Abstract
Blue rot disease caused by Penicillium expansum is one of the most widespread fungal diseases that affects apples worldwide. This work was to verify the effect of chitosan (2 and 4 g/L) and its nano-form (0.2 and 0.4 g/L) against blue rot disease on apples and their effect on the expression of six defense-related genes as well as fruit quality parameters. Regarding disease incidence, in most cases, chitosan NPs performed better as compared to their raw materials for both artificial and natural infections. The highest efficacy was obtained for chitosan NPs at 0.4 g/L for artificial and natural infection in both 2019 and 2020 seasons. All treatments kept fruit quality parameters regarding firmness, total soluble solids, and titratable acidity for artificial and natural infection in both seasons. As expected, the exogenous application of chitosan NPs and bulk form triggered an increase in the expression levels of six defense-related genes including chitinase, peroxidase, β-1,3-glucanase, Xyloglucan endotransglycosylase (XET), pathogenesis-related protein (PR8), and phenylalanine ammonia lyase-1 (PAL1). Moreover, the highest mRNA quantity of all the studied genes was detected in leaves treated with chitosan NPs at both concentrations compared to other treatments. Chitosan NPs can be considered an eco-friendly and effective approach against blue mold of apples and can be integrated into management programs to maintain postharvest quality and extend the shelf life of fruits.
1. Introduction
During 2018, Egypt produced around 704,727 tons of apples and the harvested area was almost 28,085 ha, with a yield of 250,926 hg/ha [1]. Anna apples (Malus domestica Borkh.) are considered to be one of the fruit crops whose cultivated area has increased rapidly in Egypt due to its nutritional value and its compatibility with the prevailing environmental conditions, especially in the newly reclaimed lands. Apples are exposed during handling, transportation, and storage to many economically influencing fungal diseases, the most important of which are fruit rot, especially those caused by Penicillium fungi.
Research conducted in this field has shown that blue rot disease caused by Penicillium expansum (Link) thorn is one of the most widespread fungal diseases that affects apples in all countries of the world after they are collected during handling or in storage. In addition to causing fruit rot, some strains of P. expansum produce the mycotoxin, patulin [2]. Ref. [3] reported that this pathogen caused a soft rot, which led to a complete rot of the fruit within 5–7 days at the appropriate temperature. Ref. [4] mentioned that the economic losses resulting from infection with P. expansum is related to the production of the carcinogenic metabolite called patulin. Patulin is a harmful neurotoxin when the fruits are used for fresh consumption or in processing [5]. Besides, the author stated that when the fungus infects the host, it produced patulin mycotoxin citrinin, a virulence factor.
P. expansum grows most efficiently at a temperature of 15 °C, while, it grows slower at lower and higher temperatures. It needs high humidity to grow best; its growth rate is fastest at 90% RH. The conidia of P. expansum infects fruit through wounds. These wounds can occur in the fruits during harvesting, packaging, and processing, where puncturing, bruising, and limb rubs occur, all of which provide spots through which spores can enter the fruit [6]. P. expansum produces organic acids during infection to acidify host tissues, which enhance fungal development, showing a relation between environmental acidity and the virulence of the pathogen [7].
During recent years, the fight against this disease has relied on the use of chemical systemic fungicides, which have raised many objections after it was confirmed that they damage the environment and human health and that their residues lead to the formation of strains resistant to fungicides used in controlling the causal pathogen. To overcome these limitations, several safe alternatives have been proposed to reduce/stop the use of chemical pesticides. A number of non-chemical postharvest treatments as alternative agents against postharvest diseases in fruit crops were recently reviewed and their high efficiency was proven [8].
Nanomaterials have been suggested due to their particular properties and their serious applications in the field of agriculture and plant protection. Different nanomaterials have been tested by several scientists successfully to manage plant diseases, especially those attacking fruit crops [9]. Among alternative methods, chitosan nanoparticles (100–300 nm) are used as coatings and antimicrobial agents for ‘Golab Kohanz’ and ‘Gala’ apples [10,11]. A clay-chitosan nanocomposite was examined against green mold on ‘Valencia late’ sweet orange [12]. Chitosan nanoparticles were a potent abiotic elicitor of plant resistance to different pathogens attacks [13].
In the systemic acquired resistance (SAR) mechanism, plant defenses are induced by prior treatments that consequently improve resistance against later pathogen attacks. However, the molecular details of the signaling machinery are poorly understood [14,15]. The final outcome that is linked with the beginning of disease resistance is the potentiated expression and the following accumulation of pathogenesis-related proteins (PRs) [16]. Some of these pathogenesis-related proteins, chitinases (PR2), β-1, 3 glucanase (PR3), and PR8, can directly hydrolyze fungal cell walls [17]. Lignin accumulation and the synthesis of phenols are regulated by Phenylalanine ammonia lyase (PAL), where they play an important role in the pathway of phenylpropanoid [16,18]. Global analysis of mRNA expression for various defense-related genes has developed as a valuable means for explaining gene expression affected by a varied range of biological processes, including biotic and abiotic stresses, as well as fruit development [16,19].
This research was designed to verify the effect of the preharvest application of chitosan and its nano-form against blue rot disease on apples caused by P. expansum after harvesting. Additionally, their effect on apple fruit quality and the expression levels of six defense-related genes, including chitinase, peroxidase, β-1,3-gluc, XET, PR8, and PAL1, were investigated.
