Agronomic Biofortification of Garlic through Selenium and Arbuscular Mycorrhizal Fungi Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growing Conditions
2.2. AMF Propagation with Maize-Trap Culture
2.3. Experimental Design
2.3.1. Trial 1
2.3.2. Trial 2
2.4. Sampling and Growth Index Measurement
2.5. Selenium Content Analysis
2.6. Nutrient Acquisition Measurement
2.7. Statistical Analysis
3. Results
3.1. Effects of Exogenous Se Spraying on Selenium Enrichment and Quality of Garlic Bulbs (Trial 1)
3.1.1. Wide-Ranging Gradients of Se Treatments on Garlic
3.1.2. Agronomic Parameters
3.1.3. Se Enrichment
3.1.4. Nutritional Qualities
3.2. The Garlic Growth and Quality of Bulbs under AMF Inoculation and Se Application (Trial 2)
3.2.1. Plant Growth and Agronomic Parameters
3.2.2. Nutritional Qualities
3.2.3. Se Enrichment
4. Discussion
4.1. Effects of Exogenous Se on Garlic
4.2. Effects of AMF Inoculation on Garlic
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gupta, M.; Gupta, S. An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant. Sci. 2017, 7, 2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conversa, G.; Lazzizera, C.; Chiaravalle, A.E.; Miedico, O.; Bonasia, A.; La Rotonda, P.; Elia, A. Selenium fern application and arbuscular mycorrhizal fungi soil inoculation enhance Se content and antioxidant properties of green asparagus (Asparagus officinalis L.) spears. Sci. Hortic. 2019, 252, 176–191. [Google Scholar] [CrossRef]
- Bodnar, M.; Szczyglowska, M.; Konieczka, P.; Namiesnik, J. Methods of selenium supplementation: Bioavailability and determination of selenium compounds. Crit. Rev. Food Sci. 2016, 56, 36–55. [Google Scholar] [CrossRef]
- Kahakachchi, C.; Boakye, H.T.; Uden, P.C.; Tyson, J.F. Chromatographic speciation of anionic and neutral selenium compounds in Se-accumulating Brassica juncea (Indian mustard) and in selenized yeast. J. Chromatogr. 2004, 1054, 303–312. [Google Scholar] [CrossRef]
