzi, M.; Aroca, R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- do Prado, D.Z.; Oliveira, S.L.; Okino-Delgado, C.H.; Auer, S.; Ludwig-Müller, J.; da Silva, M.R.; da Costa Fernandes, C.; Carbonari, C.A.; Zambuzzi, W.F.; Fleuri, L.F. Aspergillus flavipes as a novel biostimulant for rooting-enhancement of Eucalyptus. J. Clean. Prod. 2019, 234, 681–689. [Google Scholar] [CrossRef]
- Franco, J.A.; Cros, V.; Bañón, S.; González, A.; Abrisqueta, J.M. Effects of nursery irrigation on postplanting root dynamics of Lotus creticus in semiarid field conditions. HortScience 2002, 37, 525–528. [Google Scholar] [CrossRef]
- Green, J.J.; Baddeley, J.A.; Cortina, J.; Watson, C.A. Root development in the Mediterranean shrub Pistacia lentiscus as affected by nursery treatments. J. Arid Environ. 2005, 61, 1–12. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clouston, A.M.; Hill, R.A.; Minchin, R.; Braithwaite, M.; Stewart, A. A bioassay screening Trichoderma isolates for enhancement of root development in Impatiens walleriana cuttings. N. Z. Plant Prot. 2010, 63, 33–38. [Google Scholar] [CrossRef]
- Fardous, M.A.; Hegazi, M.A.; El-Bably, S.Z.; Hana, M.R. Response of Mexican Petunia (Ruellia brittoniana L.) to salinity, organic and bio-materials. Appl. Ecol. Environ. Res. 2020, 18, 5789–5802. [Google Scholar] [CrossRef]
- Gomes, E.N.; Vieira, L.M.; Tomasi, J.D.C.; Tomazzoli, M.M.; Grunennvaldt, R.L.; Fagundes, C.D.M.; Machado, R.C.B. Brown seaweed extract enhances rooting and roots growth on Passiflora actinia Hook stem cuttings. Ornam. Hortic. 2018, 24, 269–276. [Google Scholar] [CrossRef] [Green Version]
- Ferrante, A.; Trivellini, A.; Vernieri, P.; Piaggesi, A. Application of Actiwave® for improving the rooting of Camellia cuttings. Acta Hortic. 2013, 1009, 213–218. [Google Scholar] [CrossRef]
- Monder, M.J. Rooting and growth of root cuttings of two old rose cultivars ‘Harison’s Yellow’and ‘Poppius’ treated with IBA and biostimulants. Acta Agrobot. 2019, 72, 1774. [Google Scholar] [CrossRef]
- Monder, M.J.; WoliŃSki, K.; Niedzielski, M. The propagation of Rosa gallica ‘Tuscany Superb’ by root cuttings with the use of IBA and biostimulants. Not. Bot. Horti Agrobot. Cluj Napoca 2019, 47, 691–698. [Google Scholar] [CrossRef] [Green Version]
- Hurrell, J.A. Ornamental plants. In Introduction to Ethnobiology; Albuquerque, U.P., Alves, R.R.N., Eds.; Springer: Cham, Switzerland, 2016; pp. 171–176. [Google Scholar]
- Savé, R. What is stress and how to deal with it in ornamental plants? Acta Hort. 2009, 813, 241–254. [Google Scholar] [CrossRef]
- Naylor, L.A.; Viles, H.A.; Carter, N.E.A. Biogeomorphology revisited: Looking towards the future. Geomorphology 2002, 47, 3–14. [Google Scholar] [CrossRef]
- Wainwright, J.; Parsons, A.J.; Schlesinger, W.H. Hydrology–vegetation interactions in areas of discontinuous flow on a semi-arid bajada, Southern New Mexico. J. Arid Environ. 2002, 51, 319–338. [Google Scholar] [CrossRef]
- de Baets, S.; Poesen, J.; Knapen, A.; Barbera, G.G.; Navarro, J.A. Root characteristics of representative Mediterranean plant species and their erosion-reducing potential during concentrated runoff. Plant Soil 2007, 294, 169–183. [Google Scholar] [CrossRef]
- Francia, J.R.; Durán, Z.V.H.; Martínez, R.A. Environmental impact from mountainous olive orchards under different soil-management systems (SE Spain). Sci. Total Environ. 2006, 358, 46–60. [Google Scholar] [CrossRef]
- Gyssels, G.; Poesen, J.; Bochet, E.; Li, Y. Impact of plant roots on the resistance of soils to erosion by water: A review. Prog. Phys. Geogr. 2005, 29, 189–217. [Google Scholar] [CrossRef] [Green Version]
- De Baets, S.; Poesen, J. Empirical models for predicting the erosion-reducing effects of plant roots during concentrated flow erosion. Geomorphology 2010, 118, 425–432. [Google Scholar] [CrossRef]
