Ginkgo biloba Seeds—An Environmental Pollutant or a Functional Food
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Reagents and Instruments
2.3. Micro Elemental Analysis
2.4. Fat and Protein Analyses
2.5. Analyses of Ginkgo Starch
2.6. Analyses of Secondary Metabolites in Ginkgo Seeds
2.7. Antimicrobial Analysis
3. Results
3.1. Microelements in Ginkgo biloba Seeds
3.2. Proteins and Lipids in Ginkgo biloba Seeds
3.3. Properties of Ginkgo Starch
3.4. Secondary Metabolites in Ginkgo Kernels
3.5. Antimicrobial Studies
4. Discussion
5. Further Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- DeFeudis, F.V.; Drieu, K. Ginkgo biloba extract (EGb 761) and CNS functions: Basic studies and clinical applications. Curr. Drug Targets 2000, 1, 25–58. [Google Scholar] [CrossRef]
- Wieland, L.S.; Feinberg, T.M.; Ludeman, E.; Prasad, N.K.; Amri, H. Ginkgo biloba for cognitive impairment and dementia (Protocol). Cochrane Database Syst. Rev. 2002, 4, CD003120. [Google Scholar] [CrossRef]
- Belwal, T.; Giri, L.; Bahukhandi, A.; Tariq, M.; Kewlani, P.; Bhatt, I.D.; Rawal, R.S. Chapter 3.19. Ginkgo biloba. In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 241–250. [Google Scholar] [CrossRef]
- Ivanova, V.; Natcheva, L.; Gercheva, P. Use of Ginko biloba, L. in green system of the town of Plovdiv. Sci. Works USB Plovdiv. Ser. C Tech. Technol. 2015, 13, 241–244. [Google Scholar]
- American Oil Chemists Society (AOAC). Kjeldahl’s method for protein determination in cereals and feed. In Official Methods of AnalysisSM, 16th ed.; Method 945; AOAC International: Rockville, MD, USA, 1996; p. 18-B. [Google Scholar]
- International Organization for Standardization (ISO Standard No. 659:2009). Oilseeds—Determination of Oil Content (Reference Method); International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- International Organization for Standardization (ISO Standard No. 5509:2000). Animal and Vegetable Fats and Oils. Preparation of Methyl Esters of Fatty Acids; International Organization for Standardization: Geneva, Switzerland, 2000. [Google Scholar]
- International Organization for Standardization (ISO Standard No. 5508:2004). Animal and Vegetable Fats and Oils. Analysis by Gas Chromatography of Methyl Esters of Fatty Acids; International Organization for Standardization: Geneva, Switzerland, 2004. [Google Scholar]
- International Organization for Standardization (ISO Standard No. ISO 9936). Animal and Vegetable Fats and Oils. Determination of Tocopherols and Tocotrienols Contents. Method Using High-Performance Liquid Chromatography; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- Knutson, C.A.; Grove, M.J. Rapid Method for Estimation of Amylose in Maize Starches. Cereal Chem. 1994, 71, 469–471. [Google Scholar]
