Growth Response of Ginger (Zingiber officinale), Its Physiological Properties and Soil Enzyme Activities after Biochar Application under Greenhouse Conditions
Abstract
:1. Introduction
2. Materials and Methods
3. Results
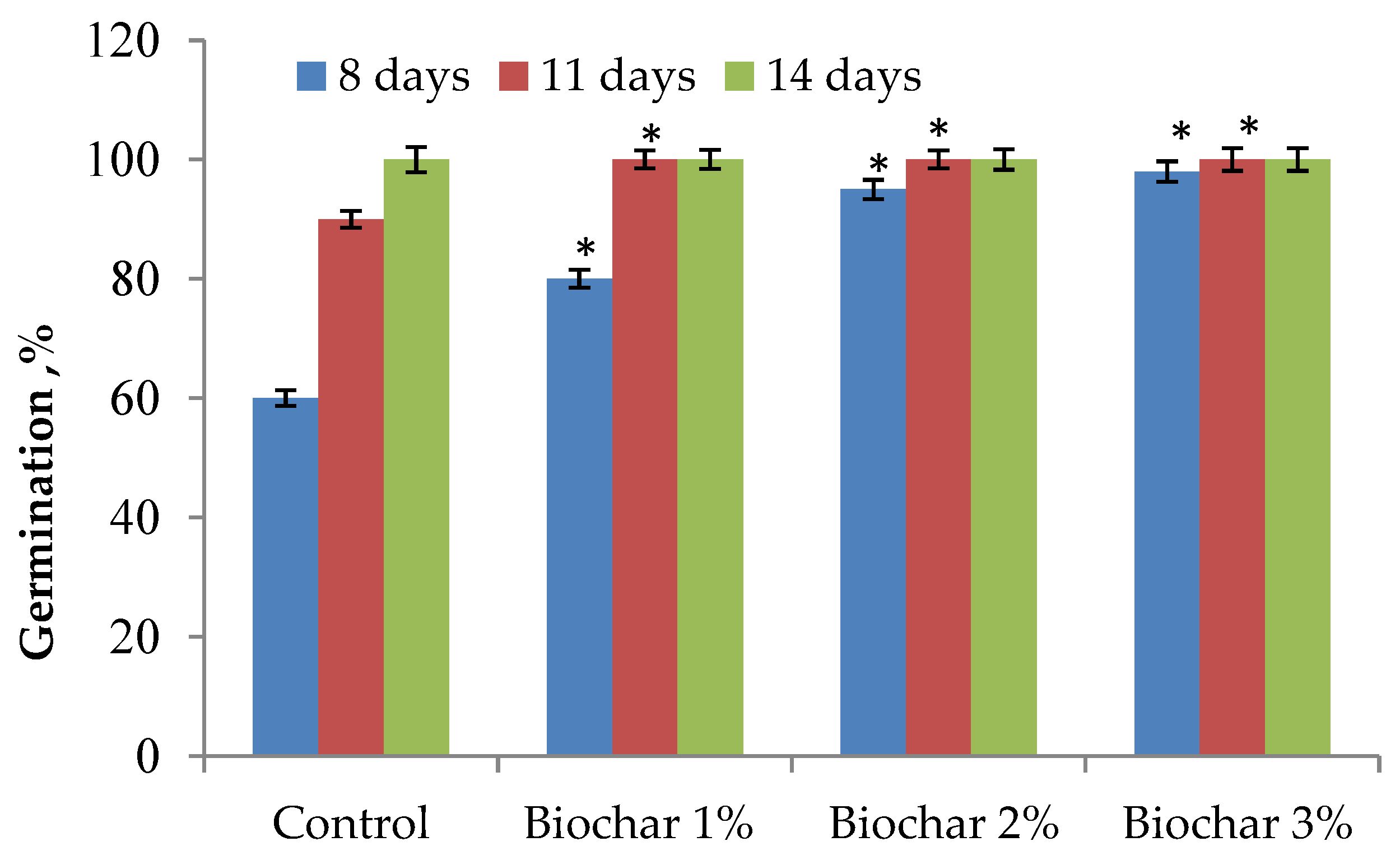
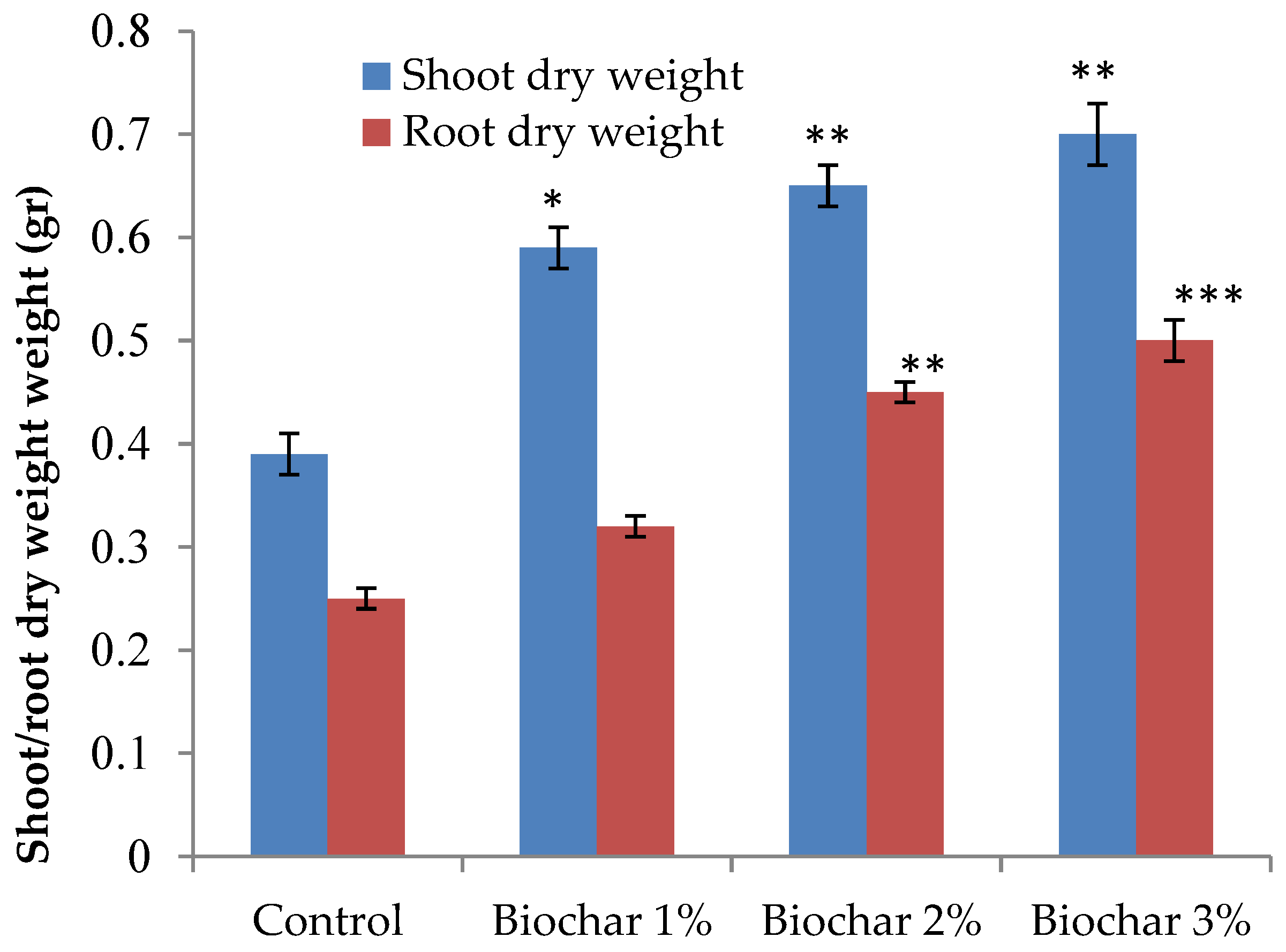
3.1. Plant Growth and Root Morphological Traits of Ginger
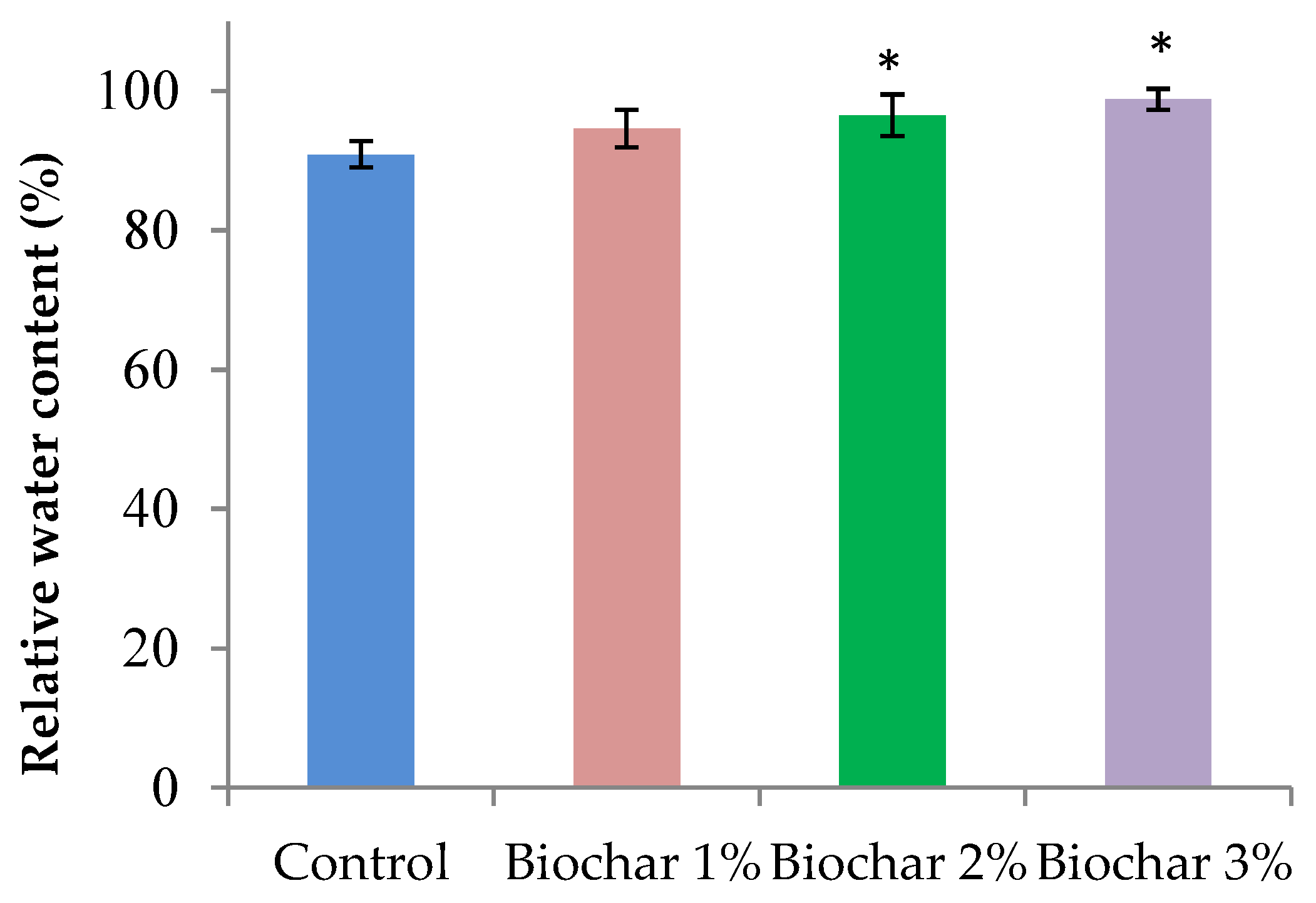
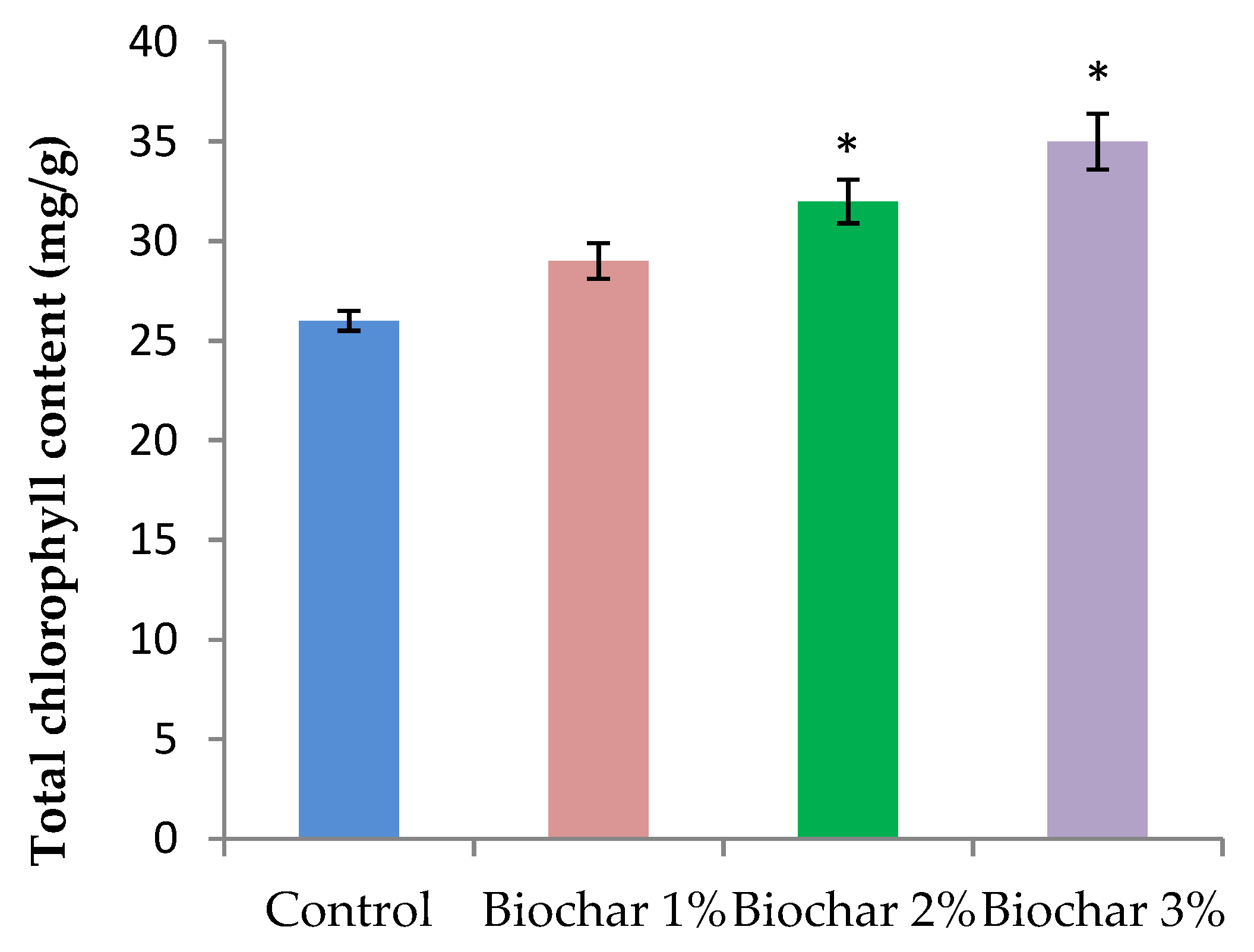
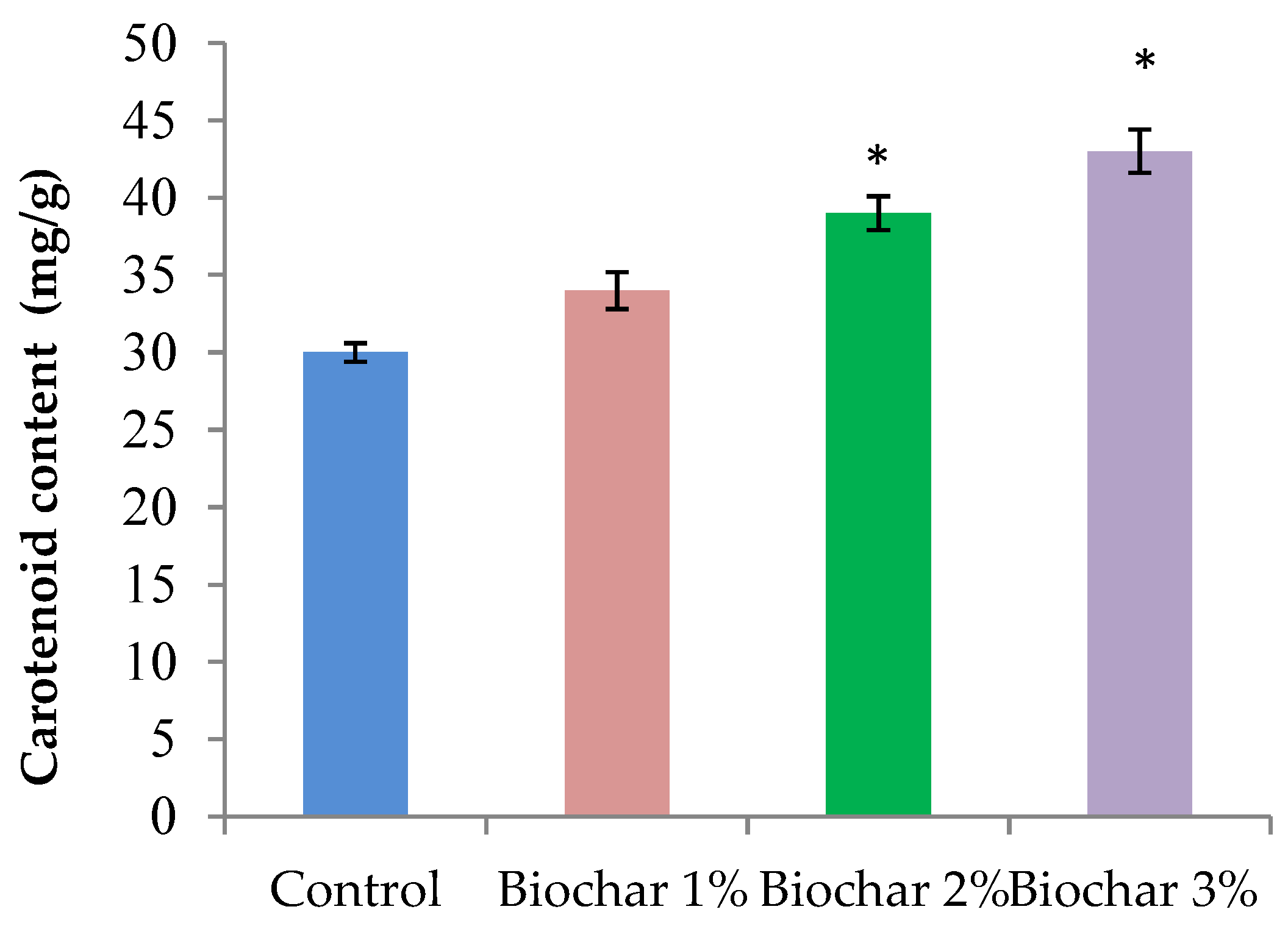
3.2. Physiological Properties of Ginger
3.3. Soil Enzyme Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dedov, V.N.; Tran, V.H.; Duke, C.C.; Connor, M.; Christie, M.J.; Mandadi, S.; Roufogalis, B.D. Gingerols: A novel class of vanilloid receptor (VR1) agonists. Br. J. Pharmacol. 2002, 137, 793–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borrelli, F.; Capasso, R.; Pinto, A.; Izzo, A.A. Inhibitory effect of ginger (Zingiber officinale) on rat ileal motility in vitro. Life Sci. 2004, 74, 2889–2896. [Google Scholar] [CrossRef]
- Newerli-Guz, J.; Pych, A. Antixodiant properties of ginger (Zingiber officinale Roscoe). Zesz. Nauk. AMG 2012, 73, 28–33. [Google Scholar]
- Vimala, S.; Norhanom, A.W.; Yadav, M. Anti-tumor promoter activity in Malaysian ginger rhizome used in traditional medicine. Br. J. Cancer 1999, 80, 110–116. [Google Scholar] [CrossRef]
- Akoachere, J.F.; Ndip, R.N.; Chenwi, E.B.; Ndip, L.M.; Njock, T.E.; Anong, D.N. Antibacterial effect of Zingiber officinale and Garcinia kola on respiratory tract pathogens. East Afr. Med. J. 2002, 79, 588–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzanna, R.; Lindmark, L.; Frondoza, C. Ginger-A herbal medicinal product with broad anti-inflammatory actions. J. Med. Food 2005, 8, 125–132. [Google Scholar] [CrossRef]
- Jabborova, D.; Sayyed, R.Z.; Azimov, A.; Jabbarov, Z.; Matchanov, A.; Enakiev, Y.; Baazeem, A.; Sabagh, A.E.; Danish, S.; Datta, R. Impact of mineral fertilizers on mineral nutrients in the ginger rhizome and on soil enzymes activities and soil properties. Saudi J. Biol. Sci. 2021, in press. [Google Scholar] [CrossRef]
