‘Garnem’ and Myrobalan ‘P.2175’: Two Different Drought Responses and Their Implications in Drought Tolerance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Stress Conditions
2.2. Plant Water Status Evaluation
2.3. Abscisic Acid Determination
2.4. RNA Extraction and Gene Expression Analysis
2.5. Statistical Analyses
3. Results and Discussion
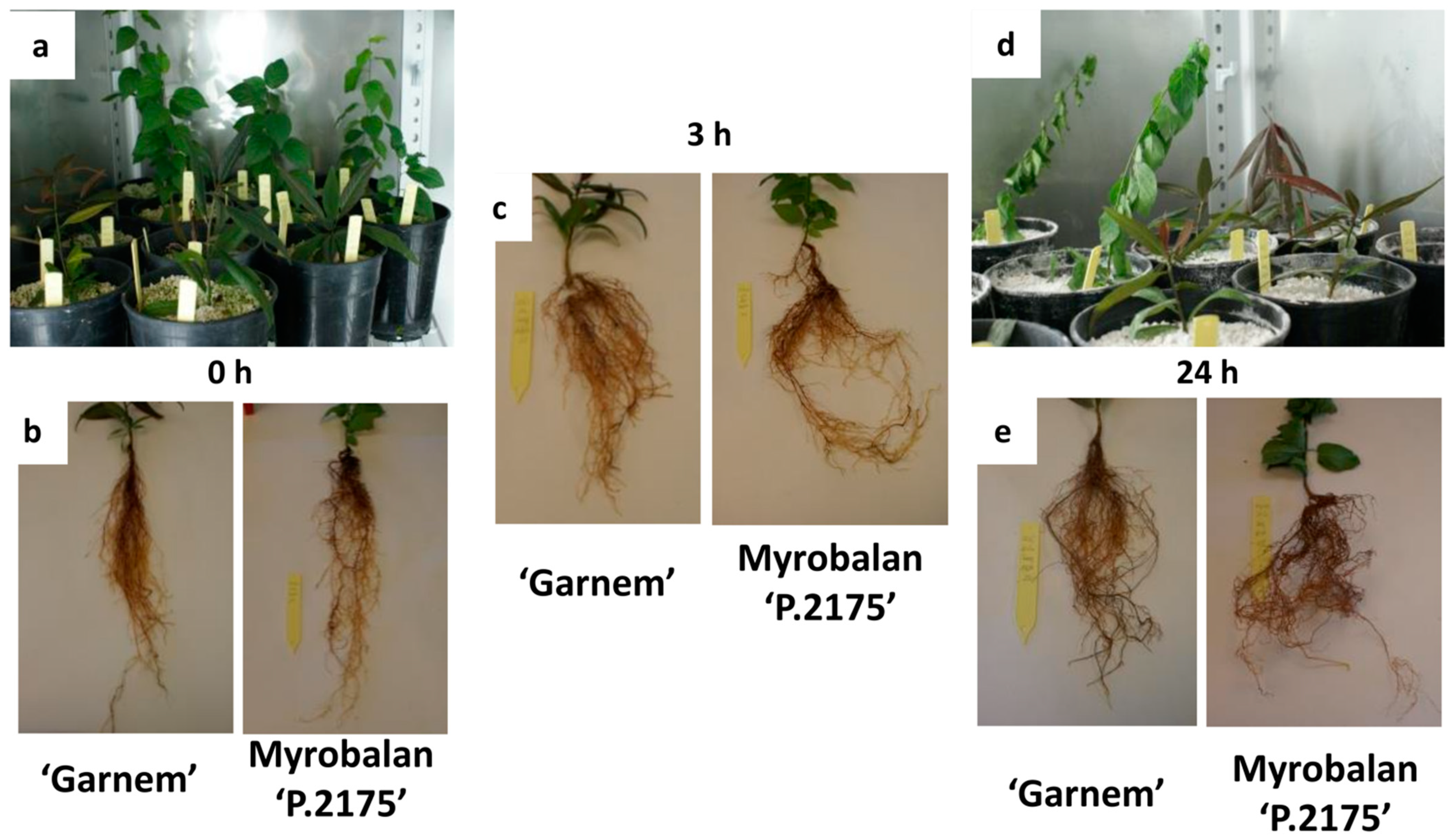
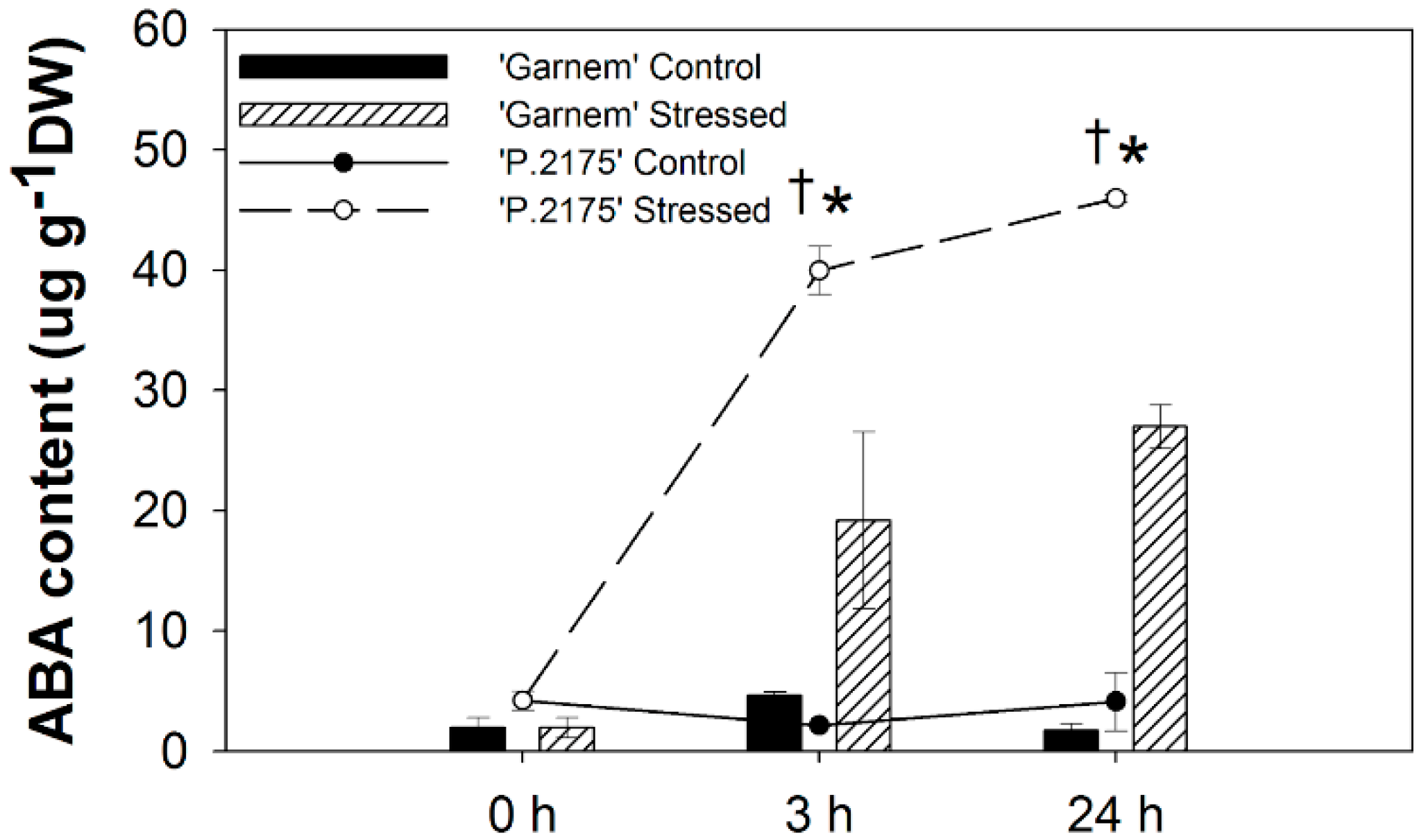
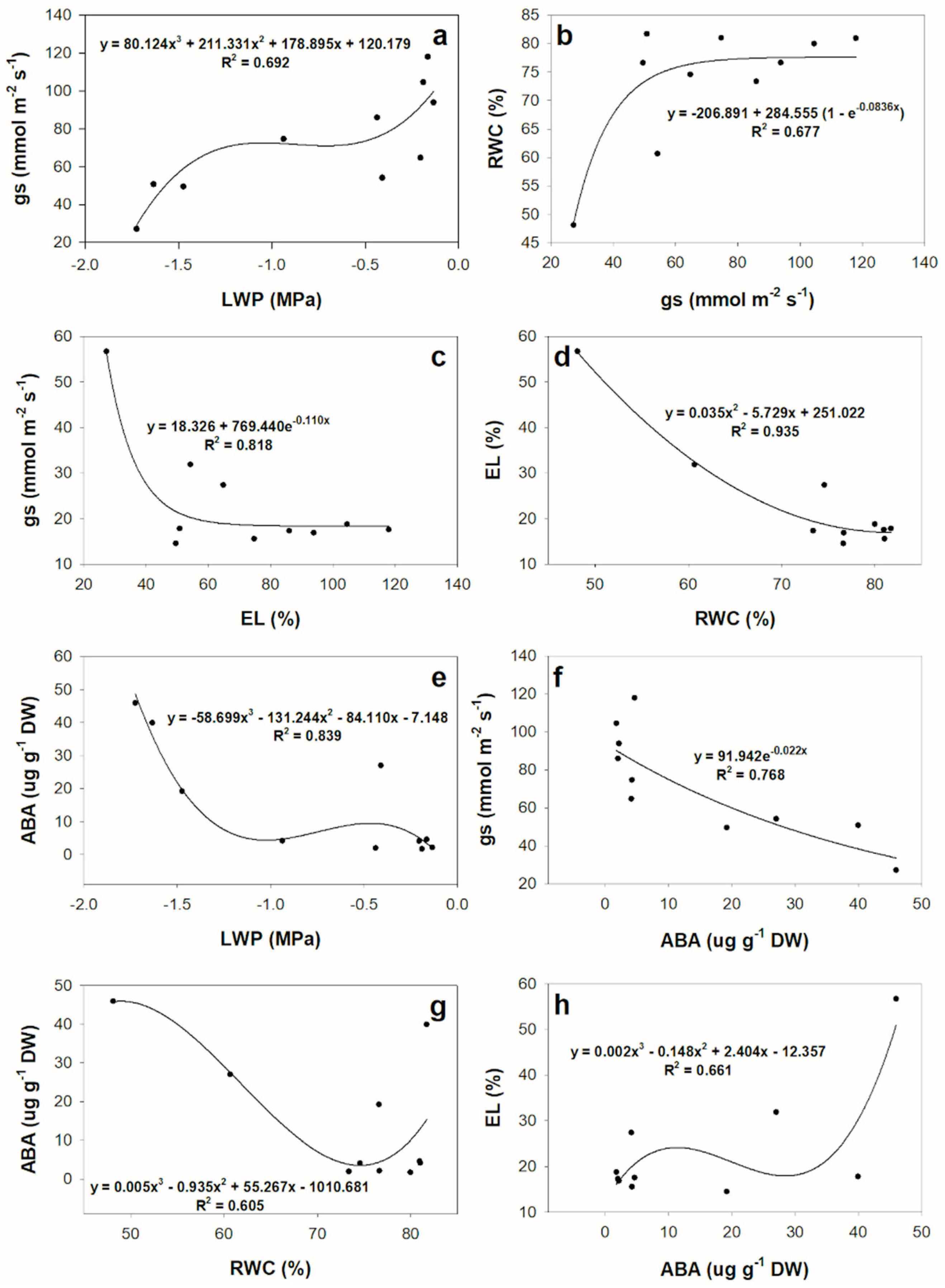
3.1. Physiological and Biochemical Changes in Response to Drought at Short-Term
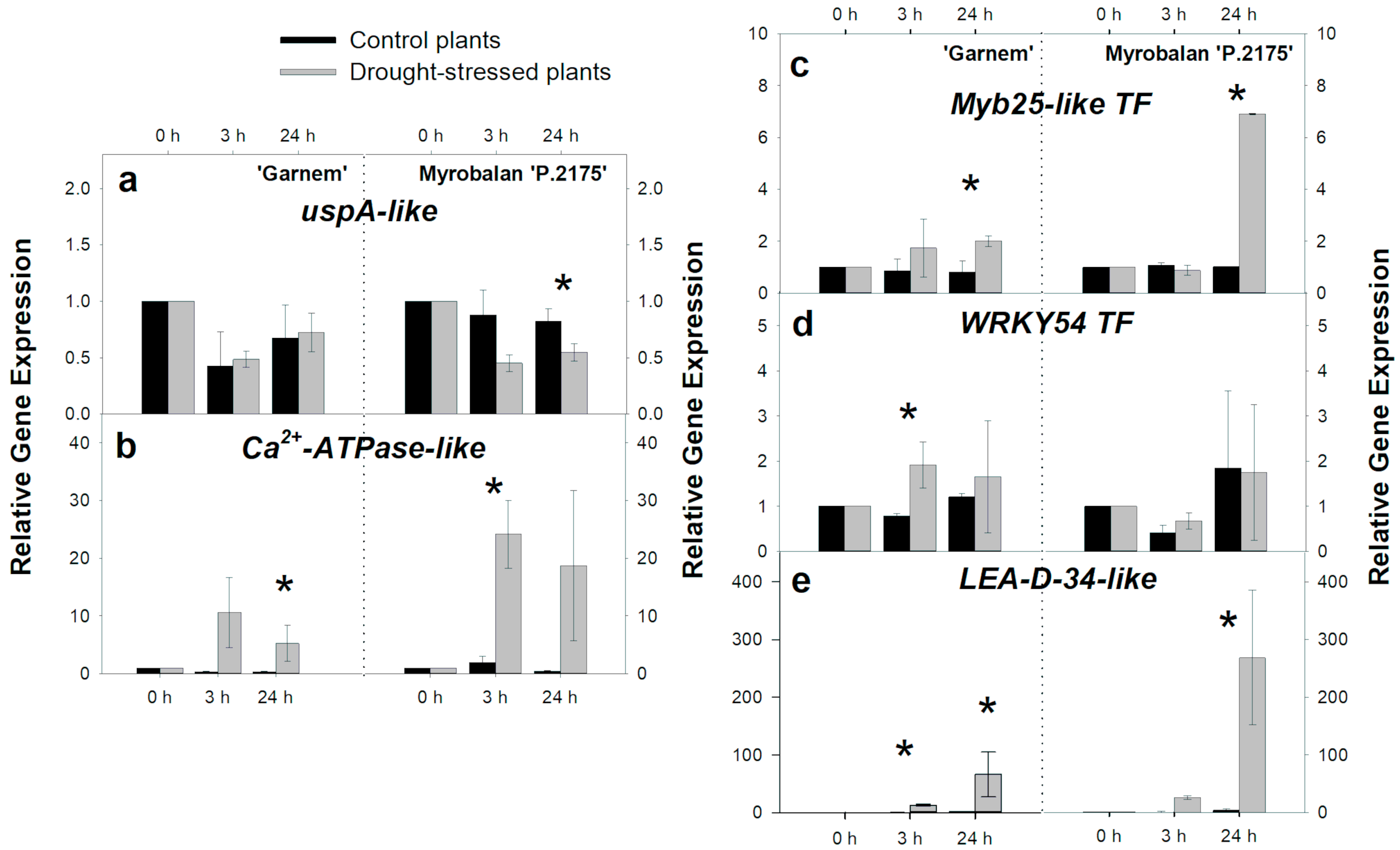
3.2. Gene Regulation during Short-Term-Drought Response
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rehder, A. A Manual of Cultivated Trees and Shrubs Hardy in North America; The MacMillan Company: New York, NY, USA, 1940. [Google Scholar]
- Layne, R.E.C. Peach Rootstocks. In Rootstocks for Fruit Crops; Rom, R.C., Carlson, R.F., Eds.; Wiley: New York, NY, USA, 1987; pp. 185–216. [Google Scholar]
- Felipe, A.J. ‘Felinem’, ‘Garnem’, and ‘Monegro’ almond × peach hybrid rootstocks. HortScience 2009, 44, 196–197. [Google Scholar] [CrossRef] [Green Version]
- Rubio-Cabetas, M.J.; Felipe, A.J.; Reighard, G.L. Rootstock Development. In Almonds. Botany, Production and Uses; Rafel Socias i Company, Gradziel, T.M., Eds.; CABI: Wallingford, UK, 2017; pp. 209–227. ISBN 9781845933869. [Google Scholar]
- IPCC. Summary for Policymakers. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Effors to Erradicate Poverty; Masson-Delmotte, V., Zhai, P., Ho, P., Roberts, D., Skea, J., Shukla, P., Pirani, A., Moufouma-Okia, W.C.P., Pidcock, R., Connors, S., et al., Eds.; World Meteorological Organization: Geneva, Switzerland, 2018; ISBN 9788578110796. [Google Scholar]