- Morado-Castillo, R.; Quintanilla-Licea, R.; Gomez-Flores, R.; Blaschek, W. Total Phenolic and Flavonoid Contents and Flavonoid Composition of Flowers and Leaves from the Mexican Medicinal Plant Gymnosperma glutinosum (Spreng.) Less. Eur. J. Med. Plants 2016, 15, 1–8. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Butler, L.G. Protein precipitation method for the quantitative determination of tannins. J. Agric. Food Chem. 1978, 26, 809–812. [Google Scholar] [CrossRef]
- Haldar, D.; Sen, D.; Gayen, K. Development of Spectrophotometric Method for the Analysis of Multi-component Carbohydrate Mixture of Different Moieties. Appl. Biochem. Biotechnol. 2017, 181, 1416–1434. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.G.; Vrancheva, R.Z.; Marchev, A.S.; Petkova, N.T.; Aneva, I.Y.; Denev, P.P.; Georgiev, V.G.; Pavlov, A.I. Antioxidant activities and phenolic compounds in Bulgarian Fumaria species. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 296–306. [Google Scholar]
- Feodorova, Y.; Tomova, T.; Minchev, D.; Turiyski, V.; Draganov, M.; Argirova, M. Cytotoxic effect of Ginkgo biloba kernel extract on HCT116 and A2058 cancer cell lines. Heliyon 2020, 6, e04941. [Google Scholar] [CrossRef]
- Tumbarski, Y.; Lincheva, V.; Petkova, N.; Nikolova, R.; Vrancheva, R.; Ivanov, I. Antimicrobial activity of extract from aerial parts of potentilla (Potentilla reptans L.). Ind. Technol. 2017, 4, 37–43. [Google Scholar]
- Wang, H.-Y.; Zhang, Y.-Q. The main active constituents and detoxification process of Ginkgo biloba seeds and their potential use in functional health foods. J. Food Comp. Anal. 2019, 83, 103247. [Google Scholar] [CrossRef]
- Zhou, G.; Yao, X.; Tang, Y.; Qian, D.; Su, S.; Zhang, L.; Jin, C.; Qin, Y.; Duan, J. An optimized ultrasound-assisted extraction and simultaneous quantification of 26 characteristic components with four structure types in functional foods from ginkgo seeds. Food Chem. 2014, 158, 177–185. [Google Scholar] [CrossRef]
- Chen, X.; Zhong, W.; Shu, C.; Yang, H.; Li, E. Comparative analysis of chemical constituents and bioactivities of the extracts from leaves, seed coats and embryoids of Ginkgo biloba L. Nat. Prod. Res. 2020, 35, 1–4. [Google Scholar] [CrossRef]
- Goh, L.M.; Barlow, P.J. Antioxidant capacity in Ginkgo biloba. Food Res. Int. 2002, 35, 815–820. [Google Scholar] [CrossRef]
- Brufau, G.; Boatella, J.; Rafecas, M. Nuts: Source of energy and macronutrients. Br. J. Nutr. 2006, 96, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Hierro, M.T.G.; Robertson, G.; Christie, W.W.; Joh, Y.-G. The fatty acid composition of the seeds of Ginkgo biloba. J. Am. Oil Chem. Soc. 1996, 73, 575–579. [Google Scholar] [CrossRef]
- Mahadevan, S.; Park, Y.; Park, Y. Modulation of cholesterol metabolism by Ginkgo biloba L. nuts and their extract. Food Res. Int. 2008, 41, 89–95. [Google Scholar] [CrossRef]
- Miao, M.; Jiang, H.; Jiang, B.; Cui, S.W.; Jin, Z.; Zhang, T. Structure and functional properties of starches from Chinese ginkgo (Ginkgo biloba L.) nuts. Food Res. Int. 2012, 49, 303–310. [Google Scholar] [CrossRef]