- Pohl, M.; Alig, D.; Körner, C.; Rixen, C. Higher plant diversity enhances soil stability in disturbed alpine ecosystems. Plant Soil 2009, 324, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Gao, G.; Wang, S.; Jiao, L.; Wu, X.; Fu, B. The effects of vegetation on runoff and soil loss: Multidimensional structure analysis and scale characteristics. J. Geogr. Sci. 2018, 28, 59–78. [Google Scholar] [CrossRef] [Green Version]
- Greenway, D.R. Vegetation and slope stability. In Slope Stability: Geotechnical Engineering and Geomorphology; Anderson, M.G., Richards, K.S., Eds.; John Wiley and Sons: Chichester, UK, 1987; pp. 187–230. [Google Scholar]
- Francis, C.F.; Thornes, J.B. Runoff hydrographs from three Mediterranean vegetation cover types. In Vegetation and Erosion, Processes and Environments; Thornes, J.B., Ed.; Wiley: Chichester, UK, 1990; pp. 363–384. [Google Scholar]
- Romero, D.M.A.; Cammeraat, L.H.; Vacca, A.; Kosmas, C. Soil erosion at three experimental sites in the Mediterranean. Earth Surf. Proc. Landf. 1999, 24, 1243–1256. [Google Scholar] [CrossRef]
- Durán Zuazo, V.H.; Francia, M.J.R.; Rodríguez Rodríguez, C.R.; Martínez, R.A.; Cárceles, R.B. Soil erosion and runoff prevention by plant covers in a mountainous area (SE Spain): Implications for sustainable agriculture. Environmentalist 2006, 26, 309–319. [Google Scholar] [CrossRef]
- González, H.J.C.; de Luis, M.; Raventós, J.; Cortina, J.; Sánchez, J.R. Hydrological response of Mediterranean gorse shrubland under extreme rainfall simulation event. Z. Geomorphol. 2004, 48, 293–304. [Google Scholar] [CrossRef]
- de Baets, S.; Poesen, J.; Reubens, B.; Wemans, K.; De Baerdemaeker, J.; Muys, B. Root tensile strength and root distribution of typical Mediterranean plant species and their contribution to soil shear strength. Plant Soil 2008, 305, 207–226. [Google Scholar] [CrossRef]
- de Baets, S.; Poesen, J.; Reubens, B.; Muys, B.; De Baerdemaeker, J.; Meersmans, J. Methodological framework to select plant species for controlling rill and gully erosion: Application to a Mediterranean ecosystem. Earth Surf. Process. Landf. 2009, 34, 1374–1392. [Google Scholar] [CrossRef]
- Bochet, E.; Rubio, J.L.; Poesen, J. Relative efficiency of three representative matorral species in reducing water erosion at the microscale in a semi-arid climate (Valencia, Spain). Geomorphology 1998, 23, 139–150. [Google Scholar] [CrossRef]
- Burylo, M.; Rey, F.; Mathys, N.; Dutoit, T. Plant root traits affecting the resistance of soils to concentrated flow erosion. Earth Surf. Proc. Landf. 2012, 37, 1463–1470. [Google Scholar] [CrossRef]
- Chau, N.L.; Chu, L.M. Fern cover and the importance of plant traits in reducing erosion on steep soil slopes. Catena 2017, 151, 98–106. [Google Scholar] [CrossRef]
- Bautista, S.; Mayor, A.G.; Bourakhouadar, J.; Bellot, J. Plant spatial pattern predicts hillslope runoff and erosion in a semiarid Mediterranean landscape. Ecosystems 2007, 10, 987–998. [Google Scholar] [CrossRef] [Green Version]
- Xiao, P.; Yao, W.; Shen, Z.; Yang, C.; Lyu, X.; Jiao, P. Effects of shrub on runoff and soil loss at loess slopes under simulated rainfall. Chinese Geogr. Sci. 2017, 27, 589–599. [Google Scholar] [CrossRef]
- Atasoy, M. A check list of Mediterranean plants to control erosion in Turkey. Int. J. Appl. Sci. Eng. 2018, 11, 147–152. [Google Scholar] [CrossRef]
- Kervroëdan, L.; Armand, R.; Saunier, M.; Ouvry, J.F.; Faucon, M.P. Plant functional trait effects on runoff to design herbaceous hedges for soil erosion control. Ecol. Eng. 2018, 118, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Hallock, B.G.; Kimmelshue, C.; Rein, S.; Curto, M.; Scharff, M. Overland Flow and Rainfall Simulation Studies on Ornamental Vegetation, Compost, and Jute Netting. In Proceedings of the International Erosion Control Association (IECA), Reno, Nevada, 9–13 February 2009; Available online: https://digitalcommons.calpoly.edu/cgi/viewcontent.cgi?article=1026&context=ersc_fac/ (accessed on 14 May 2021).