- Tejedor, E.; de Luis, M.; Cuadrat, J.M.; Esper, J.; Saz, M.Á. Tree-ring-based drought reconstruction in the Iberian Range (east of Spain) since 1694. Int. J. Biometeorol. 2016, 60, 361–372. [Google Scholar] [CrossRef] [Green Version]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Belin, C.; Thomine, S.; Schroeder, J.I. Water Balance and the Regulation of Stomatal Movements. In Abiotic Stress Adaptation in Plants: Physiological, Molecular and Genomic Foundation; Pareek, A., Sopory, S.K., Bohnert, H.J., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 283–305. ISBN 978-90-481-3111-2. [Google Scholar]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lind, C.; Dreyer, I.; López-Sanjurjo, E.J.; von Meyer, K.; Ishizaki, K.; Kohchi, T.; Lang, D.; Zhao, Y.; Kreuzer, I.; Al-Rasheid, K.A.S.; et al. Stomatal Guard Cells Co-opted an Ancient ABA-Dependent Desiccation Survival System to Regulate Stomatal Closure. Curr. Biol. 2015, 25, 928–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.-K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Bielsa, B.; Sanz, M.; Rubio-Cabetas, M. Uncovering early response to drought by proteomic, physiological and biochemical changes in the almond × peach rootstock ‘Garnem’. Funct. Plant Biol. 2019, 46, 994–1008. [Google Scholar] [CrossRef] [PubMed]
- Tombesi, S.; Nardini, A.; Frioni, T.; Soccolini, M.; Zadra, C.; Farinelli, D.; Poni, S.; Palliotti, A. Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Sci. Rep. 2015, 5, 12449. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Böhmer, M.; Hu, H.; Nishimura, N.; Schroeder, J.I. Guard Cell Signal Transduction Network: Advances in Understanding Abscisic Acid, CO2, and Ca2+ Signaling. Annu. Rev. Plant Biol. 2010, 61, 561–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, F.; Kuromori, T.; Sato, H.; Shinozaki, K. Regulatory gene networks in drought stress responses and resistance in plants. In Survival Strategies in Extreme Cold and Desiccation. Advances in Experimental Medicine and Biology; Springer: Singapore, 2018; Volume 1081, pp. 189–214. ISBN 9789811312441. [Google Scholar]