- Cai, J.; Cai, C.; Man, J.; Xu, B.; Wei, C. Physicochemical Properties of Ginkgo Kernel Starch. Int. J. Food Prop. 2015, 18, 380–391. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhan, H.; Yao, C.; Hu, L.; Peng, Y.; Shen, J. Study on physicochemical and in-vitro enzymatic hydrolysis properties of ginkgo (Ginkgo biloba) starch. Food Hydrocoll. 2015, 48, 312–319. [Google Scholar] [CrossRef]
- Liu, X.-W.; Yang, J.-L.; Niu, W.; Jia, W.-W.; Olaleye, O.E.; Wen, Q.; Duan, X.-N.; Huang, Y.-H.; Wang, F.-Q.; Du, F.-F.; et al. Human pharmacokinetics of ginkgo terpene lactones and impact of carboxylation in blood on their platelet-activating factor antagonistic activity. Acta Pharmacol. Sin. 2018, 39, 1935–1946. [Google Scholar] [CrossRef] [PubMed]
- Tomova, T.; Doncheva, N.; Mihaylova, A.; Kostadinov, I.; Peychev, L.; Argirova, M. An Experimental Study on Phytochemical Composition and Memory Enhancing Effect of Ginkgo biloba Seed Extract. Folia Med. 2021, 63, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Gerstmeier, J.; Seegers, J.; Witt, F.; Waltenberger, B.; Temml, V.; Rollinger, J.M.; Stuppner, H.; Koeberle, A.; Schuster, D.; Werz, O. Ginkgolic Acid is a Multi-Target Inhibitor of Key Enzymes in Pro-Inflammatory Lipid Mediator Biosynthesis. Front Pharmacol. 2019, 10, 797. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-B.; Cho, G.-S. Antimicrobial Activity of Extracts and Fractions of Ginkgo biloba Leaves, Seed and Outer Seedcoat. J. Korean Soc. Food Sci. Nutr. 2011, 40, 7–13. [Google Scholar] [CrossRef][Green Version]
- Chassagne, F.; Huang, X.; Lyles, J.T.; Quave, C.L. Validation of a 16th Century Traditional Chinese Medicine Use of Ginkgo biloba as a Topical Antimicrobial. Front. Microbiol. 2019, 10, 775. [Google Scholar] [CrossRef]
- Bigliardi, B.; Galati, F. Innovation trends in the food industry: The case of functional foods. Trends Food Sci. Technol. 2013, 31, 118–129. [Google Scholar] [CrossRef]
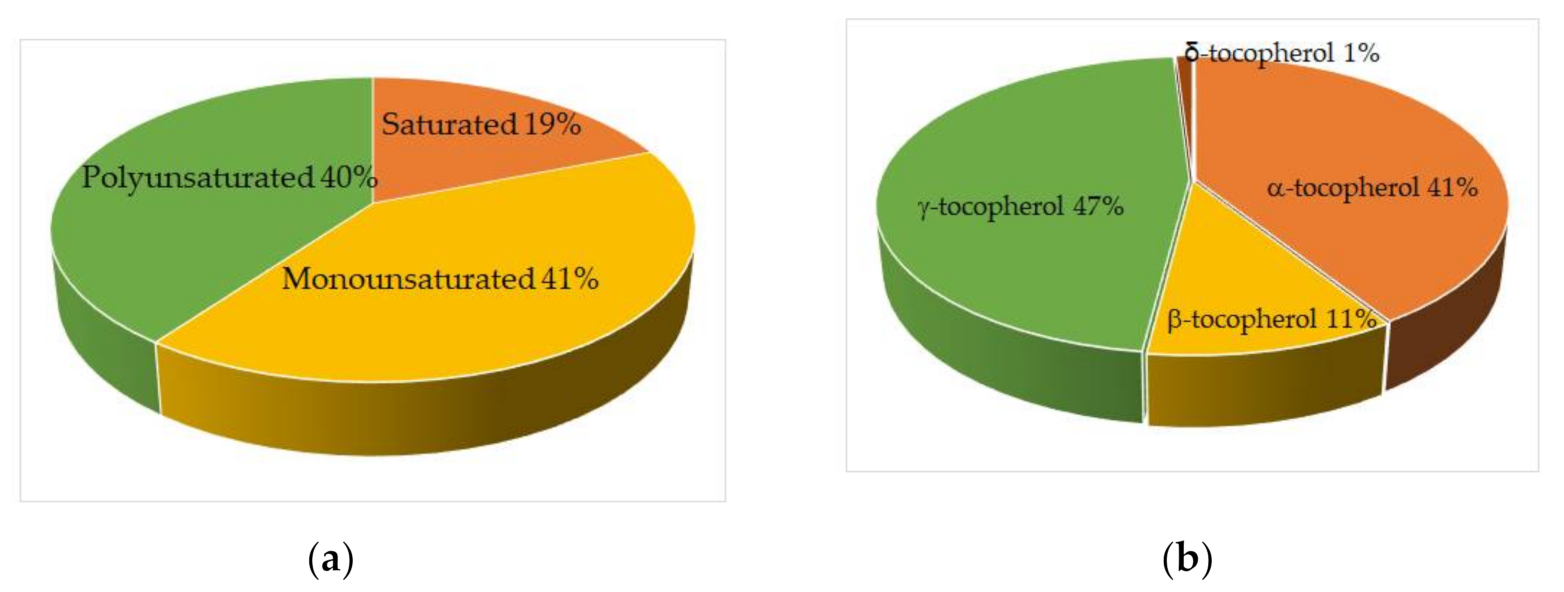
| Sample | Year of Collection | Fe, ppm | Cu, ppm | Zn, ppm |
|---|---|---|---|---|
| North region | 2013 | 29.5 ± 0.2 | 7.5 ± 0.1 | 8.8 ± 0.2 |
| 2014 | 23.8 ± 0.2 | 9.3 ± 0.4 | 23.7 ± 0.5 | |
| 2015 | 31.4 ± 1.2 | 9.0 ± 0.6 | 16.7 ± 1.1 | |
| 2016 | 64.4 ± 2.2 | 11.4 ± 0.3 | 20.4 ± 0.8 | |
| Central region | 2017 | 41.8 ± 0.8 | 9.67 ± 0.2 | 24.07 ± 0.9 |
| Medical college | ||||
| (South region) | 2017 | 18.3 ± 0.2 | 6.5 ± 0.2 | 13.5 ± 0.05 |
| 2018 | 26.0 ± 1.4 | 6.8 ± 0.4 | 17.2 ± 0.8 |
| Fatty Acids | % |
|---|---|
| C10:0 | 0.1 |
| C12:0 | 0.1 |
| C14:0 | 0.6 |
| C14:1 | 0.5 |
| C15:0 | 0.4 |
| C16:0 | 14.9 |
| C16:1 | 3.5 |
| C17:0 | 0.2 |
| C17:1 | 0.3 |
| C18:0 | 2.0 |
| C18:1 | 24.5 |
| C18:1 trans | 11.6 |
| C18:2 (ω-6) | 32.2 |
| C18:2 trans | 1.8 |
| C18:3 (ω-3) | 0.9 |
| C20:0 | 0.5 |
| C20:1 | 0.3 |