- Ruchala, S.L.; Zhang, D.; Mitchell, W.; Jianhau, L. Improving vegetative propagation techniques of sweet fern (Comptonia peregrina). Comb. Proc. Int. Plant Propagators Soc. 2002, 52, 381–387. [Google Scholar]
- Thomas, S.C.L.; Schroeder, W.R. Sea buckthorn (Hippophae rhamnoides L.): A multipurpose plant. HortTechnology 1996, 6, 370–380. [Google Scholar] [CrossRef]
- Andreu, V.; Rubio, J.L.; Cerni, R. Effect of Mediterranean shrub on water erosion control. In Desertification in Developed Countries; Mouat, D.A., Hutchinson, C.F., Eds.; Springer: Dordrecht, The Netherlands, 1995; pp. 5–15. [Google Scholar] [CrossRef]
- Le Houérou, H.N. The role of cacti (Opuntia spp.) in erosion control, land reclamation, rehabilitation and agricultural development in the Mediterranean Basin. J. Arid Environ. 1996, 33, 135–159. [Google Scholar] [CrossRef] [Green Version]
- Aydin, M.; Celik, I.; Berkman, A. Use of Some Natural Plant Species for Erosion Control in Southern Turkey. In Proceedings of the 10th International Soil Conservation Organisation Meeting, West Lafayette, IN, USA, 24–29 May 1999; pp. 452–458. Available online: https://topsoil.nserl.purdue.edu/nserlweb-old/isco99/pdf/ISCOdisc/SustainingTheGlobalFarm/P187-Aydin.pdf (accessed on 14 May 2021).
- Bochet, E.; Poesen, J.; Rubio, J.L. Runoff and soil loss under individual plants of a semi-arid Mediterranean shrubland: Influence of plant morphology and rainfall intensity. Earth Surf. Process. Landf. 2006, 31, 536–549. [Google Scholar] [CrossRef]
- Zegeye, A.D.; Langendoen, E.J.; Tilahun, S.A.; Mekuria, W.; Poesen, J.; Steenhuis, T.S. Root reinforcement to soils provided by common Ethiopian highland plants for gully erosion control. Ecohydrology 2018, 11, e1940. [Google Scholar] [CrossRef]
- Berendse, F.; van Ruijven, J.; Jongejans, E.; Keesstra, S. Loss of plant species diversity reduces soil erosion resistance. Ecosystems 2015, 18, 881–888. [Google Scholar] [CrossRef]
- McDonald, M.B.; Kwong, F.Y. Introduction to flower seeds and the flower seed industry. In Flower Seeds: Biology and Technology; McDonald, M.B., Kwong, F.Y., Eds.; CABI Publishing: Wallingford, UK, 2005; pp. 1–5. [Google Scholar] [CrossRef]
- Bretzel, F.; Vannucchi, F.; Romano, D.; Malorgio, F.; Benvenuti, S.; Pezzarossa, B. Wildflowers: From conserving biodiversity to urban greening—A review. Urban For. Urban Green. 2016, 20, 428–436. [Google Scholar] [CrossRef]
- Tinsley, M.J.; Simmons, M.T.; Windhager, S. The establishment success of native versus non-native herbaceous seed mixes on a revegetated roadside in Central Texas. Ecol. Eng. 2006, 26, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Karim, M.N.; Mallik, A.U. Roadside revegetation by native plants: I. Roadside microhabitats, floristic zonation and species traits. Ecol. Eng. 2008, 32, 222–237. [Google Scholar] [CrossRef]
- García-Fayos, P.; Bochet, E. Indication of antagonistic interaction between climate change and erosion on plant species richness and soil properties in semiarid Mediterranean ecosystems. Glob. Chang. Biol. 2009, 15, 306–318. [Google Scholar] [CrossRef]
- Fattet, M.; Fu, Y.; Ghestem, M.; Ma, W.; Foulonneau, M.; Nespoulous, J.; Le Bissonnais, Y.; Stokes, A. Effects of vegetation type on soil resistance to erosion: Relationship between aggregate stability and shear strength. Catena 2011, 87, 60–69. [Google Scholar] [CrossRef]
| Species | Family | Plant Habitus | References |