- Sauter, A.; Davies, W.; Hartung, W. The long-distance abscisic acid signal in the droughted plant: The fate of the hormone on its way from root to shoot. J. Exp. Bot. 2001, 52, 1991–1997. [Google Scholar] [CrossRef] [Green Version]
- Sharp, R.E.; LeNoble, M.E. ABA, ethylene and the control of shoot and root growth under water stress. J. Exp. Bot. 2002, 53, 33–37. [Google Scholar] [CrossRef]
- Kuromori, T.; Seo, M.; Shinozaki, K. ABA Transport and Plant Water Stress Responses. Trends Plant Sci. 2018, 23, 513–522. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Paul, S.; Basu, S. Cross-talk between abscisic acid-dependent and abscisic acid-independent pathways during abiotic stress. Plant Cell Rep. 2013, 32, 985–1006. [Google Scholar] [CrossRef] [PubMed]
- Arbona, V.; Iglesias, D.J.; Jacas, J.; Primo-Millo, E.; Talon, M.; Gómez-Cadenas, A. Hydrogel substrate amendment alleviates drought effects on young citrus plants. Plant Soil 2005, 270, 73–82. [Google Scholar] [CrossRef]
- Scholander, P.F.; Hammel, H.T.; Hemmingsen, E.A.; Bradstreet, E.D. Hydrostatic Pressure and Osmotic Potential in Leaves of Mangroves and Some Other Plants. Proc. Natl. Acad. Sci. USA 1964, 52, 119–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef] [Green Version]
- Blum, A.; Ebercon, A. Call membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci. 1981, 21, 43–47. [Google Scholar] [CrossRef]
- Gómez-Cadenas, A.; Pozo, O.J.; García-Augustín, P.; Sancho, J. V Direct analysis of abscisic acid in crude plant extracts by liquid chromatography-electrospray/tandem mass spectrometry. Phytochem. Anal. 2002, 13, 228–234. [Google Scholar] [CrossRef]