- Saidi, I.; Chtourou, Y.; Djebali, W. Selenium alleviates cadmium toxicity by preventing oxidative stress in sunflower (Helianthus annuus) seedlings. J. Plant Physiol. 2014, 171, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Govasmark, E.; Salbu, B. Translocation and re-translocation of selenium taken up from nutrient solution during vegetative growth in spring wheat. J. Sci. Food Agr. 2011, 91, 1367–1372. [Google Scholar] [CrossRef]
- Duran, P.; Acuna, J.J.; Jorquera, M.A.; Azcon, R.; Borie, F.; Cornejo, P.; Mora, M.L. Enhanced selenium content in wheat grain by co-inoculation of selenobacteria and arbuscular mycorrhizal fungi: A preliminary study as a potential Se biofortification strategy. J. Cereal Sci. 2013, 57, 275–280. [Google Scholar] [CrossRef]
- Golubkina, N.; Kekina, H.; Caruso, G. Yield, quality and antioxidant properties of Indian mustard (Brassica juncea L.) in response to foliar biofortification with selenium and iodine. Plants 2018, 7, 80. [Google Scholar] [CrossRef] [Green Version]
- Lyons, G. Selenium in cereals: Improving the efficiency of agronomic biofortification in the UK. Plant Soil 2010, 332, 1–4. [Google Scholar] [CrossRef]
- Wei, J. Effects of Exogenous Selenium on Root Morphology and Nutrient Absorption of Maize and Its Role in Salt Stress. Master’s Thesis, Hebei University, Baoding, China, 2019. [Google Scholar]
- De Feudis, M.; D’Amato, R.; Businelli, D.; Guiducci, M. Fate of selenium in soil: A case study in a maize (Zea may L.) field under two irrigation regimes and fertilized with sodium selenite. Sci. Total Environ. 2019, 659, 131–139. [Google Scholar] [CrossRef]
- Tai, P.D.; Li, P.J. Toxic effects of selenium on plants. J. Agro Environ. Sci. 2002, 06, 496–498. [Google Scholar]