2. Materials and Methods
2.1. The Pathogen and Pathogenicity Test
P. expansum was isolated from naturally infected apples at Giza governorate and maintained on potato dextrose agar (PDA) medium under sterilized conditions. The purified isolate was identified based on its morphological and cultural characteristics utilizing the descriptions of P. expansum [20]. The fungal culture was identified at Mycology Research and Disease Survey Dept., Plant Pathology Research Institute, ARC, Giza governorate, Egypt. Using Koch’s postulates, the pathogenicity of fungal strains was carried out and confirmed on Anna apples.
2.2. Conidial Suspension
To produce inoculum, P. expansum was grown on PDA plates at 24 °C in the dark. Conidia were collected from one-week-old plates by scraping them with a sterile spatula and then suspending them in sterile distilled water containing 0.05% Tween 20 (v/v). The resulting suspension was filtered through two layers of sterile gauze. The spore counts were made by a Thoma counting chamber (HGB Henneberg-Sander GmbH, Lutzellinden, Germany), and the suspension was diluted with sterile distilled water to obtain a final concentration of 106 conidia/mL.
2.3. Source of Compounds
Chitosan and acetic acid were brought from Sigma-Aldrich Co. (St Louis, MO, USA) (purity 99%), while chitosan nanoparticles were obtained from the nanotechnology laboratory and advanced materials, plant pathology research institute, ARC, Egypt, and the sizes were <100 nm. In this respect, a stock solution of chitosan (10 mg/mL) was prepared by dissolving 2 g of purified chitosan powder in 100 mL of distilled water with 2 mL of acetic acid (stirred for 24 h), and the volume was supplemented up to 200 mL with distilled water [21], then two concentrations, i.e., 2 and 4 g/L were made by appropriate dilution. The pH of the prepared solution was adjusted to pH 5.6 using NaOH (1 N) and autoclaved at 121 °C for 15 min [22]. Chitosan nanoparticles were prepared to get 0.2 and 0.4 g/L concentrations.
2.4. Characterization of Chitosan Nanoparticles
Chitosan nanoparticle dispersibility was determined using a transmission electron microscope (TEM) (FEI Tecnai G2, FEI Company, Hillsboro, OR, USA). Different forms of imaging were engaged: bright field at electron accelerating voltage 200 kV using lanthanum hexaboride (LaB6) electron source gun, and diffraction example imaging. An Eagle CCD camera with 4k picture resolution was utilized to obtain transmitted electron pictures [9]. The TEM micrograph of chitosan nanoparticles showed that their shape is spherical with average sizes between 100 and 160 nm (Figure 1).

Figure 1.
Transmission electron microscopic image of chitosan NPs, ranging from 50–100 nm.
2.5. Antifungal Effect of the Tested Chemical on P. expansum Growth In Vitro
Tested chemicals, i.e., chitosan nanoparticles at 0.2 and 0.4 g/L, chitosan at 2 and 4 g/L, and acetic acid at 2 m/L, were added to 250 mL conical flasks containing 100 mL sterilized PDA medium before its solidifying and rotated gently to ensure equal distribution of added chemicals. A separate PDA flask free of tested chemicals was used as control. The supplemented media were poured into sterilized Petri dishes (9 cm). Mycelial discs (5 mm Ø) of P. expansum taken from seven day old cultures were placed at the center of the prepared Petri dishes, then incubated at 25 ± 2 °C. Four replicates were used for each treatment. The average linear growth diameter of colonies was measured when fungal mycelium covered one plate in the control treatment. Inhibition percentage was determined by the following equation [23]:
where
- Xc: Mean of colony diameter in control plates.
- Xi: Mean of colony diameter of test plates.
2.6. The Effect of Pre-Harvest Spray with Tested Chemicals on the Development of Blue Mold on Apples under Storage Conditions
2.6.1. Field Application
Pre-harvest experiments were conducted on 13-year-old apple trees cv. Anna grown in an experimental farm at El-Qanater El-Khairia Horticultural Research Station, Agricultural Research Centre, Qalyubia governorate during two successive seasons 2019 and 2020. The field experiment was designed as a complete randomized trial, with four replicates for each treatment (each replicate consisted of three trees). Four sprays of the studied chemical as inducers for SAR were applied on the vegetative growth and fruits of apple trees cv. Anna, at bloom stage and repeated every 20 days until two days before harvesting. Chitosan nanoparticles were sprayed at concentrations of 0.2 and 0.4 g/L whereas chitosan was sprayed at 2 and 4 g/L. Acetic acid was used for comparison at 2 m/L. Apple trees treated with water served as a negative control.
Apples were harvested at physiological maturity (firmness was 12.68 lb/in2, TSS content was 7.8% and titratable acidity 0.82%) and screened for uniform size and lack of visible defects. Then, they were transported to the laboratory on the same day and divided into two groups. The first group was used for artificial infection with P. expansum while the second group was used for naturally occurring infections.