- Meisel, L.; Fonseca, B.; González, S.; Baeza-Yates, R.; Cambiazo, V.; Campos, R.; González, M.; Orellana, A.; Retamales, J.; Silva, H. A Rapid and Efficient Method for Purifying High Quality Total RNA from Peaches (Prunus persica) for Functional Genomics Analyses. Biol. Res. 2005, 38, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.; Puryear, J.; Cairney, J. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 1993, 11, 113–116. [Google Scholar] [CrossRef]
- Salzman, R.A.; Fujita, T.; Zhu-Salzman, K.; Hasegawa, P.M.; Bressan, R.A. An Improved RNA Isolation Method for Plant Tissues Containing High Levels of Phenolic Compounds or Carbohydrates. Plant Mol. Biol. Rep. 1999, 17, 11–17. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, T. RNA Isolation from Highly Viscous Samples Rich in Polyphenols and Polysaccharides. Plant Mol. Biol. Rep. 2002, 20, 417. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Research 2016, 5, 1554. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, S.; Rabara, R. Abscisic acid—An enigma in the abiotic stress tolerance of crop plants. Plant Gene 2017, 11, 90–98. [Google Scholar] [CrossRef]
- Negin, B.; Moshelion, M. The evolution of the role of ABA in the regulation of water-use efficiency: From biochemical mechanisms to stomatal conductance. Plant Sci. 2016, 251, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Transcriptional Regulatory Networks in Cellular Responses and Tolerance to Dehydration and Cold Stresses. Annu. Rev. Plant Biol. 2006, 57, 781–803. [Google Scholar] [CrossRef] [Green Version]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Bolat, I.; Dikilitas, M.; Ercisli, S.; Ikinci, A.; Tonkaz, T. The effect of water stress on some morphological, physiological, and biochemical characteristics and bud success on apple and quince rootstocks. Sci. World J. 2014, 2014, 769732. [Google Scholar] [CrossRef]
- Christmann, A.; Grill, E.; Huang, J. Hydraulic signals in long-distance signaling. Curr. Opin. Plant Biol. 2013, 16, 293–300. [Google Scholar] [CrossRef]