- Lehmann, J.; Skjemstad, J.O.; Sohi, S.; Carter, J.; Barson, M.; Falloon, P.; Coleman, K.; Woodbury, P.; Krull, E. Australian climate-carbon cycle feedback reduced by soil black carbon. Nat. Geosci. 2008, 1, 832–835. [Google Scholar] [CrossRef]
- Stanton, C.Y.; Mach, K.J.; Turner, P.A.; Lalonde, S.J.; Sanchez, D.L.; Field, C.B. Managing cropland and rangeland for climate mitigation: An expert elicitation on soil carbon in California. Clim. Chang. 2018, 147, 633–646. [Google Scholar] [CrossRef] [Green Version]
- Suddick, E.C.; Six, J. An estimation of annual nitrous oxide emissions and soil quality following the amendment of high temperature walnut shell biochar and compost to a small scale vegetable crop rotation. Sci. Total. Environ. 2013, 465, 298–307. [Google Scholar] [CrossRef]
- Scisłowska, M.; Włodarczyk, R.; Kobyłecki, R.; Bis, Z. Biochar to improve the quality and productivity of soils. J. Ecol. Eng. 2015, 16, 31–35. [Google Scholar] [CrossRef]
- Laird, D.A.; Fleming, P.; Davis, D.D.; Horton, R.; Wang, B.; Karlen, D.L. Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 2010, 158, 443–449. [Google Scholar] [CrossRef] [Green Version]
- Major, J.; Rondon, M.; Molina, D.; Riha, S.J.; Lehmann, J. Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol. Plant Soil 2010, 333, 117–128. [Google Scholar] [CrossRef]
- Carter, S.; Shackley, S.; Sohi, S.; Suy, T.B.; Haefele, S. The Impact of Biochar Application on Soil Properties and Plant Growth of Pot Grown Lettuce (Lactuca sativa) and Cabbage (Brassica chinensis). Agronomy 2013, 3, 404–418. [Google Scholar] [CrossRef] [Green Version]
- Agegnehu, G.; Bass, A.M.; Nelson, P.N.; Muirhead, B.; Wright, G.; Bird, M.I. Biochar and biochar-compost as soil amendments: Effects on peanut yield, soil properties and greenhouse gas emissions in tropical North Queensland, Australia. Agric. Ecosyst. Environ. 2015, 213, 72–85. [Google Scholar] [CrossRef]