2.6.2. Artificial Infection Experiments
Regarding artificial infections, fruits were surface-sterilized in 1% sodium hypochlorite solution for 2 min, washed with sterile distilled water, and allowed to dry at room temperature. Sterilized fruits were wounded by making two injuries (1 mm width, 2 mm depth) with a nail on two opposite sides of each fruit at the central equatorial region of the fruit, then inoculated with the prepared spore suspension of P. expansum (106 conidia/mL) using an atomizer. Treated fruits were packed in polypropylene baskets (1 kg). Four replicates were used for each treatment (each replicate consisted of 13 apples). All fruits were cold stored at 4 ± 1 °C with 90% RH for 45 days. At the end of cold storage, the incidence of blue mold (%), fruit firmness, TSS, and titratable acidity were determined.
2.7. Assessments
2.7.1. Disease Incidence
The incidence of decay was calculated using the formula as follows:
2.7.2. Natural Infection Experiments
Concerning the second group, which was related to a naturally occurring infection, treated fruits in the field were harvested and submitted to the same procedures without any artificial inoculation. The experimental design, storage conditions, and assessments were also the same as described above.
2.7.3. Fruit Firmness (lb/in2)
Fruit firmness was measured on two opposite sides of the equatorial fruit zone after removing the peel by using a hand Magness Taylor pressure tester (Model FT 327, Italy), and the results were expressed in lb/in2.
2.7.4. Fruit Total Soluble Solids
Total soluble solids (TSS) were measured in fruit juice using a digital pocket refractometer (PAL 1, Atago Co., Ltd., Tokyo, Japan) and expressed as percentages.
2.7.5. Fruit Titratable Acidity
Titratable acidity (TA) was measured in fruit juice by titration against calibrated 0.1 N NaOH solution in the presence of phenolphthalein as an indicator. TA was calculated as a percentage of malic acid according to A.O.A.C. (https://archive.org/details/gov.law.aoac.methods.1.1990, accessed on 10 June 2021).
2.8. Total RNA Isolation and cDNA Synthesis
Total RNA was extracted from the plant leaf samples of Anna apple trees treated with chitosan nanoparticles at 0.2 and 0.4 mg/mL, chitosan bulk form at 2.0 and 4.0 mg/mL, acetic acid at 2.0 mg/mL, as well as apple trees treated with water as a control. The plant leaf samples were collected from the plants after 48 h from the last field application of the tested chemicals as well as control, each was pooled from at least five different trees (five biological replicates) and immediately placed in liquid nitrogen. Total RNA was extracted using the RNeasy® Plant Mini Kit (QIAGEN, Cat No.74903, Hilden, Germany) according to the manufacturer’s protocol. The extracted RNA samples were purified from genomic DNA and treated with gDNAWipeout Buffer that was included in the QuantiTect® Reverse Transcription Kit, according to the manufacturer’s recommendations. The reverse transcription reaction was performed using the High Capacity cDNA reverse transcriptase kit (COSMO cDNA synthesis Kit, Willowfort, UK). The cDNA samples were stored at −20 °C until use.
2.9. Differential Expression Analysis Using Quantitative Real-Time PCR
Triplicate PCR reactions were performed as well as cDNA template negative and non-template control. Each PCR reaction consisted of 2.5 μL of cDNA (except for NTC and cDNA control), 12.5 μL of SYBR Green Mix (Cat. No. 204143; Qiagen, Hilden, Germany), and 0.3 μM of each forward and reverse primer of the six studied genes (Table 1). A total of 1 μL of RNase inhibitor was added and the final volume was adjusted to 25 μL by adding RNase-free water. Reactions were assessed using a Prime Q real-time qPCR machine (Techne, UK) with a two-step cycling protocol under the following conditions: 95 °C for 10 min, 40 cycles of 95 °C for 15 s, thereafter 60 °C for 60 s. The EF-1α gene (GenBank XM008387060) was used as the internal reference gene for qPCR data normalization. All experimentally induced changes in the expression of the studied genes are presented as n-fold changes relative to the corresponding controls. Relative gene expression ratios (RQ) between the treated and control groups were calculated using the formula: RQ = 2−ΔΔCT.

Table 1.
Name and sequence of primer pairs used for quantitative real-time polymerase chain reaction (qRT-PCR) analysis.
2.10. Statistical Analysis
Obtained data were statistically analyzed through Co-Stat 3.4 software as the usual technique of analysis of variance [24]. The mean was compared using the least significant difference (LSD) at p = 0.05 as outlined by [25].
3. Results
3.1. Antifungal Effect of Some Chemical on the Colony Growth of P. expansum In Vitro
All tested treatments reduced P. expansum growth on PDA medium compared with control Table 2. Acetic acid was the best effective treatment where it completely inhibited the growth of tested fungus. Nano-chitosan was more effective at reducing the growth of P. expansum than their natural forms. On the other hand, the mycelial growth of P. expansum was more sensitive to high concentrations of nano-chitosan compared with other treatments. Chitosan at 2 g/L was the least effective at reducing P. expansum growth.

Table 2.
Effect of chitosan and its nanoform on the growth (mm) of P. expansum in vitro.
