Optical Characteristics of Greenhouse Plastic Films Affect Yield and Some Quality Traits of Spinach (Spinacia oleracea L.) Subjected to Different Nitrogen Doses
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up and Design, and Plastic Films Properties
2.2. Plant Management
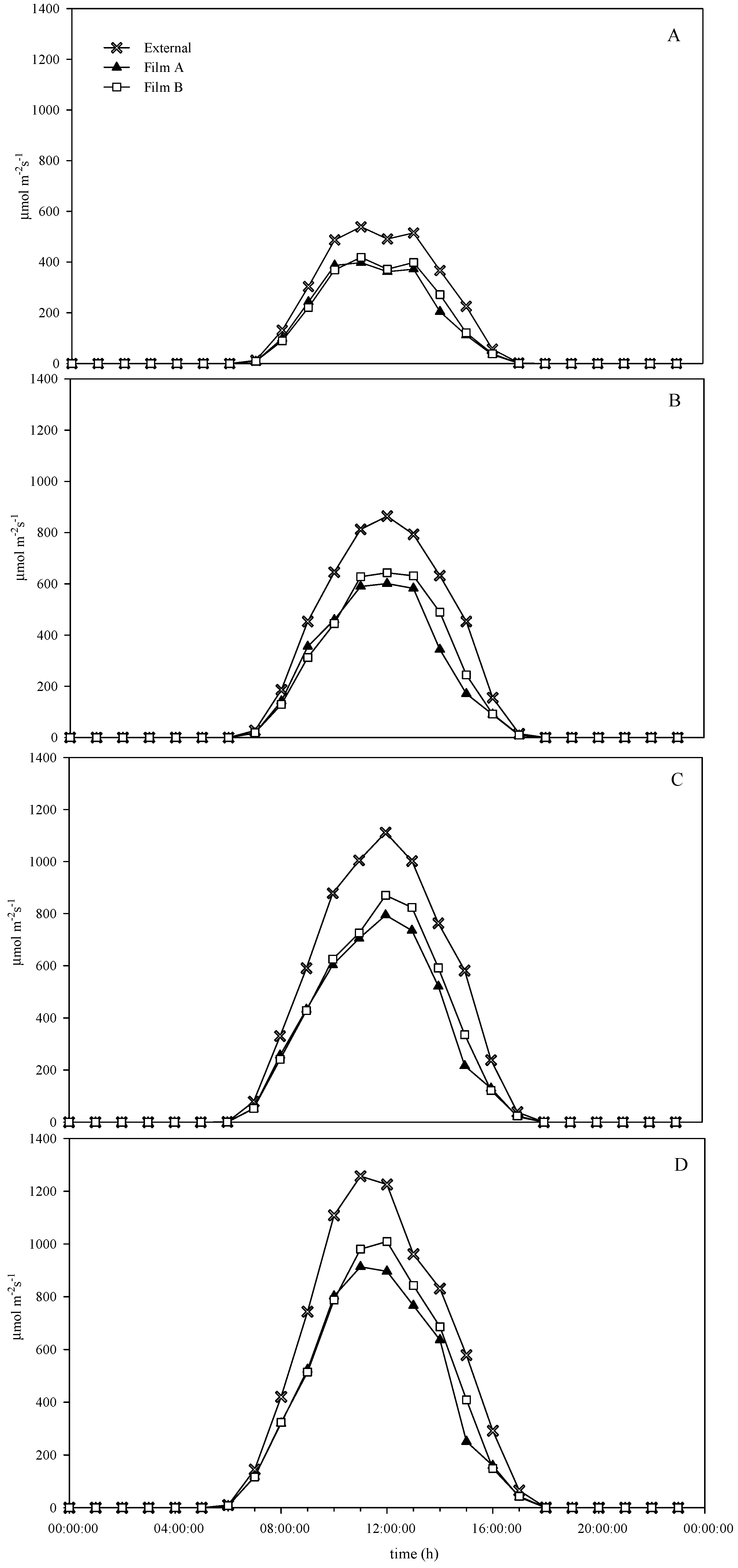
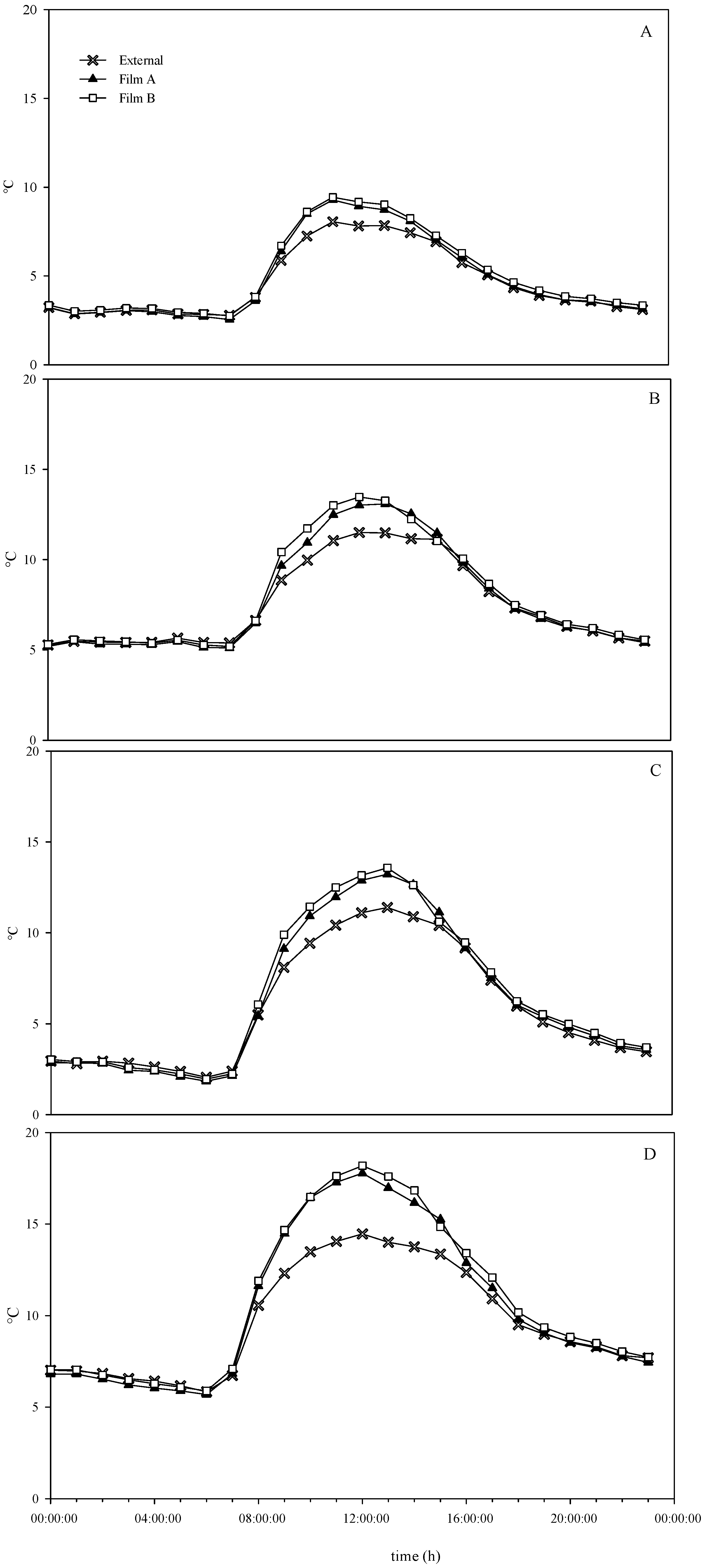