- Trupiano, D.; Cocozza, C.; Baronti, C.; Amendola, C.; Vaccari, F.P.; Lustrato, G.; Lonardo, S.D.; Fantasma, F.; Tognetti, R.; Scippa, G.S. The Effects of Biochar and Its Combination with Compost on Lettuce (Lactuca sativa L.) Growth, Soil Properties, and Soil Microbial Activity and Abundance. Int. J. Agron. 2017, 2017, 3158207. [Google Scholar] [CrossRef] [Green Version]
- Głodowska, M.; Schwinghamer, T.; Husk, B.; Smith, D. Biochar based inoculants improve soybean growth and nodulation. Agric. Sci. 2017, 8, 1048–1064. [Google Scholar] [CrossRef] [Green Version]
- Sarma, B.; Borkotoki, B.; Narzari, R.; Kataki, R.; Gogoi, N. Organic amendments: Effect on carbon mineralization and crop productivity in acidic soil. J. Clean. Prod. 2017, 152, 157–166. [Google Scholar] [CrossRef]
- Ma, H.; Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D. Effect of biochar and irrigation on soybean-Rhizobium symbiotic performance and soil enzymatic activity in field rhizosphere. Agronomy 2019, 9, 626. [Google Scholar] [CrossRef] [Green Version]
- Zhaoxiang, W.; Huihu, L.; Qiaoli, L.; Changyan, Y.; Yu, F. Application of bio-organic fertilizer, not biochar, in degraded red soil improves soil nutrients and plant growth. Rhizosphere 2020, 16, 100264. [Google Scholar] [CrossRef]
- Qayyum, M.F.; Haider, G.; Raza, M.A.; Abdel, K.S.H.; Mohamed, A.K.S.N.; Rizwan, M.; El-Sheikh, M.A.; Alyemeni, M.N.; Ali, S. Straw-based biochar mediated potassium availability and increased growth and yield of cotton (Gossypium hirsutum L.). J. Saudi Chem. Soc. 2020, 24, 963–973. [Google Scholar] [CrossRef]
- Speratti, A.B.; Johnson, M.S.; Sousa, H.M.; Dalmagro, H.; Couto, E.G. Biochars from local agricultural waste residues contribute to soil quality and plant growth in a Cerrado region (Brazil) Arenosol. GCB Bioenergy 2018, 10, 272–286. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yin, R.; Liu, R. Characterization of biochar from fast pyrolysis and its effect on chemical properties of the tea garden soil. J. Anal. Appl. Pyrol. 2014, 110, 375–381. [Google Scholar] [CrossRef]