- Nawaz, F.; Zul, B.; Ahmad, K.S.; Majeed, S.; Shehzad, M.A.; Javeed, H.M.R.; Tahir, M.N.; Ahsan, M. Pretreatment with selenium and zinc modulates physiological indices and antioxidant machinery to improve drought tolerance in maize (Zea mays L.). S. Afr. J. Bot. 2021, 138, 209–216. [Google Scholar] [CrossRef]
- Cabral, L.; Soares, C.R.F.S.; Giachini, A.J.; Siqueira, J.O. Arbuscular mycorrhizal fungi in phytoremediation of contaminated areas by trace elements: Mechanisms and major benefits of their applications. World J. Microb. Biot. 2015, 31, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Sessitsch, A.; Kuffner, M.; Kidd, P.; Vangronsveld, J.; Wenzel, W.W.; Fallmann, K.; Puschenreiter, M. The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol. Biochem. 2013, 60, 182–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd Allah, E.F.; Tabassum, B.; Alqarawi, A.A.; Alshahrani, T.S.; Malik, J.A.; Hashem, A. Physiological Markers Mitigate Drought Stress in Panicum Turgidum Forssk. By Areuscular Mycorrhizal Fungi. Pak. J. Bot. 2019, 51, 2003–2011. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Rajkumar, M.; Oliveira, R.S.; Zhang, C.; Freitas, H. Potential of plant beneficial bacteria and arbuscular mycorrhizal fungi in phytoremediation of metal-contaminated saline soils. J. Hazard. Mater. 2019, 379, 120813. [Google Scholar] [CrossRef]
- Wang, P.; Wang, T.; Wu, S.; Wen, M.; Lu, L.; Ke, F.; Wu, Q. Effect of arbuscular mycorrhizal fungi on rhizosphere organic acid content and microbial activity of trifoliate orange under different low P conditions. Arch. Agron. Soil Sci. 2019, 65, 2029–2042. [Google Scholar] [CrossRef]
- Romero-Munar, A.; Baraza, E.; Gulias, J.; Cabot, C. Arbuscular mycorrhizal fungi confer salt tolerance in giant reed (Arundo donax L.) plants grown under low phosphorus by reducing leaf Na+ concentration and improving phosphorus use efficiency. Front. Plant Sci. 2019, 10, 843. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Zhang, S.; Wen, B.; Huang, H.; Luo, L. Accumulation and Speciation of Selenium in Plants as Affected by Arbuscular Mycorrhizal Fungus Glomus mosseae. Biol. Trace. Elem. Res. 2011, 143, 1789–1798. [Google Scholar] [CrossRef]