2.3. Photosynthetically Active Radiation and Temperature Measurements
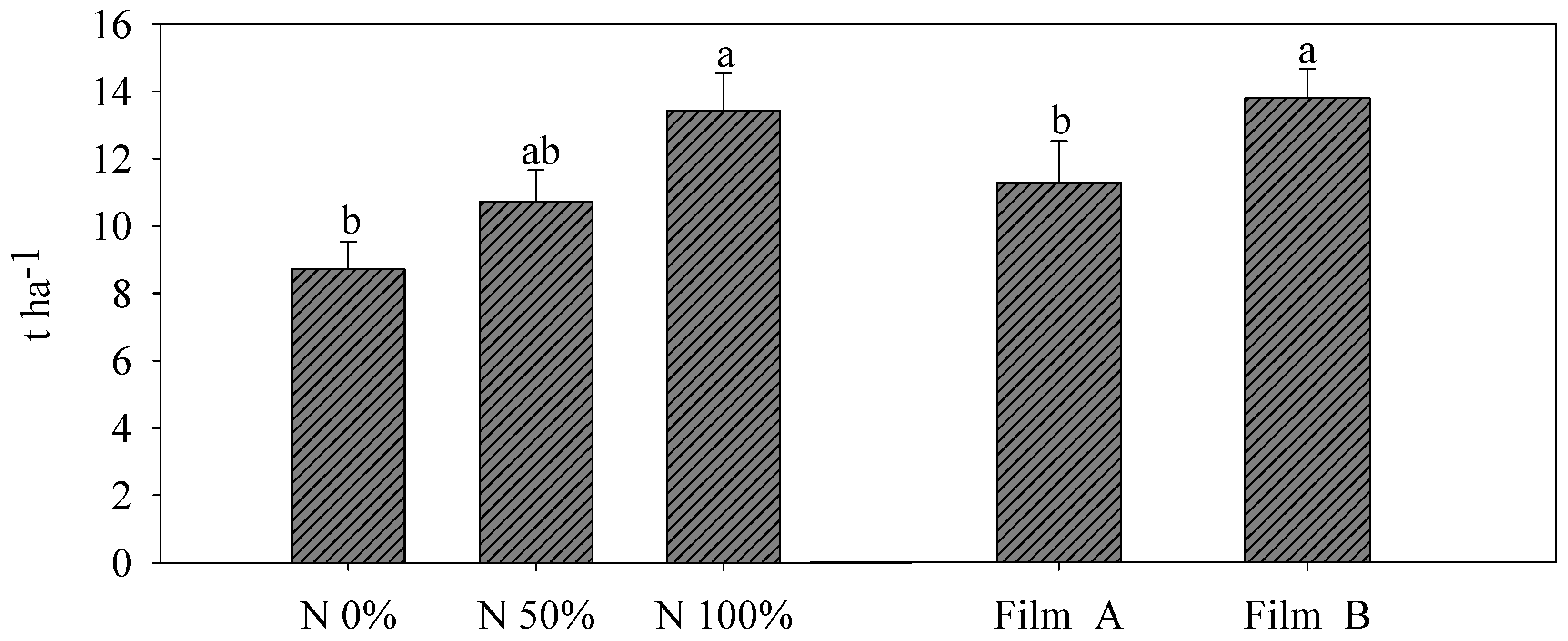
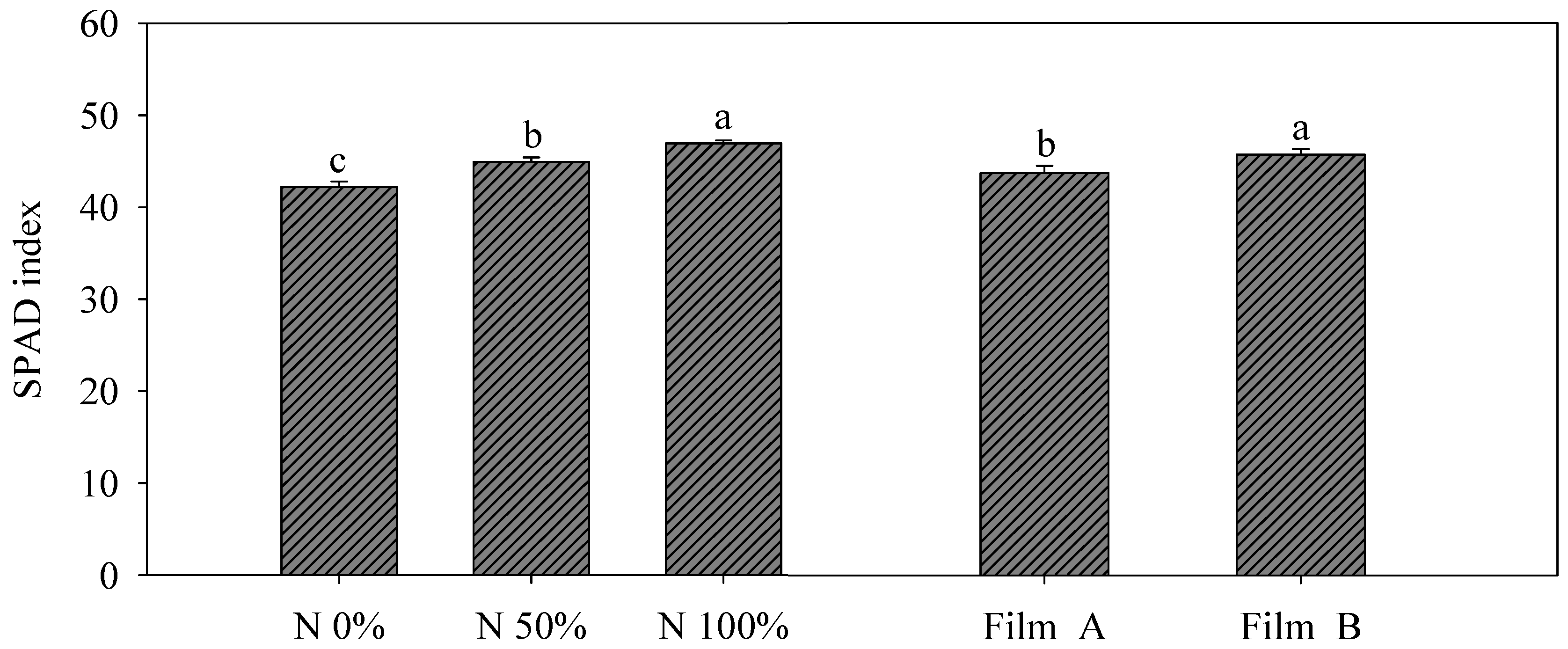
2.4. Yield Measurements, SPAD Index and Color Parameters
2.5. Qualitative Parameters Assessments
2.6. Statistical Analysis
3. Results
3.1. Environmental Conditions
3.2. Yield and SPAD Index
3.3. Spinach Colorimetric Indices, Bioactive Qualities and Dry Matter Percentage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maeda, N.; Yoshida, H.; Mizushina, Y. Chapter 26-Spinach and Health: Anticancer Effect. In Bioactive Foods in Promoting Health; Fru Vege; Academic Press: Cambridge, MA, USA, 2010; pp. 393–405. [Google Scholar] [CrossRef]
- Hanif, R.; Iqbal, Z.; Iqbal, M.; Hanif, S.; Rasheed, M. Use of vegetables as nutritional food: Role in human health. J. Agric. Biol. Sci. 2006, 1, 18–22. [Google Scholar]
- FAOSTAT 2018. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 2 March 2021).
- ISTAT 2019. Available online: https://www.istat.it/it/agricoltura?dati (accessed on 2 March 2021).