- Gaskin, J.W.; Speir, R.A.; Harris, K.; Das, K.C.; Lee, R.D.; Morris, L.A.; Fisher, D.S. Effect of peanut hull and pine chip biochar on soil nutrients, corn nutrient status, and yield. Agron. J. 2010, 102, 623–633. [Google Scholar] [CrossRef] [Green Version]
- Saxena, J.; Rana, G.; Pandey, M.J. Impact of addition of biochar along with Bacillus sp. on growth and yield of French beans. Sci. Hortic. 2013, 162, 351–356. [Google Scholar] [CrossRef]
- Anderson, C.R.; Condron, I.M.; Clough, T.J.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Biochar induced soil microbial community change: Implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 2011, 54, 309–320. [Google Scholar] [CrossRef]
- Jabborova, D.; Wirth, S.; Kannepalli, A.; Narimanov, A.; Desouky, S.; Davranov, K.; Sayyed, R.Z.; El Enshasy, H.; Malek, R.A.; Syed, A.; et al. Co-Inoculation of Rhizobacteria and Biochar Application Improves Growth and Nutrientsin Soybean and Enriches Soil Nutrients and Enzymes. Agronomy 2020, 10, 1142. [Google Scholar] [CrossRef]
- Zhou, H.M.; Zhang, D.X.; Wang, P.; Liu, X.Y.; Cheng, K.; Li, L.; Zheng, J.; Zhang, X.; Zheng, J.; Crowley, D.; et al. Changes in microbial biomass and the metabolic quotient with biochar addition to agricultural soils: A meta-analysis. Agric. Ecosyst. Environ. 2017, 239, 80–89. [Google Scholar] [CrossRef] [Green Version]
- Farrar, M.B.; Wallace, H.M.; Xu, C.Y.; Nguyen, T.T.; Tavakkoli, E.; Joseph, S.; Bai, S.H. Short-term effects of organo-mineral enriched biochar fertiliser on ginger yield and nutrient cycling. J. Soils Sediments 2019, 19, 668–682. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A reexamination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef] [Green Version]