- Larsen, E.H.; Lobinski, R.; Burger-Meyer, K.; Hansen, M.; Ruzik, R.; Mazurowska, L.; Rasmussen, P.H.; Sloth, J.J.; Scholten, O.; Kik, C. Uptake and speciation of selenium in garlic cultivated in soil amended with symbiotic fungi (mycorrhiza) and selenate. Anal. Bioanal. Chem. 2006, 385, 1098–1108. [Google Scholar] [CrossRef]
- Munier-Lamy, C.; Deneux-Mustin, S.; Mustin, C.; Merlet, D.; Berthelin, J.; Leyval, C. Selenium bioavailability and uptake as affected by four different plants in a loamy clay soil with particular attention to mycorrhizae inoculated ryegrass. J. Environ. Radioact. 2007, 97, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, N.; Garmendia, I.; Fabbrin, E.G.; Bettoni, M.M.; Palop, J.A.; Sanmartin, C. Selenium fertilization and mycorrhizal technology may interfere in enhancing bioactive compounds in edible tissues of lettuces. Sci. Hortic. 2015, 195, 163–172. [Google Scholar] [CrossRef]
- Petropoulos, S.; Fernandes, A.; Barros, L.; Ciric, A.; Sokovic, M.; Ferreira, I.C.F.R. Antimicrobial and antioxidant properties of various Greek garlic genotypes. Food Chem. 2018, 245, 7–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hitchcock, J.; Schaefer, G.; Katz, A.; Kaschula, H. The immunomodulating and anti-inflammatory effects of garlic organosulfur compounds in cancer prevention. Eur. J. Cancer 2014, 505, S238–S239. [Google Scholar] [CrossRef]
- Khatua, T.N.; Borkar, R.M.; Mohammed, S.A.; Dinda, A.K.; Srinivas, R.; Banerjee, S.K. Novel sulfur metabolites of garlic attenuate cardiac hypertrophy and remodeling throughiInduction of Na+/K+-ATPase expression. Front. Pharmacol. 2017, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega-Hissi, E.G.; Andrada, M.F.; Diaz, M.G.; Garro Martinez, J.C. Computational study of the hydrogen peroxide scavenging mechanism of allyl methyl disulfide, an antioxidant compound from garlic. Mol. Divers. 2019, 23, 985–995. [Google Scholar] [CrossRef]