3.2. Effect of Pre-Harvest Spray with Tested Chemicals on the Development of Blue Mold on Apples under Storage Condition
The effect of chitosan and its nanoform against artificial and natural infection of blue mold of Anna apples is shown in Table 3. Overall, the highest percentage of decay incidence was recorded for water check treatment. The results revealed that all treatments were significantly better than water control in reducing the percentages of blue mold. In most cases, chitosan nanoform performed better as compared to its raw material for both artificial and natural infections. The highest efficacy was obtained for nanochitosan at 0.4 g/L. In particular, the efficacy was 63.71 and 95.56% for artificial and natural infections in 2019, respectively. The same trend was also observed in the 2020 season. In in the in vivo test, all concentrations of acetic acid vapors and chitosan solutions significantly reduced blue mold incidence or rotted part tissues of apples. The most effective treatment was combined treatment between acetic acid vapor followed by chitosan solutions which reduced the disease and rotted part tissues by more than 87.0%. In this study, acetic acid was the most effective at reducing the linear growth of P. expansum in vitro, but it was less effective at controlling blue mold in apples than other treatments.

Table 3.
Effect of pre-harvest sprays of chitosan and its nanoform against artificial and natural infection of blue mold of Anna apples after 45 days of cold storage at 4 ± 1 °C during seasons 2019 and 2020.
3.3. Effects of the Tested Chemicals on the Quality of Postharvest Apples
Fruit Firmness
The effect of chitosan and its nanoform on firmness (lb/in2) of Anna apples is shown in Table 4. Fruit firmness ranged from 7.5–9.1 and 8.1–10.7 lb/in2 for artificial and natural infection, respectively, in the 2019 season. Fruit firmness ranged from 7.7–9.4 and 8.0–10.5 lb/in2 for artificial and natural infection, respectively, in the 2020 season. The results showed that the highest firmness value was recorded for nano-chitosan at 0.2 g/mL. Additionally, control fruit show the lowest firmness values in all cases.

Table 4.
Effect of chitosan and its nanoform on firmness (lb/in2) of Anna apples after 45 days of cold storage at 4 ± 1 °C during seasons 2019 and 2020.
3.4. Fruit Total Soluble Solids
The effect of chitosan and its nanoform on the TSS of Anna apples is shown in Table 5. TSS values ranged from 8.1–10.1 and 8.8–11.0% for artificial and natural infection, respectively, in the 2019 season. TSS ranged from 8.5–10.6 and 8.4–10.9% for artificial and natural infection, respectively, in the 2020 season. In most cases, chitosan in raw material at 2 g/L gave the highest TSS values.

Table 5.
Effect of chitosan and its nanoform on TSS% of Anna apples after 45 days of cold storage at 4 ± 1 °C during seasons 2019 and 2020.
3.5. Fruit Titratable Acidity
The effect of chitosan and its nanoform on the titratable acidity of Anna apples is shown in Table 6. TA values ranged from 0.31–0.64 and 0.46–0.72% for artificial and natural infection, respectively, in the 2019 season. TA ranged from 0.34–0.67 and 0.43–0.74% for artificial and natural infection, respectively, in the 2020 season. It is well known that quality parameters are very important when researchers are investigating antifungal agents.

Table 6.
Effect of chitosan and its nanoform on titratable acidity of Anna apples after 45 days of cold storage at 4 ± 1 °C during seasons 2019 and 2020.
3.6. Expression Profiling of Some Defense-Related Genes in Leaf Samples of Apple Trees (cv. Anna) Responding to Application with Different Treatments
To investigate the molecular role of the field application of chitosan NPs at 0.2 and 0.4 mg/mL and chitosan bulk form at 2.0 and 4.0 mg/mL on plant systemic acquired resistance (SAR) induction, six defense-related genes’ accumulation were quantified in apple leaves in this study. In addition, acetic acid at 2.0 mg/mL treatment was studied for comparison to confirm the chitosan effect where it was used as a dissolving agent for chitosan preparation. The studied genes were chitinase, peroxidase, β-1,3-glu, XET, PR8, and PAL1. In general, both chitosan NPs and bulk material at both concentrations upregulated the six defense-related genes’ expression with different patterns higher than the other treatments (Figure 2). From the results, the most pronounced increase of the studied genes’ accumulation was observed in leaves of apple trees treated in the field with chitosan NPs compared to the treatment with chitosan bulk and acetic acid after 48 h from the treatments. Data revealed that the mRNA quantity of chitinase, peroxidase, β-1,3-glu, XET, PR8, and PAL1 genes were much higher in apple tissues treated with chitosan NPs at 0.4 mg/mL (8.3, 5.4, 7.8, 27.5, 6.8, 7.9-fold increase, respectively) than other treatments (Figure 2A–F). The expression of XET defense-related gene recorded the highest mRNA quantity in response to chitosan NPs at both concentrations 0.2 and 0.4 mg/mL, as well as chitosan bulk at 4 mg/mL (18.6, 27.5, 12.1-fold increase, respectively) (Figure 2D). All studied genes recorded the lowest mRNA quantity in response to acetic acid, which confirmed the chitosan efficiency at SAR induction against the investigated pathogen. Noteworthy, there was a great difference between the levels of expression in response to chitosan NPs and chitosan bulk materials at 0.4 and 4 mg/mL, respectively (Figure 2). Our results confirmed the long-lasting effect of the field applied treatments on the expression of the defense-related studied genes.
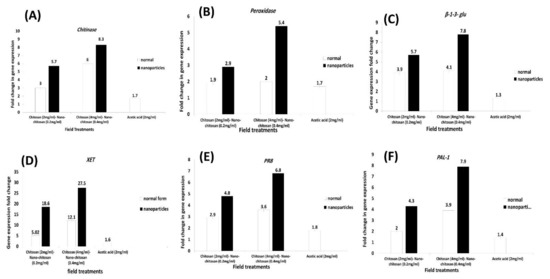
Figure 2.