- Hiscox, J.; Israelstam, G. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979, 57, 1332–1334. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenol phosphate for the assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Green, V.S.; Stott, D.E.; Diack, M. Assay for Fluorescein Diacetate Hydrolytic Activity: Optimization for Soil Samples. Soil Biol. Biochem. 2006, 38, 693–701. [Google Scholar] [CrossRef]
- Floch, C.; Gutiérrez, E.A.; Criquet, S. ABTS assay of phenol oxidase activity in soil. J. Microbiol. Methods 2007, 71, 319–324. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis: Microbiological and Biochemical Properties; Weaver, R.W., Angle, J.S., Bottomley, P.S., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 775–833. [Google Scholar]
- Jabborova, D.; Choudhary, R.; Karunakaran, R.; Ercisli, S.; Ahlawat, J.; Sulaymanov, K.; Azimov, A.; Jabbarov, Z. The Chemical Element Composition of Turmeric Grown in Soil–Climate Conditions of Tashkent Region, Uzbekistan. Plants 2021, 10, 1426. [Google Scholar] [CrossRef] [PubMed]
- Kun, X.; Feng, X. Effect of nitrogen on the growth and yield of ginger. China Veg. 1999, 6, 12–14. [Google Scholar]
- Haque, M.M.; Rahman, A.K.; Ahmed, M.; Masud, M.M.; Sarker, M.M. Effect of nitrogen and potassium on the yield and quality of ginger in hill slope. J. Soil Nat. 2007, 1, 36–39. [Google Scholar]
- Singh, M.; Khan, M.M.; Naeem, M. Effect of nitrogen on growth, nutrient assimilation, essential oil content, yield and quality attributes in Zingiber officinale Rosc. J. Saudi Soc. Agric. Sci. 2016, 15, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Jabborova, D.; Enakiev, Y.; Sulaymanov, K.; Kadirova, D.; Ali, A.; Annapurna, K. Plant growth promoting bacteria Bacillus subtilis promote growth and physiological parameters of Zingiber officinale Roscoe. Plant Sci. Today 2021, 8, 66–71. [Google Scholar] [CrossRef]
- Kanwal, S.; Ilyas, N.; Shabir, S.; Saeed, M.; Gul, R.; Zahoor, M.; Batool, N.; Mazhar, R. Application of biochar in mitigation of negative effects of salinity stress in wheat (Triticum aestivum L.). J. Plant Nutr. 2018, 41, 526–538. [Google Scholar] [CrossRef]