- Bellvert, J.; Nieto, H.; Pelechá, A.; Jofre-Čekalović, C.; Zazurca, L.; Miarnau, X. Remote Sensing Energy Balance Model for the Assessment of Crop Evapotranspiration and Water Status in an Almond Rootstock Collection. Front. Plant Sci. 2021, 12, 288. [Google Scholar] [CrossRef]
- Spinelli, G.M.; Snyder, R.L.; Sanden, B.L.; Shackel, K.A. Water stress causes stomatal closure but does not reduce canopy evapotranspiration in almond. Agric. Water Manag. 2016, 168, 11–22. [Google Scholar] [CrossRef]
- Bielsa, B.; Hewitt, S.; Reyes-Chin-Wo, S.; Dhingra, A.; Rubio-Cabetas, M.J. Identification of water use efficiency related genes in ‘Garnem’ almond-peach rootstock using time-course transcriptome analysis. PLoS ONE 2018, 13, e205493. [Google Scholar] [CrossRef]
- Isokpehi, R.D.; Simmons, S.S.; Cohly, H.H.P.; Ekunwe, S.I.N.; Begonia, G.B.; Ayensu, W.K. Identification of drought-responsive universal stress proteins in Viridiplantae. Bioinform. Biol. Insights 2011, 5, 41–58. [Google Scholar] [CrossRef] [Green Version]
- Sinha, P.; Pazhamala, L.T.; Singh, V.K.; Saxena, R.K.; Krishnamurthy, L.; Azam, S.; Khan, A.W.; Varshney, R.K. Identification and Validation of Selected Universal Stress Protein Domain Containing Drought-Responsive Genes in Pigeonpea (Cajanus cajan L.). Front. Plant Sci. 2016, 6, 1065. [Google Scholar] [CrossRef]
- Udawat, P.; Jha, R.K.; Sinha, D.; Mishra, A.; Jha, B. Overexpression of a Cytosolic Abiotic Stress Responsive Universal Stress Protein (SbUSP) Mitigates Salt and Osmotic Stress in Transgenic Tobacco Plants. Front. Plant Sci. 2016, 7, 518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhuria, M.; Goel, P.; Kumar, S.; Singh, A.K. The Promoter of AtUSP Is Co-regulated by Phytohormones and Abiotic Stresses in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 1957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Che, S.; Zhang, Y.; Wang, H.; Wei, T.; Yan, G.; Song, W.; Yu, W. Universal stress protein in Malus sieversii confers enhanced drought tolerance. J. Plant Res. 