Relative expression profiles of six defense-related genes in apple leaves (Anna cv.) using qRT-PCR analysis. Plants were treated with chitosan NPs at 0.2 and 0.4 mg/mL, chitosan bulk materials 2.0 and 4.0 mg/mL, and acetic acid at 2.0 mg/mL. The figure showed the expression profile of; (A) chitinase gene; (B) peroxidase gene; (C) β-1,3-glucanase (β-1,3-gluc); (D) Xyloglucan endotransglycosylase (XET); (E) pathogenesis-related protein (PR8); and (F) Phenylalanine ammonia lyase-1 (PAL1). The qPCR data were normalized with the EF-1α gene, the reference, (XM008387060). Error bars represent standard errors.
4. Discussion
Nanotechnology is employed in agriculture to boost food production, nutritious value, quality, and safety. Low-cost, environmentally friendly NPs can help plants resist disease [26]. Systemic acquired resistance (SAR) is one of plant’s defense responses when facing attack by different pathogens that threaten plant production and survival. It can be induced by prior treatments with different types of elicitors, including non-chemical methods [8,27]. Many studies that investigated the effect of chitosan on the colony growth of fungi, ref. [14] observed that chitosan moderately suppressed the growth of Alternaria alternata in vitro. Chitosan treatment significantly inhibited spore germination and mycelia growth of P. expansum, also, it induced noticeable changes in P. expansum morphology characterized by hyphal agglomeration, the presence of big vesicles in the mycelium, and abnormal bifurcated and turgid shapes [28]. P. expansum isolates were entirely inhibited at all tested concentrations of silver/chitosan nanocomposites of 0.30 mg·mL−1 after 7 days of incubation. The developed nanocomposites showed a low inhibition zone [29]. Chitosan polymer and chitosan nanoparticles (CS NPs) at a concentration of 0.6% (w/v) severely delayed the mycelial growth of Rhizopus sp., Colletotrichum capsici, C. gloeosporioides, and A. niger [30].
The highest efficacy was obtained for chitosan NPs at 0.4 g/L, which was explained by [31], who found that the use of materials with nanoparticles due to their diffusion over a large area of the treated surface led in turn to high activity and an effective stimulus for plant metabolism, better cell penetration, and increased plant activity. Furthermore, other researchers have demonstrated that chitosan nanocomposites have numerous advantages, such as being non-toxic, stable, and capable of serving as a matrix for a variety of food, medicinal, and plant extracts [32,33]. Furthermore, because of its non-toxic characteristics, biodegradability, and biocompatibility, the usage of chitosan-based coating has gotten a lot of attention. Chitosan has been shown to help preserve the quality of fruits and vegetables by preventing moisture and odor loss, as well as lowering respiratory rates, ethylene production, and transpiration. According to [34,35], acetic acid vapors, either alone or in combination with chitosan coating, are effective in suppressing blue mold in apples during storage via preventing oxygen access in plant tissues and microbial growth. In an in vivo test, acetic acid vapors and all concentrations of chitosan significantly reduced blue mold incidence or rotted part tissues of apples. Acetic acid was the most effective at reducing the linear growth of P. expansum in vitro, but it was less effective at controlling the blue mold of apples than other treatments during storage. This is in line with the findings of [36], who found that while acetic acid inhibited the growth of B. cinerea and P. aphanidermatum in vitro, it was the least effective treatment at suppressing grey mold and cottony rot of green bean pods during storage when applied in the field. The highest firmness value was recorded for chitosan NPs at 0.2 g/mL. Coating apples cv. Lady William with 1.0% chitosan after harvesting sustains quality features for up to 80 days in storage at 18 ± 2 °C and 56 ± 2% RH, according to the findings of [37]. In fresh-cut ‘Gala’ apple, nano-chitosan was tested in terms of firmness and microbiological profile [11].
In most cases, chitosan in raw material at 2 g/L gave the highest TSS values. This is consistent with the findings of [37], who found that fruits coated with 1.5% chitosan were firmer, had higher juice content, titratable acidity, and ascorbic acid, as well as less weight loss, fruit juice Ph, TSS, and TSS–acid ratio, all of which were comparable to the effects of 1.0% chitosan on the fruits. Nanochitosan prolonged the quality and avoided the mass loss of apples during storage [10]. In terms of firmness and titratable acidity during storage, apple cv. Red Delicious treated with nanocalcium performed better than apples treated with calcium chloride [38]. The antioxidant and antimicrobial properties of nanomaterials such as silver-chitosan nanocomposites, as well as the formation of chitosan film on fruit crops, which keeps them fresh by acting as a barrier for oxygen uptake and thus slowing the metabolic action and ripening process [38], could be responsible for the improved quality. Recently, nanochitosan did not show any negative impact when used on table grapes in terms of TSS and TA or their ratio [9,39].