- Maass, H.I.; Klaas, M. Infraspecific differentiation of garlic (Allium-sativum L.) by isozyme and RAPD markers. Theor. Appl. Genet. 1995, 91, 89–97. [Google Scholar] [CrossRef]
- Fritsch, R.M.; Friesen, N. Evolution, Domestication and Taxonomy: Allium Crop Science Recent Advances; CABI Press: Oxford, UK, 2002. [Google Scholar]
- Ni, R.; Luo, K. Determination of total selenium and arsenic in coal by wet digestion hydride generation atomic fluorescence spectrometry (HG-AFS). Spectrosc. Spect Anal. 2015, 35, 1404–1408. [Google Scholar] [CrossRef]
- Ali, A.; Ghani, M.I.; Ding, H.; Fan, Y.; Cheng, Z.; Iqbal, M. Co-amended synergistic interactions between arbuscular mycorrhizal fungi and the organic substrate-induced cucumber yield and fruit quality associated with the regulation of the AM-Fungal community structure under anthropogenic cultivated soil. Int. J. Mol. Sci. 2019, 20, 1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 3, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Baghalian, K.; Ziai, S.A.; Naghavi, M.R.; Badi, H.N.; Khalichi, A. Evaluation of allicin content and botanical traits in Iranian garlic (Allium satimm L.) ecotypes. Sci. Hortic. 2005, 103, 155–166. [Google Scholar] [CrossRef]
- Quang, T.D.; Cui, Z.; Huang, J.; Thi, A.T.T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium distribution in the Chinese environment and its relationship with human health: A review. Environ. Int. 2018, 112, 294–309. [Google Scholar]
- Dumont, E.; Vanhaecke, F.; Cornelis, R. Selenium speciation from food source to metabolites: A critical review. Anal. Bioanal. Chem. 2006, 385, 1304–1323. [Google Scholar] [CrossRef]