2019, 132, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Cai, C.; Fan, S.; Wang, L.; Zeng, X. Spatial and temporal calcium signaling and its physiological effects in Moso Bamboo under drought stress. Forests 2019, 10, 224. [Google Scholar] [CrossRef] [Green Version]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene Expression and Signal Transduction in Water-Stress Response. Plant Physiol. 1997, 115, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Knight, H. Calcium signaling during abiotic stress in plants. Int. Rev. Cytol. 1999, 195, 269–324. [Google Scholar] [CrossRef]
- Huda, K.M.K.; Yadav, S.; Banu, M.S.A.; Trivedi, D.K.; Tuteja, N. Genome-wide analysis of plant-type II Ca2+ ATPases gene family from rice and Arabidopsis: Potential role in abiotic stresses. Plant Physiol. Biochem. 2013, 65, 32–47. [Google Scholar] [CrossRef]
- Geisler, M.; Frangne, N.; Gomès, E.; Martinoia, E.; Palmgren, M.G. The ACA4 gene of Arabidopsis encodes a vacuolar membrane calcium pump that improves salt tolerance in yeast. Plant Physiol. 2000, 124, 1814–1827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boursiac, Y.; Lee, S.M.; Romanowsky, S.; Blank, R.; Sladek, C.; Chung, W.S.; Harper, J.F. Disruption of the Vacuolar Calcium-ATPases in Arabidopsis Results in the Activation of a Salicylic Acid-Dependent Programmed Cell Death Pathway. Plant Physiol. 2010, 154, 1158–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vendramin, E.; Pea, G.; Dondini, L.; Pacheco, I.; Dettori, M.T.; Gazza, L.; Scalabrin, S.; Strozzi, F.; Tartarini, S.; Bassi, D.; et al. A unique mutation in a MYB gene cosegregates with the nectarine phenotype in peach. PLoS ONE 2014, 9, e90574. [Google Scholar] [CrossRef] [Green Version]
- Machado, A.; Wu, Y.; Yang, Y.; Llewellyn, D.J.; Dennis, E.S. The MYB transcription factor GhMYB25 regulates early fibre and trichome development. Plant J. 2009, 59, 52–62. [Google Scholar] [CrossRef]