In the systemic acquired resistance (SAR) mechanism, plant defenses are preconditioned by a prior treatment that induced different defense genes expressions, which enhanced resistance against subsequent pathogen infections. Although the molecular details of the signaling machinery are poorly understood [14,15,27], chitosan can provide an effective approach to maintaining postharvest quality and extending the shelf life of fruits [40]. In this study, the activation of SAR by chitosan and chitosan NPs resulted in the enhanced expression of six defense-related genes (chitinase, peroxidase, 1,3-gluc, XET, PR8, and PAL1). The increase in XET, a cell wall-related gene, activity caused by chitosan and chitosan NPs treatments during this study reflects a higher xyloglucan endotransglucosylation that, together with the decrease in endoglucanases, would prevent fungal access to the cellulose-xyloglucan network, hence decreasing the cell wall colonization [40,41]. A similar high expression level of AcXET1 and AcXET2 genes on days six and eight in cherimoya fruits coated with 20.0 mM citric acid combined with 1.00% chitosan has been observed [40]. All studied genes recorded the lowest mRNA quantity in response to acetic acid, which confirmed the chitosan efficiency on SAR induction against the investigated pathogen. Different members of β-1,3-glucanase are probably involved in various biological functions in plant defense responses against a wide variety of pathogens [14,15,42]. Chitinase in combination with β-1,3-glucanase can directly degrade the fungal cell wall or act indirectly by releasing oligosaccharide elicitors of defense reaction, both of which are potential defense mechanisms against fungal infection in fruit [15,43], which explain the decrease in the disease severity in response to our studied treatments. Additionally, our results were supported and explained by [40], with their results stating that PR8 (chitinase type 3) is toxic to invading fungal pathogens. Pathogenesis-related proteins are over-expressed following pathogen attack or stress. Our results confirmed the long-lasting effect of the field applied treatments on the expression of the defense-related genes that is supported by the results recorded in table grapes [40], jujube fruits [44], apple [19,45], common beans [26,46], and in tomatoes and sweet peppers [14,15]. The present study suggests that the exogenous application of chitosan NPs or bulk form could effectively induce strong systemic acquired resistance (SAR) against P. expansum infection in apples (cv. Anna) through enhancing the expression of the defense-related genes.
5. Conclusions
Chitosan NPs are a promising agent against P. expansum, the causative agent of apple blue mold, and can be introduced into management programs. At high concentrations, however, chitosan NPs demonstrated minimal degradation on apples caused by P. expansum. In addition, all of the treatments studied could be valuable materials for fruit quality preservation postharvest. Exogenous application of chitosan NPs or bulk form could effectively induce strong systemic acquired resistance (SAR) against P. expansum infection in apples (cv. Anna) by increasing the expression of the studied defense-associated genes. To better its applicability, more research into the mode of action of such a substance is required.
Author Contributions
Conceived and designed the study, F.A.A.-R. and M.H.R.; conceptualization, H.A.S.E.-G. and Y.A.; methodology, F.A.A.-R. and Y.A.; analysis, H.A.S.E.-G. and Y.A.; investigation, F.A.A.-R., M.H.R. and M.S.S.H.; resources, G.A.M.; data curation, M.S.S.H.; writing—original draft preparation, H.A.S.E.-G. and I.A.I.; writing—review and editing, F.A.A.-R. and H.A.S.E.-G.; supervision, I.A.I.; funding acquisition, I.A.I. All authors have read and agreed to the published version of the manuscript.
Funding
The current work was funded by Taif University Researchers Supporting Project number (TURSP–2020/120), Taif University, Taif, Saudi Arabia.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to privacy concerns.
Acknowledgments
The authors are grateful to Taif University for providing funds to publish the current work by Taif University Researchers Supporting Project number (TURSP-2020/120), Taif University, Taif, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAOSTAT 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 29 October 2020).
- Errampalli, D. Penicillium expansum (Blue Mold). In Postharvest Decay; Bautista-Baños, S., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 189–231. [Google Scholar] [CrossRef]
- Larous, L.; Hendel, N.; Abood, J.K.; Ghoul, M. The growth and production of patulin mycotoxin by Penicillium expansum on apple fruits and its control by the use of propionic acid and sodium benzoate. Arab J. Plant Prot. 2007, 25, 123–128. [Google Scholar]