- Bu, X.; Xue, J.; Wu, Y.; Ma, W. Effect of Biochar on Seed Germination and Seedling Growth of Robinia pseudoacacia L. In Karst Calcareous Soils. Commun. Soil Sci. Plant Anal. 2020, 51, 352–363. [Google Scholar] [CrossRef]
- Hilioti, Z.; Michailof, C.M.; Valasiadis, D.; Iliopoulou, E.F.; Koidou, V.; Lappas, A.A. Characterization of castor plant-derived biochars and their effects as soil amendments on seedlings. Biomass Bioenergy 2017, 105, 96–106. [Google Scholar] [CrossRef]
- Jabborova, D.; Annapurna, K.; Paul, S.; Kumar, S.; Ibrahim, M.; Elkelish, A.A. Beneficial features of biochar and AMF for improving spinach plant growth, root morphological traits, physiological properties and soil enzymatic activities. J. Fungi. 2021, 7, 571. [Google Scholar] [CrossRef]
- Agboola, K.; Moses, S. Effect of biochar and cowdung on nodulation, growth and yield of soybean (Glycine max L. Merrill). Int. J. Agric. Biosci. 2015, 4, 154–160. [Google Scholar]
- Uzoma, K.; Inoue, M.; Andry, H.; Fujimaki, H.; Zahoor, A.; Nishihara, E. Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manag. 2011, 27, 205–212. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Prasad, M.; Kavanagh, A.; Tzortzakis, N. Biochar type, ratio, and nutrient levels in growing media affects seedling production and plant performance. Agronomy 2020, 10, 1421. [Google Scholar] [CrossRef]
- Petruccelli, R.; Bonetti, A.; Traversi, M.L.; Faraloni, C.; Valagussa, M.; Pozz, A. Influence of biochar application on nutritional quality of tomato (Lycopersicon esculentum). Crop. Pasture Sci. 2015, 66, 747–755. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Yao, Y.; Ji, Y.; Deng, J.; Zhou, G.; Liu, R.; Shao, J.; Zhou, L.; Li, N.; Zhou, X. Biochar amendment boosts photosynthesis and biomass in C3 but not C4 plants: A global synthesis. GCB Bioenergy 2020, 12, 605–617. [Google Scholar] [CrossRef]