- Jung, C.; Jun, S.S.; Sang, W.H.; Yeon, J.K.; Chung, H.K.; Sang, I.S.; Baek, H.N.; Yang, D.C.; Cheong, J.J. Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis. Plant Physiol. 2008, 146, 623–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaradat, M.R.; Feurtado, J.A.; Huang, D.; Lu, Y.; Cutler, A.J. Multiple roles of the transcription factor AtMYBR1/AtMYB44 in ABA signaling, stress responses, and leaf senescence. BMC Plant Biol. 2013, 13, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, P.; Rabara, R.C.; Rushton, P.J. A systems biology perspective on the role of WRKY transcription factors in drought responses in plants. Planta 2014, 239, 255–266. [Google Scholar] [CrossRef]
- Chen, J.; Nolan, T.M.; Ye, H.; Zhang, M.; Tong, H.; Xin, P.; Chu, J.; Chu, C.; Li, Z.; Yina, Y. Arabidopsis WRKY46, WRKY54, and WRKY70 transcription factors are involved in brassinosteroid-regulated plant growth and drought responses. Plant Cell 2017, 29, 1425–1439. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, F.; Xie, Q. Balancing growth and adaptation to stress: Crosstalk between brassinosteroid and abscisic acid signaling. Plant Cell Environ. 2020, 43, 2325–2335. [Google Scholar] [CrossRef] [PubMed]
- Hundertmark, M.; Hincha, D.K. LEA (Late Embryogenesis Abundant) proteins and their encoding genes in Arabidopsis thaliana. BMC Genom. 2008, 9, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, D.; Zhang, Q.; Cheng, T.; Pan, H.; Yang, W.; Sun, L. Genome-wide identification and analysis of late embryogenesis abundant (LEA) genes in Prunus mume. Mol. Biol. Rep. 2013, 40, 1937–1946. [Google Scholar] [CrossRef] [PubMed]
- Magwanga, R.O.; Lu, P.; Kirungu, J.N.; Lu, H.; Wang, X.; Cai, X.; Zhou, Z.; Zhang, Z.; Salih, H.; Wang, K.; et al. Characterization of the late embryogenesis abundant (LEA) proteins family and their role in drought stress tolerance in upland cotton. BMC Genet. 2018, 19, 6. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Cao, J. Late Embryogenesis Abundant (LEA) Gene Family in Maize: Identification, Evolution, and Expression Profiles. Plant Mol. Biol. Rep. 2016, 34, 15–28. [Google Scholar] [CrossRef]