| C20:2 (ω-6) | 0.3 |
| C20:3 (ω-3) | 3.4 |
| C20:4 (ω-6) | 1.3 |
| C22:0 | 0.1 |
| C22:1 | 0.1 |
| C22:2 (ω-6) | 0.2 |
| C20:5 (ω-3) | 0.2 |
| C20:1 | 0.3 |
| C20:2 (ω-6) | 0.3 |
| C20:3 (ω-3) | 3.4 |
| C20:4 (ω-6) | 1.3 |
| C22:0 | 0.1 |
| C22:1 | 0.1 |
| C22:2 (ω-6) | 0.2 |
| C20:5 (ω-3) | 0.2 |
| C20:3 (ω-3) | 3.4 |
| Starch | RDS | SDS | TDS | RS | Total Starch |
|---|---|---|---|---|---|
| Ginkgo biloba (crop 2019) | 7.3 | 26.3 | 42.1 | 33.4 | 75.5 |
| Wheat | 26.3 | 43.0 | 67.1 | 1.7 | 68.8 |
| Maize | 12.17 | 55.1 | 71.9 | 0.8 | 72.7 |
| Standard (manufacturer’s data) | 6.9 | 16.2 | 35.9 | 47.4 | 83.3 |
| Standard (values found) | 6.0 | 16.8 | 32.8 | 48.9 | 81.7 |
| Compound | Own Results | [18] | [19] |
|---|---|---|---|
| Quercetin | 1.4–14.4 | na | nd |
| Kaempferol | nd–20.0 | na | nd |
| Isorhamnetin | 4.5–30.0 | 0.45 | nd–3.5 |
| Rutin | 2–20 | na | nd–1.9 |
| Ginkgolide A | 242 | 165.17 | 0.92–3.47 |
| Ginkgolide B | 388 * | 199.67 | 575–1988 |
| Ginkgolide J | – | na | 15–47 |
| Ginkgolide C | 143 | 136.3 | 152–600 |
| Bilobalide | 122 | 72.52 | 22–46 |
| Ginkgotoxin | 335 | na | na |
| Ginkgolic acid | 18 | 51.7 | 56.5–172.2 |
| Strain | MIC |
|---|---|
| Gram (+) bacteria | |
| Bacillus subtilis ATCC 6633 | 0.875 |
| Bacillus cereus | 0.875 |
| Bacillus amyloliquefaciens | 1.75 |
| Staphylococcus aureus ATCC 25923 | 0.875 |
| Listeria monocytogenes ATCC 8632 | – |
| Enterococcus faecalis | 3.5 |
| Micrococcus luteus | 3.5 |
| Gram (-) bacteria | |
| Salmonella enteritidis | 1.75 |
| Salmonella abony | – |
| Klebsiella sp. | – |
| Escherichia coli ATCC 8739 | 1.75 |
| Proteus vulgaris ATCC 6380 | – |
| Pseudomonas aeruginosa ATCC 9027 | 1.75 |
| Yeasts | |
| Candida albicans NBIMCC 74 | – |
| Saccharomyces cerevisiae | – |
| Fungi | |
| Aspergillus niger ATCC 1015 | 0.875 |
| Aspergillus flavus | – |
| Penicillium sp. | 1.75 |
| Rhizopus sp. | 0.109 |
| Fusarium moniliforme ATCC 38932 | 3.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomova, T.; Slavova, I.; Tomov, D.; Kirova, G.; Argirova, M.D. Ginkgo biloba Seeds—An Environmental Pollutant or a Functional Food. Horticulturae 2021, 7, 218. https://doi.org/10.3390/horticulturae7080218
Tomova T, Slavova I, Tomov D, Kirova G, Argirova MD. Ginkgo biloba Seeds—An Environmental Pollutant or a Functional Food. Horticulturae. 2021; 7(8):218. https://doi.org/10.3390/horticulturae7080218
Chicago/Turabian StyleTomova, Teodora, Iva Slavova, Desislav Tomov, Gergana Kirova, and Mariana D. Argirova. 2021. "Ginkgo biloba Seeds—An Environmental Pollutant or a Functional Food" Horticulturae 7, no. 8: 218. https://doi.org/10.3390/horticulturae7080218
APA StyleTomova, T., Slavova, I., Tomov, D., Kirova, G., & Argirova, M. D. (2021). Ginkgo biloba Seeds—An Environmental Pollutant or a Functional Food. Horticulturae, 7(8), 218. https://doi.org/10.3390/horticulturae7080218