- Bailey, V.L.; Fansler, S.J.; Smith, J.L.; Bolton, H., Jr. Reconciling apparent variability in effects of biochar amendment on soil enzyme activities by assay optimization. Soil Biol. Biochem. 2011, 43, 296–301. [Google Scholar] [CrossRef]
- Wang, L.; Xue, C.; Nie, X.; Liu, Y.; Chen, F. Effects of biochar application on soil potassium dynamics and crop uptake. J. Plant Nutr. Soil Sci. 2018, 181, 635–643. [Google Scholar] [CrossRef]
- Oladele, S.O. Effect of biochar amendment on soil enzymatic activities, carboxylate secretions and upland rice performance in a sandy clay loam Alfisol of Southwest Nigeria. Sci. Afr. 2019, 4, e00107. [Google Scholar] [CrossRef]
- Ouyang, L.; Tang, Q.; Yu, L.; Zhang, R. Effects of amendment of different biochars on soil enzyme activities related to carbon mineralisation. Soil Res. 2014, 52, 706–716. [Google Scholar] [CrossRef]
| Total (g kg−1) | Available (mg kg−1) | C/N | pH (H2O) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C | N | S | Ca | K | Mg | P | |||
| Biochar | 415.0 | 3.75 | 0.58 | 8893 | 1151 | 471 | 326 | 110.6 | 8.41 |
| Soil | 9.18 | 0.99 | 0.23 | 2103 | 1080 | 954 | 419 | 9.26 | 6.26 |
| Treatments | Plant Height (cm) | Leaf Length (cm) | Leaf Number | Leaf Width (cm) |
|---|---|---|---|---|
| Control | 18.0 ± 0.27 | 5.8 ± 0.01 | 3.3 ± 0.01 | 1.1 ± 0.01 |
| Biochar 1% | 22.3 ± 0.22 | 6.9 ± 0.03 | 4.0 ± 0.02 | 1.5 ± 0.01 * |
| Biochar 2% | 27.5 ± 0.18 * | 7.9 ± 0.01 * | 4.8 ± 0.1 * | 1.7 ± 0.01 * |
| Biochar 3% | 32.1 ± 0.72 ** | 9.1 ± 0.03 * | 6.1 ± 0.02 ** | 1.9 ± 0.01 ** |
| Treatments | Total Root Length (cm) | Root Surface Area (cm2) | Projected Area (cm2) | Root Diameter (mm) | Root Volume (cm3) |
|---|---|---|---|---|---|
| Control | 114.1 ± 2.05 | 30.9 ± 1.12 | 23.0 ± 0.11 | 1.06 ± 0.01 | 1.17 ± 0.01 |
| Biochar 1% | 149.5 ± 2.83 * | 46.7 ± 1.09 * | 29.7 ± 0.13 | 1.20 ± 0.01 | 1.43 ± 0.01 |
| Biochar 2% | 167.8 ± 10.11 * | 50.3 ± 2.00 ** | 38.8 ± 1.00 ** | 1.62 ± 0.02 * | 1.68 ± 0.02 * |
| Biochar 3% | 198.4 ± 12.00 ** | 58.6 ± 2.08 *** | 44.8 ± 1.07 *** | 2.09 ± 0.02 *** | 1.99 ± 0.01 *** |