|---|---|---|---|
| Amorpha fruticosa L. | Leguminosae | Shrub | [145] |
| Anthyllis cytisoides L. | Leguminosae | Shrub | [128,139,140,146] |
| Artemisia vulgaris L. | Compositae | Herb | [146] |
| Atriplex halimus L. | Amaranthaceae | Shrub | [128,139,140,147] |
| Carpobrotus edulis (L.) N.E.Br. | Aizoaceae | Succulent | [148] |
| Comptonia peregrina (L.) J. M. Coult. | Myricaceae | Shrub | [149] |
| Dorycnium pentaphyllum Scop. | Leguminosae | Shrub | [128,146] |
| Hedera helix L. | Araliaceae | Climber | [148] |
| Hippophae rhamnoides L. | Rhamnaceae | Shrub | [150] |
| Lantana montevidensis (Spreng.) Briq. | Verbenaceae | Shrub | [148] |
| Lavandula lanata L. | Lamiaceae | Shrub | [7] |
| Limonium supinum (Girard) Pignatti | Plumbaginaceae | Herb | [139,140] |
| Lonicera japonica Thunb. ‘Repens’ | Caprifoliaceae | Climber | [148] |
| Medicago arborea L. | Leguminosae | Shrub | [151] |
| Myoporum parvifolium R. Br. ‘Prostratus’ | Scrophulariaceae | Shrub | [148] |
| Nephrolepis auriculata (L.) Trimen | Nephrolepidaceae | Fern | [143] |
| Nerium oleander L. | Apocynaceae | Shrub | [128,140,146] |
| Opuntia ficus-indica (L.) Miller f. amyclaea and f. elongata | Cactaceae | Succulent | [152] |
| Origanum bastetanum L. | Lamiaceae | Herb | [7] |
| Origanum vulgare L. | Lamiaceae | Herb | [147] |
| Psolarea bituminosa L. | Leguminosae | Herb | [151] |
| Putoria calabrica (L.) DC. | Rubiaceae | Shrublet | [153] |
| Retama shaerocarpa (L.) Boiss | Leguminosae | Shrub | [128,139,140,146] |
| Robinia pseudoacacia L. | Leguminosae | Tree | [142] |
| Rosa abyssinica R. Br. ex Lindl. | Rosaceae | Shrub | [153] |
| Rosmarinus officinalis L. | Lamiaceae | Shrub | [128,140,141,146,153,154] |
| Rosmarinus officinalis L. ‘Prostratus’ | Lamiaceae | Shrub | [148] |
| Salsola genistoides Juss. ex Poir. | Amaranthaceae | Shrub | [128,139,140,146] |
| Salvia lavandulifolia Vahl | Lamiaceae | Shrub | [7] |
| Santolina rosmarinifolia L. | Compositae | Shrub | [7] |
| Senecio jacobaea L. | Compositae | Herb | [147] |
| Tamarix canariensis Willd. | Tamaricaceae | Tree | [128,139,140,146] |
| Tanacetum vulgare L. | Compositae | Herb | [147] |
| Tephrosia vogelii Hook. f. | Leguminosae | Tree | [155] |
| Vinca major L. | Apocynaceae | Herb | [148] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francini, A.; Toscano, S.; Romano, D.; Ferrini, F.; Ferrante, A. Biological Contribution of Ornamental Plants for Improving Slope Stability along Urban and Suburban Areas. Horticulturae 2021, 7, 310. https://doi.org/10.3390/horticulturae7090310
Francini A, Toscano S, Romano D, Ferrini F, Ferrante A. Biological Contribution of Ornamental Plants for Improving Slope Stability along Urban and Suburban Areas. Horticulturae. 2021; 7(9):310. https://doi.org/10.3390/horticulturae7090310
Chicago/Turabian StyleFrancini, Alessandra, Stefania Toscano, Daniela Romano, Francesco Ferrini, and Antonio Ferrante. 2021. "Biological Contribution of Ornamental Plants for Improving Slope Stability along Urban and Suburban Areas" Horticulturae 7, no. 9: 310. https://doi.org/10.3390/horticulturae7090310
APA StyleFrancini, A., Toscano, S., Romano, D., Ferrini, F., & Ferrante, A. (2021). Biological Contribution of Ornamental Plants for Improving Slope Stability along Urban and Suburban Areas. Horticulturae, 7(9), 310. https://doi.org/10.3390/horticulturae7090310