- Liu, J.; Wu, L.; Wei, C.; Li, F.; Tan, Y. Effects of two ways of applying selenium on camellia oleifera abel yield and oil quality. J. Biobased. Mater. Bio. 2019, 13, 863–869. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B.; Matraszek, R.; Pogorzelec, M. The dual effects of two inorganic selenium forms on the growth, selected physiological parameters and macronutrients accumulation in cucumber plants. Acta Physiol. Plant 2015, 37, 41. [Google Scholar] [CrossRef] [Green Version]
- Lyons, G.H.; Genc, Y.; Soole, K.; Stangoulis, J.C.R.; Liu, F.; Graham, R.D. Selenium increases seed production in Brassica. Plant Soil 2009, 318, 73–80. [Google Scholar] [CrossRef]
- Astaneh, R.K.; Bolandnazar, S.; Nahandi, F.Z.; Oustan, S. Effects of selenium on enzymatic changes and productivity of garlic under salinity stress. S. Afr. J. Bot. 2019, 121, 447–455. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, N.; Liang, X.; Zheng, L.; Zhang, C.; Li, Y.; Zhang, Z.; Gao, Y.; Zhao, J. A comparative study on the accumulation, translocation and transformation of selenite, selenate, and SeNPs in a hydroponic-plant system. Ecotox. Environ. Safe 2020, 189, 109955. [Google Scholar] [CrossRef]
- Ramos, S.J.; Rutzke, M.A.; Hayes, R.J.; Faquin, V.; Guilherme, L.R.G.; Li, L. Selenium accumulation in lettuce germplasm. Planta 2011, 233, 649–660. [Google Scholar] [CrossRef]
- Hajiboland, R.; Joudmand, A.; Aliasgharzad, N.; Tolra, R.; Poschenrieder, C. Arbuscular mycorrhizal fungi alleviate low-temperature stress and increase freezing resistance as a substitute for acclimation treatment in barley. Crop Pasture Sci. 2019, 70, 218–233. [Google Scholar] [CrossRef]
- Al-Arjani, A.F.; Hashem, A.; Abd Allah, E.F. Arbuscular mycorrhizal fungi modulates dynamics tolerance expression to mitigate drought stress in Ephedra foliata Boiss. Saudi J. Biol. Sci. 2020, 27, 380–394. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Anand, G.; Singh, N.; Kapoor, R. Arbuscular mycorrhiza augments arsenic tolerance in wheat (Triticum aestivum L.) by strengthening antioxidant defense system and thiol metabolism. Front. Plant Sci. 2017, 8, 906. [Google Scholar] [CrossRef] [Green Version]