| Gene Name | Forward Sequence (5′→3′) | Reverse Sequence (5′→3′) | Annealing Temperature (°C) | Size (bp) |
|---|---|---|---|---|
| Ca2+-transporting ATPase plasma membrane-type-like | GCAACCTCTGTTCCTGCAAT | ACAGTTGAGGGATGGGTTCA | 60.31 | 120 |
| Myb25 Transcription factor | TGTGCTGTGGAGATGGAAGA | AGCGTTACGGATCATTTTGG | 60.18 | 154 |
| WRKY54 Transcription factor | TTTCCGTCTTCCTCTCATGG | ATGACCTGTGGGCAGTTGTT | 60.31 | 132 |
| Universal stress protein A-like | AGCCTCCCAAAGCTACCAAT | TATCAAGAACCTCCGGATCG | 60.07 | 135 |
| Late embryogenesis abundant protein D-34-like | TCTCGGGTGCTACTGAGAAA | ACGGCAGGAAATCACATCTT | 59.08 | 181 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bielsa, B.; Sanz, M.Á.; Rubio-Cabetas, M.J. ‘Garnem’ and Myrobalan ‘P.2175’: Two Different Drought Responses and Their Implications in Drought Tolerance. Horticulturae 2021, 7, 299. https://doi.org/10.3390/horticulturae7090299
Bielsa B, Sanz MÁ, Rubio-Cabetas MJ. ‘Garnem’ and Myrobalan ‘P.2175’: Two Different Drought Responses and Their Implications in Drought Tolerance. Horticulturae. 2021; 7(9):299. https://doi.org/10.3390/horticulturae7090299
Chicago/Turabian StyleBielsa, Beatriz, María Ángeles Sanz, and María José Rubio-Cabetas. 2021. "‘Garnem’ and Myrobalan ‘P.2175’: Two Different Drought Responses and Their Implications in Drought Tolerance" Horticulturae 7, no. 9: 299. https://doi.org/10.3390/horticulturae7090299
APA StyleBielsa, B., Sanz, M. Á., & Rubio-Cabetas, M. J. (2021). ‘Garnem’ and Myrobalan ‘P.2175’: Two Different Drought Responses and Their Implications in Drought Tolerance. Horticulturae, 7(9), 299. https://doi.org/10.3390/horticulturae7090299