- Zhong, L.; Carere, J.; Lu, Z.; Lu, F.; Zhou, T. Patulin in Apples and Apple-Based Food Products: The Burdens and the Mitigation Strategies. Toxins 2018, 10, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales, H.; Marín, S.; Rovira, A.; Ramos, A.J.; Sanchis, V. Patulin accumulation in apples by Penicillium expansum during postharvest stages. Lett. Appl. Microbiol. 2007, 44, 30–35. [Google Scholar] [CrossRef]
- Torres, R.; Teixidó, N.; Viñas, I.; Mari, M.; Casalini, L.; Giraud, M.; Usall, J. Efficacy of Candida sake CPA-1 formulation for controlling Penicillium expansum decay on pome fruit from different Mediterranean regions. J. Food Prot. 2006, 69, 2703–2711. [Google Scholar] [CrossRef]
- Prusky, D.; McEvoy, J.L.; Saftner, R.; Conway, W.S.; Jones, R. Relationship between host acidification and virulence of Penicillium spp. on apple and citrus fruit. Phytopathology 2004, 94, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Papoutsis, K.; Mathioudakis, M.M.; Hasperué, J.H.; Ziogas, V. Non-chemical treatments for preventing the postharvest fungal rotting of citrus caused by Penicillium digitatum (green mold) and Penicillium italicum (blue mold). Trends Food Sci. Technol. 2019, 86, 479–491. [Google Scholar] [CrossRef]
- Hashim, A.F.; Youssef, K.; Abd-Elsalam, K.A. Ecofriendly nanomaterials for controlling gray mold of table grapes and maintaining postharvest quality. Eur. J. Plant Pathol. 2019, 154, 377–388. [Google Scholar] [CrossRef]
- Gardesh, A.S.K.; Badii, F.; Hashemi, M.; Ardakani, A.Y.; Maftoonazad, N.; Gorji, A.M. Effect of nanochitosanbased coating on climacteric behavior and post-harvest shelf-life extension of apple cv. Golab Kohanz. LWT 2016, 70, 33–40. [Google Scholar] [CrossRef]
- Pilon, L.; Spricigo, P.C.; Miranda, M.; de Moura, M.R.; Assis, O.B.G.; Mattoso, L.H.C.; Ferreira, M.D. Chitosannanoparticle coatings reduce microbial growth on fresh-cut apples while not aecting quality attributes. Int. J. Food Sci. Technol. 2015, 50, 440–448. [Google Scholar] [CrossRef]
- Youssef, K.; Hashim, A.F. Inhibitory effect of clay/chitosan nanocomposite against Penicillium digitatum on citrus and its possible mode of action. Jordan J. Biol. Sci. 2020, 13, 349–355. [Google Scholar]
- Pichyangkura, R.; Chatchawan, S. Bio stimulant activity of chitosan in horticulture. Sci. Hortic. 2015, 195, 49–65. [Google Scholar] [CrossRef]
- El-Garhy, H.A.; Abdel-Rahman, F.A.; Shams, A.S.; Osman, G.H.; Moustafa, M. Comparative analyses of four chemicals used to control black mold disease in tomato and its effects on defense signaling pathways, productivity and quality traits. Plants 2020, 9, 808. [Google Scholar] [CrossRef]
- Abdel-Rahman, F.A.; Eman, Y.K.; Maali, S.S.; Tahsin, S.; Yosra, A. Preharvest application of salicylic acid induces some resistant genes of sweet pepper against black mold disease. Eur. J. Plant Pathol. 2021, 159, 755–768. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, X.; Li, B.; Zhang, Q.; Liang, W.; Wang, C. Salicylic acid confers enhanced resistance to Glomerella leaf spot in apple. Plant Physiol. Biochem. 2016, 106, 64–72. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [Green Version]
- González-Candelas, L.; Alamar, S.; Sánchez-Torres, P.; Zacarias, L.; Marcos, J.F. A transcriptomic approach highlights induction ofsecondary metabolism in citrus fruit in response to Penicillium digitatum infection. BMC Plant Biol. 2010, 10, 194–211. [Google Scholar] [CrossRef] [Green Version]
- Vilanova, L.; Wisniewski, M.; Norelli, J.; Viñas, I.; Torres, R.; Usall, J.; Phillips, J.; Droby, S.; Teixidó, N. Transcriptomic profiling of apple in response to inoculation with a pathogen (Penicillium expansum) and a non-pathogen (Penicillium digitatum). Plant Mol. Biol. Rep. 2014, 32, 566–583. [Google Scholar] [CrossRef]
- Samson, R.A.; Hoekstra, E.S.; Frisvad, J.C.; Filtenborg, O. (Eds.) Introduction to Food- and Airborne Fungi, 6th ed.; Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2002; p. 389. [Google Scholar]
- Jabnoun-Khiareddine, H.; El-Mohamedy, R.S.R.; Abdel-Kareem, F.; Aydi Ben Abdallah, R.; Gueddes-Chahed, M.; Daami-Remadi, M. Variation in chitosan and salicylic acid efficacy towards soil-borne and air-borne fungi and their suppressive effect of tomato wilt severity. J. Plant Pathol. Microbiol. 2015, 6, 325. [Google Scholar] [CrossRef] [Green Version]
- Algam, S.A.E.; Xie, G.; Li, B.; Yu, S.; Su, T.; Larsen, J. Effects of Paenibacillus strains and chitosan on plant growth promotion and control of Ralstonia wilt in tomato. J. Plant Pathol. 2010, 92, 593–600. [Google Scholar]
- Guo, Z.; Chen, R.; Xing, R.; Liu, S.; Yu, H.; Wang, P.; Li, C.; Li, P. Novel derivatives of chitosan and their antifungal activities in-vitro. Carbohydr. Res. 2006, 341, 351–354. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; John Wiley and Sons: New York, NY, USA, 1984; p. 680. [Google Scholar]