- Budi, S.W.; van Tuinen, D.; Martinotti, G.; Gianinazzi, S. Isolation from the Sorghum bicolor mycorrhizosphere of a bacterium compatible with arbuscular mycorrhiza development and antagonistic towards soilborne fungal pathogens. Appl. Environ. Microb. 1999, 65, 5148–5150. [Google Scholar] [CrossRef] [Green Version]
- Barea, J.M.; Pozo, M.J.; Azcon, R.; Azcon-Aguilar, C. Microbial co-operation in the rhizosphere. J. Exp. Bot. 2005, 56, 1761–1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelhameed, R.E.; Metwally, R.A. Alleviation of cadmium stress by arbuscular mycorrhizal symbiosis. Int. J. Phytoremediat. 2019, 21, 663–671. [Google Scholar] [CrossRef]
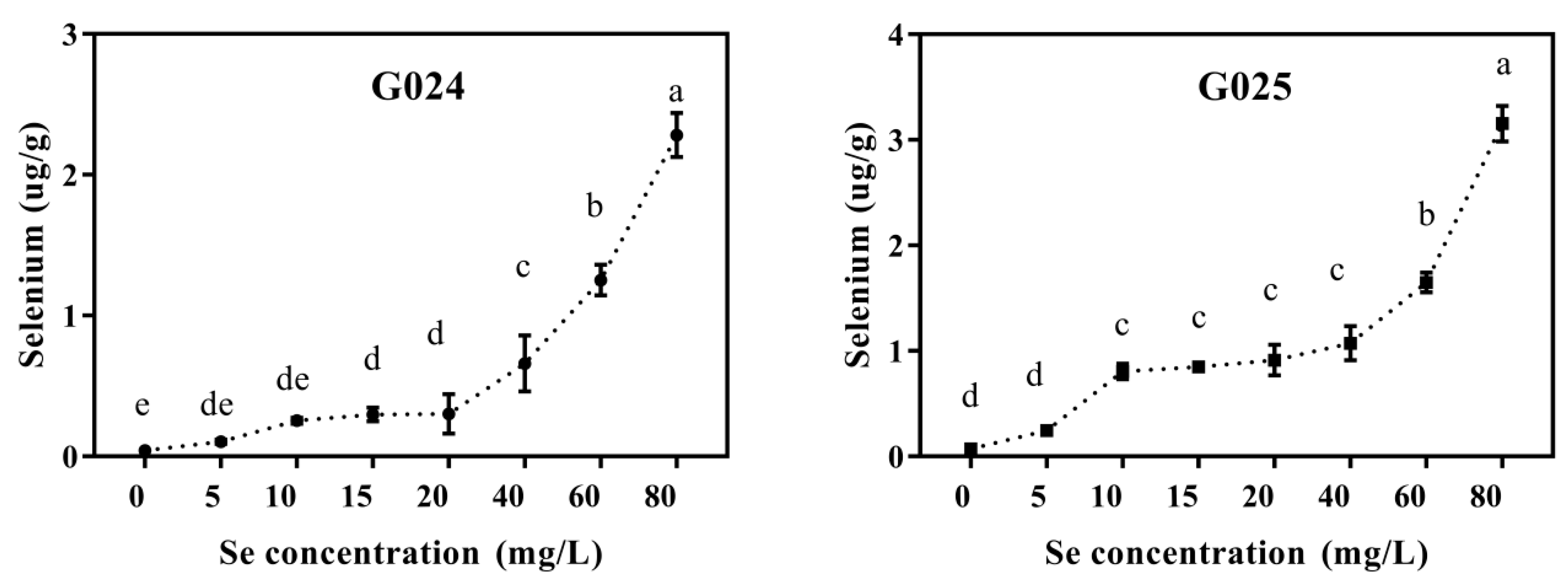
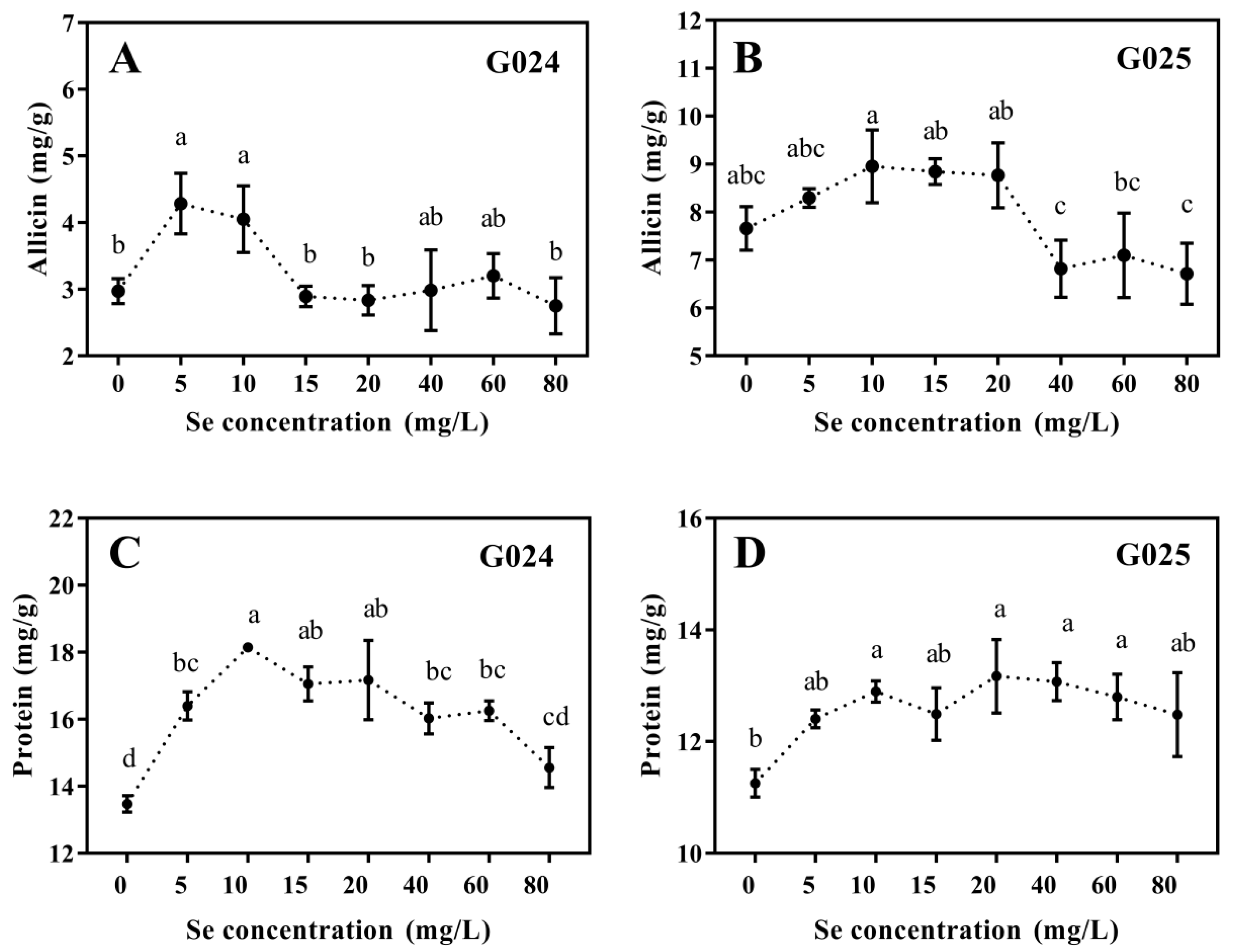
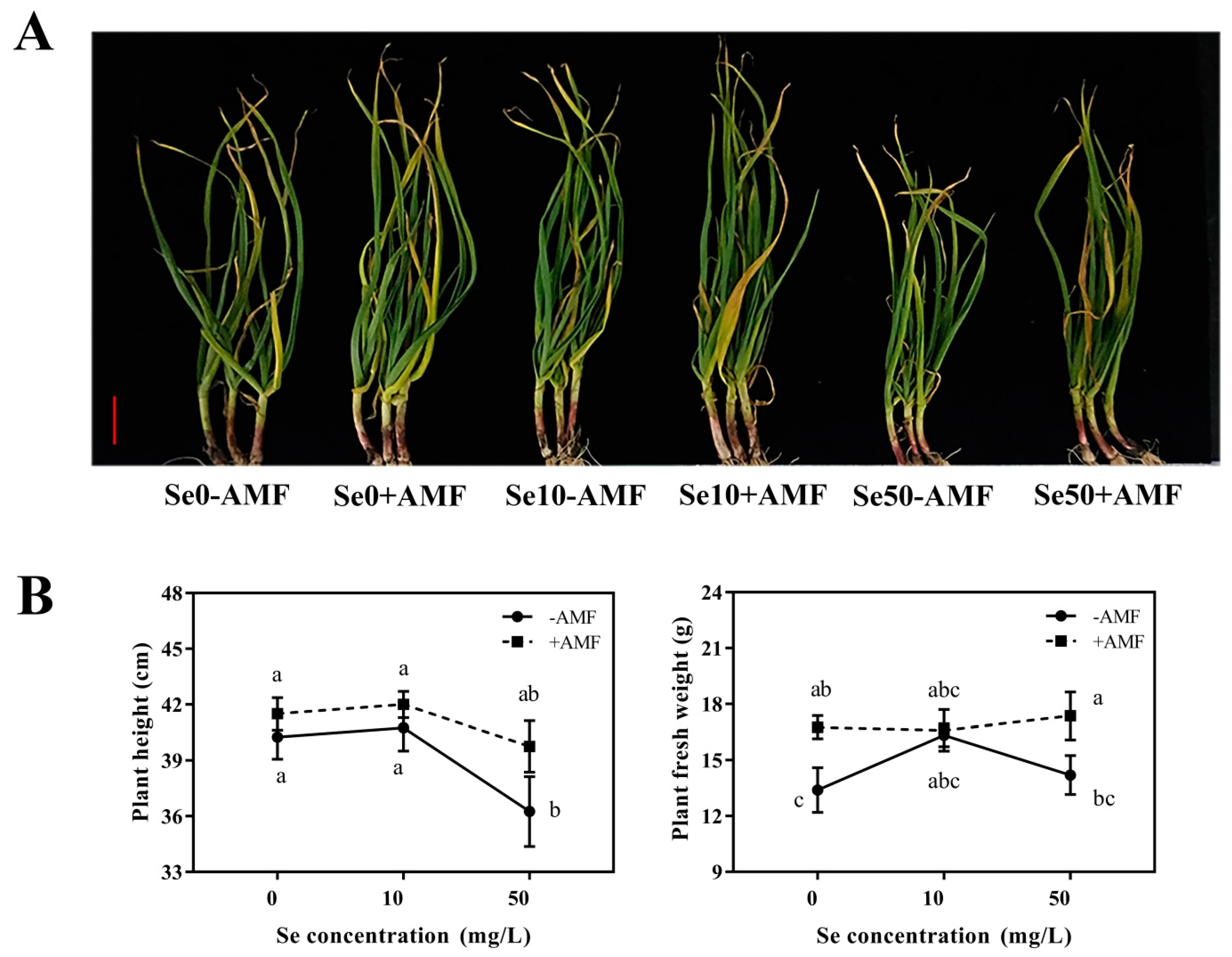
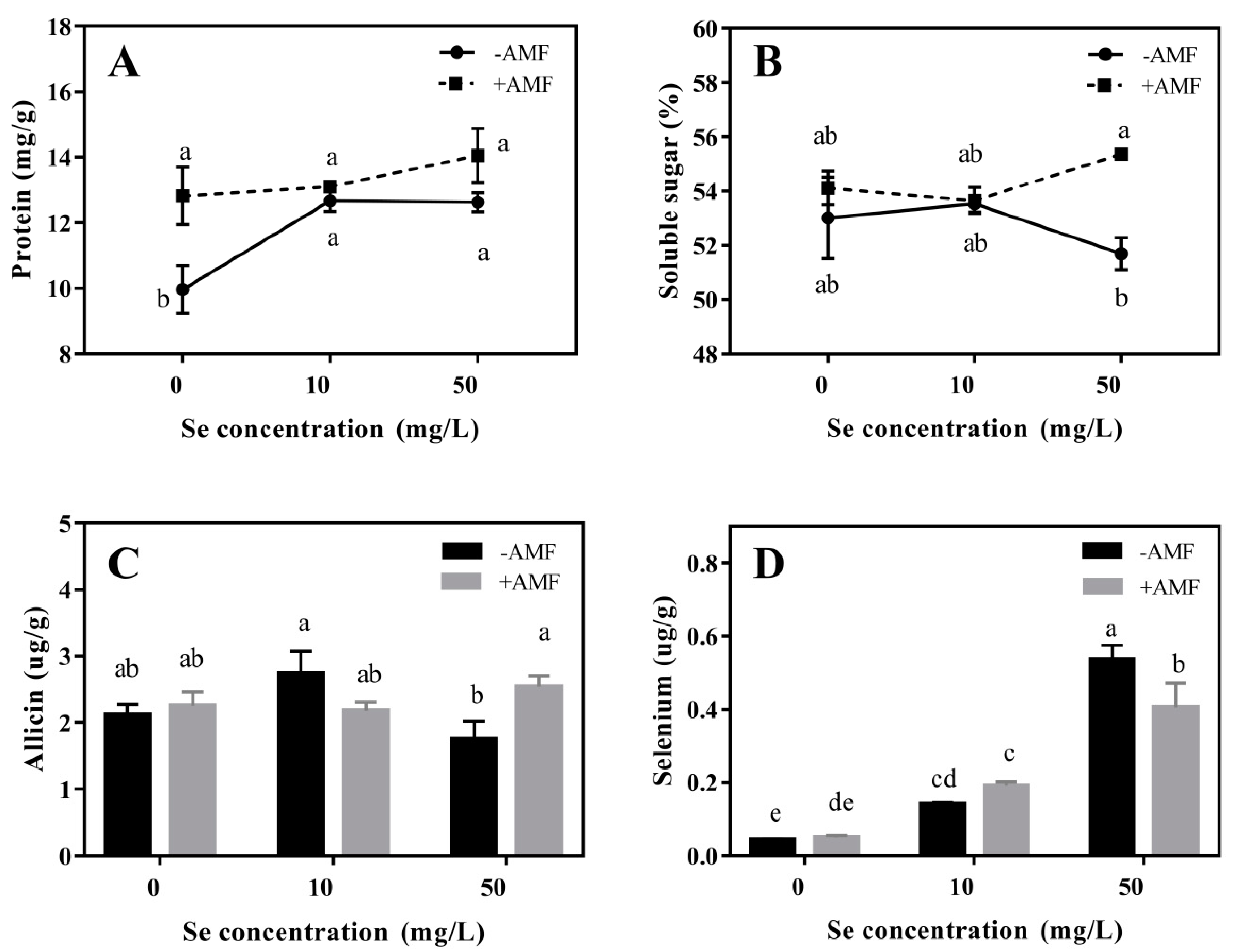
| Se (mg/L) | cv. G024 | cv. G025 | ||||
|---|---|---|---|---|---|---|
| Bulb Weight (g) | Bulb Diameter (mm) | Bulb Height (mm) | Bulb Weight (g) | Bulb Diameter (mm) | Bulb Height (mm) | |
| 0 | 36.09 ± 0.94 bc | 44.8 ± 0.4 cde | 32.5 ± 0.4 ab | 25.59 ± 0.45 cd | 40.5 ± 0.3 ef | 28.7 ± 0.3 cd |
| 5 | 39.57 ± 0.77 a | 46.6 ± 0.5 b | 32.9 ± 0.3 a | 29.07 ± 0.50 a | 43.1 ± 0.3 bc | 29.6 ± 0.3 ab |
| 10 | 41.35 ± 0.78 a | 49.2 ± 0.3 a | 33.1 ± 0.3 a | 30.17 ± 0.43 a | 45.4 ± 0.3 a | 29.9 ± 0.3 a |
| 15 | 36.80 ± 0.69 b | 46.5 ± 0.3 b | 31.7 ± 0.3 bc | 29.87 ± 0.48 a | 43.7 ± 0.3 b | 29.5 ± 0.3 abc |
| 20 | 36.16 ± 0.78 bc | 45.9 ± 0.6 bc | 31.3 ± 0.3 bc | 27.72 ± 0.44 b | 42.2 ± 0.3 cd | 28.9 ± 0.2 bcd |
| 40 | 35.86 ± 0.62 bc | 45.3 ± 0.4 bcd | 31.8 ± 0.4 bc | 26.23 ± 0.33 c | 41.0 ± 0.6 cd | 28.8 ± 0.2 bcd |
| 60 | 33.99 ± 0.74 cd | 44.3 ± 0.3 de | 31.2 ± 0.4 cd | 25.74 ± 0.46 cd | 41.4 ± 0.4 de | 28.2 ± 0.4 de |