| Treatments | Acid Phosphomono-Esterase (μg g−1 h−1) | Alkaline Phosphomono-Esterase (μg g−1 h−1) | FDA (Fluorescein Diacetate) Activity (µg g−1 h−1) | Phenol Oxidase Activity (U.g−1 DW) |
|---|---|---|---|---|
| Control | 980.1 ± 36.6 | 656.7 ± 10.5 | 40.6 ± 1.19 | 22.1 ± 0.11 |
| Biochar 1% | 1099.8 ± 38.4 | 677.1 ± 12.6 | 45.7 ± 1.30 | 30.5 ± 0.17 * |
| Biochar 2% | 1176.9 ± 43.0 | 698.2 ± 15.3 | 54.2 ± 1.12 * | 35.2 ± 1.10 * |
| Biochar 3% | 1238.4 ± 44.1 * | 753.6 ± 20.1 * | 62.8 ± 1.28 * | 39.2 ± 1.02 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabborova, D.; Wirth, S.; Halwani, M.; Ibrahim, M.F.M.; Azab, I.H.E.; El-Mogy, M.M.; Elkelish, A. Growth Response of Ginger (Zingiber officinale), Its Physiological Properties and Soil Enzyme Activities after Biochar Application under Greenhouse Conditions. Horticulturae 2021, 7, 250. https://doi.org/10.3390/horticulturae7080250
Jabborova D, Wirth S, Halwani M, Ibrahim MFM, Azab IHE, El-Mogy MM, Elkelish A. Growth Response of Ginger (Zingiber officinale), Its Physiological Properties and Soil Enzyme Activities after Biochar Application under Greenhouse Conditions. Horticulturae. 2021; 7(8):250. https://doi.org/10.3390/horticulturae7080250
Chicago/Turabian StyleJabborova, Dilfuza, Stephan Wirth, Mosab Halwani, Mohamed F. M. Ibrahim, Islam H. El Azab, Mohamed M. El-Mogy, and Amr Elkelish. 2021. "Growth Response of Ginger (Zingiber officinale), Its Physiological Properties and Soil Enzyme Activities after Biochar Application under Greenhouse Conditions" Horticulturae 7, no. 8: 250. https://doi.org/10.3390/horticulturae7080250
APA StyleJabborova, D., Wirth, S., Halwani, M., Ibrahim, M. F. M., Azab, I. H. E., El-Mogy, M. M., & Elkelish, A. (2021). Growth Response of Ginger (Zingiber officinale), Its Physiological Properties and Soil Enzyme Activities after Biochar Application under Greenhouse Conditions. Horticulturae, 7(8), 250. https://doi.org/10.3390/horticulturae7080250