- Duncan, D.B. Multiple range and multiple F tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- El-Garhy, H.A.S.; Elsisi, A.A.; Mohamed, S.A.; Morsy, O.M.; Osman, G.; Abdel-Rahman, F.A. Transcriptomic changes in green bean pods against grey mould and white rot diseases via field application of chemical elicitor nanoparticles. IET Nanobiotechnol. 2020, 14, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Esmailzadeh, M.; Soleimani, M.J.; Rouhani, H. Exogenous application of salicylic acid for inducing systemic acquired resistance against tomato stem canker disease. J. Biol. Sci. 2008, 8, 1039–1044. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Chen, C.; Xia, X.; Garba, B.; Shang, L.; Wang, Y. Proteomic analysis of the inhibitory effect of chitosan on Penicillium expansum. Food Sci. Technol. 2020, 40, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Alghuthaymi, M.A.; Abd-Elsalam, K.A.; Shami, A.; Said-Galive, E.; Shtykova, E.V.; Naumkin, A.V. Silver/chitosan nanocomposites: Preparation and characterization and their fungicidal activity against dairy cattle toxicosis Penicillium expansum. J. Fungi 2020, 6, 51. [Google Scholar] [CrossRef] [Green Version]
- Chookhongkha, N.; Sopondilok, T.; Photchanachai, S. Effect of chitosan and chitosan nanoparticles on fungal growth and chilli seed quality. Acta Hortic. 2013, 973, 231–237. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C.; Singh, S.K.; Gautam, R.; Choudhary, K.; Maurino, V.G.; Saharan, V. MgO nanoparticles biosynthesis and its effect on chlorophyll contents in the leaves of clusterbean (Cyamopsis tetragonoloba L.). Adv. Sci. Eng. Med. 2014, 6, 538–545. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances in chitosan-based micro-and nanoparticles in drug delivery. J. Control. Rel. 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Momin, J.K.; Jayakumar, C.; Prajapati, J.B. Potential of nanotechnology in functional foods. Emir. J. Food Agric. 2013, 25, 10–19. [Google Scholar] [CrossRef]
- Bautista-Baños, S.; Hernández-López, M.; Bosquez-Molina, E. Growth inhibition of selected fungi by chitosan and plant extracts. Rev. Mex. Fitopatol. 2004, 22, 178–186. [Google Scholar]
- Abd-El-Kareem, F.; Haggag, W.M.; Saied, N.M.; Elshahawy, I.E. Postharvest application of acetic acid vapours and chitosan solution for controlling gray and blue moulds of apple fruits. J. Chem. Pharm. Res. 2015, 7, 581–588. [Google Scholar]
- Abdel-Rahman, F.A. Safe Technologies For Controlling Post-Harvest Diseases of Green Bean Prepared For Exportation. Ph.D. Thesis, Faculty of Agriculture, Benha University, Benha, Egypt, 2015; p. 250. [Google Scholar]
- Zeb, S.; Muhammad, S.; Syed, T.; Mazhar, A.; Shahid, A.; Zohra, N.; Kamran, R.; Aamir, K.; Hajra, S.; Syed, A.; et al. Influence of post-harvest application of chitosan on physico-chemical changes of apple fruit during storage. Pure Appl. Biol. 2020, 9, 2554–2562. [Google Scholar] [CrossRef]
- Ranjbar, S.; Rahemi, M.; Ramezanian, A. Comparison of nano-calcium and calcium chloride spray on post-harvest quality and cell wall enzymes activity in apple cv. Red Delicious. Sci. Hortic. 2018, 240, 57–64. [Google Scholar] [CrossRef]
- Moussa, S.H.; Tayel, A.A.; Alsohim, A.S.; Abdallah, R.R. Botryticidal growth of nanosized silver–chitosan composite and its application for the control of gray mold in strawberry. J. Food Sci. 2013, 78, 1589–1594. [Google Scholar] [CrossRef]
- Youssef, K.; de Oliveira, A.G.; Tischer, C.A.; Hussain, I.; Roberto, S.R. Synergistic effect of a novel chitosan/silica nanocomposites-based formulation against gray mold of table grapes and its possible mode of action. Int. J. Biol. Macromol. 2019, 141, 247–258. [Google Scholar] [CrossRef]
- Liu, K.; Liu, J.; Li, H.; Yuan, C.; Zhong, J.; Chen, Y. Influence of postharvest citric acid and chitosan coating treatment onripening attributes and expression of cell wall related genes incherimoya (Annona cherimola Mill.) fruit. Sci. Hortic. 2016, 198, 1–11. [Google Scholar] [CrossRef]
- Miedes, E.; Lorences, E.P. Apple (Malus domestica) and tomato (Lycopersicum esculentum) fruits cell-wall hemicelluloses and xyloglucan degradation during Penicillium expansum infection. J. Agric. Food Chem. 2004, 52, 7957–7963. [Google Scholar] [CrossRef]
- De la Cova, C.; Townley, R.; Regot, S.; Greenwald, I.A. Real-time biosensor for ERK activity reveals signaling dynamics during C. elegans cell fate specification. Dev. Cell 2017, 42, 542–553.e4. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.P.; Yao, H.J.; Deng, X.; Xu, X.B.; Qin, G.Z.; Chan, Z.L. Characterization and expression of beta-1,3-glucanase genes in jujube fruit induced by the microbial biocontrol agent Cryptococcus laurentii. Phytopathology 2007, 97, 260–268. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Wang, P.; Li, M.; Ke, X.; Li, C.; Liang, D.; Wu, S.; Ma, X.; Li, C.; Zou, Y.; et al. Exogenous melatonin improves Malus resistance to Marssonina apple blotch. J. Pineal Res. 2013, 54, 426–434. [Google Scholar] [CrossRef]
- El-Garhy, H.A.S.; Rashid, I.A.S.; Abou-Ali, R.M.; Moustafa, M.M.A. Field application of safe chemical elicitors induced the expression of some resistance genes against grey mold and cottony rot diseases during snap bean pods storage. Gene 2016, 576, 358–365. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).