| 80 | 33.08 ± 0.74 d | 43.5 ± 0.4 e | 30.5 ± 0.3 d | 24.76 ± 0.46 d | 40.0 ± 0.4 f | 27.6 ± 0.3 e |
| Se (mg/L) | AMF | Bulb Weight (g) | Bulb Diameter (mm) | Bulb Height (mm) | Scape Diameter (mm) | Scape Length (cm) |
|---|---|---|---|---|---|---|
| 0 | No | 17.49 ± 0.77 bc | 33.4 ± 0.6 bc | 24.6 ± 0.2 b | 4.5 ± 0.2 b | 58 ± 1 c |
| Yes | 18.74 ± 0.36 abc | 35.8 ± 0.5 a | 27.4 ± 0.1 a | 4.7 ± 0.2 ab | 61 ± 3 abc | |
| 10 | No | 18.88 ± 0.82 ab | 34.9 ± 0.6 ab | 27.7 ± 0.4 a | 4.6 ± 0.1 b | 60 ± 1 bc |
| Yes | 19.66 ± 0.64 a | 36.2 ± 0.3 a | 27.6 ± 0.2 a | 5.1 ± 0.1 a | 67 ± 2 a | |
| 50 | No | 16.87 ± 0.56 c | 32.1 ± 0.9 c | 24.5 ± 0.4 b | 3.9 ± 0.1 c | 56 ± 3 c |
| Yes | 18.68 ± 0.47 abc | 35.5 ± 0.3 a | 27.5 ± 0.4 a | 4.9 ± 0.1 ab | 66 ± 1 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Pan, Y.; Ali, A.; Zhang, S.; Li, X.; Qi, X.; Liu, H.; Meng, H.; Cheng, Z. Agronomic Biofortification of Garlic through Selenium and Arbuscular Mycorrhizal Fungi Application. Horticulturae 2021, 7, 230. https://doi.org/10.3390/horticulturae7080230
Yang F, Pan Y, Ali A, Zhang S, Li X, Qi X, Liu H, Meng H, Cheng Z. Agronomic Biofortification of Garlic through Selenium and Arbuscular Mycorrhizal Fungi Application. Horticulturae. 2021; 7(8):230. https://doi.org/10.3390/horticulturae7080230
Chicago/Turabian StyleYang, Fan, Yupeng Pan, Ahmad Ali, Siyu Zhang, Xiaxia Li, Xiaofang Qi, Hongjiu Liu, Huanwen Meng, and Zhihui Cheng. 2021. "Agronomic Biofortification of Garlic through Selenium and Arbuscular Mycorrhizal Fungi Application" Horticulturae 7, no. 8: 230. https://doi.org/10.3390/horticulturae7080230
APA StyleYang, F., Pan, Y., Ali, A., Zhang, S., Li, X., Qi, X., Liu, H., Meng, H., & Cheng, Z. (2021). Agronomic Biofortification of Garlic through Selenium and Arbuscular Mycorrhizal Fungi Application. Horticulturae, 7(8), 230. https://doi.org/10.3390/horticulturae7080230