- Non Renseigné, U.A.; Iqbal, M. Nitrate accumulation in plants, factors affecting the process, and human health implications. A review. Agron. Sustain. Dev. 2007, 27, 45–57. [Google Scholar]
- Colla, G.; Kim, H.J.; Myriacou, M.C.; Rouphael, Y. Nitrate in fruits and vegetables. Sci. Hortic. 2018, 237, 221–238. [Google Scholar] [CrossRef]
- Salehzadeh, H.; Maleki, A.; Rezaee, R.; Shahmoradi, B.; Ponnet, K. The nitrate content of fresh and cooked vegetables and their health-related risks. PLoS ONE 2020, 15, e0227551. [Google Scholar] [CrossRef]
- Barker, A.V.; Maynard, D.N. Nutritional factors affecting nitrate accumulation in spinach. Commun. Soil Sci. Plant Anal. 1971, 2, 471–478. [Google Scholar] [CrossRef]
- Stagnari, F.; Di Bitetto, V.; Pisante, M. Effects of N fertilizers and rates on yield, safety and nutrients in processing spinach genotypes. Sci. Hortic. 2007, 114, 225–233. [Google Scholar] [CrossRef]
- Goh, K.M.; Vity Akon, P. Effects of fertilizers on vegetable production 2. Effects of nitrogen fertilizers on nitrogen content and nitrate accumulation of spinach and beetroot. N. Zeal. J. Agric. Res. 1986, 29, 485–494. [Google Scholar] [CrossRef]
- Porto, M.L.; Alves, J.C.; Souza, A.P.; Araujo, R.C.; Arruda, J.A. Nitrate production and accumulation in lettuce as affected by mineral Nitrogensupply and organic fertilization. Hortic. Bras. 2008, 26, 227–230. [Google Scholar] [CrossRef]
- Abubaker, S.M.; Abu-Zahra, T.R.; Alzubi, Y.A.; Tahboub, A.B. Nitrate accumulation in spinach (Spinacia oleracea L.) tissues under different fertilization regimes. J. Food Agric. Environ. 2010, 8, 778–780. [Google Scholar]
- Breimer, T. Environmental factors and cultural measures affecting the nitrate content in spinach. Fert. Res. 1982, 3, 191–292. [Google Scholar] [CrossRef]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Nocerino, S.; Rouphael, Y.; Colla, G.; El-Nakhel, C.; Mori, M. Nitrogen Use and Uptake Efficiency and Crop Performance of Baby Spinach (Spinacia oleracea L.) and Lamb’s Lettuce (Valerianella locusta L.) Grown under Variable Sub-Optimal N Regimes Combined with Plant-Based Biostimulant Application. Agronomy 2020, 10, 278. [Google Scholar] [CrossRef]
- Bian, Z.; Wang, Y.; Zhang, X.; Li, T.; Grundy, S.; Yang, Q.; Cheng, R. A Review of Environment Effects on Nitrate Accumulation in Leafy Vegetables Grown in Controlled Environments. Foods 2020, 9, 732. [Google Scholar] [CrossRef] [PubMed]
- Stagnari, F.; Galieni, A.; Pisante, M. Shading and nitrogen management affect quality, safety and yield of greenhouse-grown leaf lettuce. Sci. Hort. 2015, 192, 70–79. [Google Scholar] [CrossRef]
- Fu, Y.; Li, H.Y.; Yu, J.; Liu, H.; Cao, Z.Y.; Manukovsky, N.S.; Liu, H. Interaction effects of light intensity and nitrogen concentration on growth, photosynthetic characteristics and quality of lettuce (Lactuca sativa L. Var. youmaicai). Sci. Hort. 2017, 214, 51–57. [Google Scholar] [CrossRef]
- Santamaria, P. Nitrate in vegetables: Toxicity, content, intake and EC regulation (review). J. Sci. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Di Mola, I.; Rouphael, Y.; Colla, G.; Fagnano, M.; Paradiso, R.; Mori, M. Morph physiological traits and nitrate content of greenhouse lettuce as affected by irrigation with saline water. HortScience 2017, 52, 1716–1721. [Google Scholar] [CrossRef]
- Di Mola, I.; Rouphael, Y.; Ottaiano, L.; Duri, L.G.; Mori, M.; De Pascale, S. Assessing the effects of salinity on yield, leaf gas exchange and nutritional quality of spring greenhouse lettuce. Acta Hortic. 2018, 1227, 479–484. [Google Scholar] [CrossRef]
- Caruso, G.; Formisano, L.; Cozzolino, E.; Pannico, A.; El-Nakhel, C.; Rouphael, Y.; Tallarita, A.; Cenvinzo, V.; De Pascale, S. Shading affects Yield, Elemental Composition and Antioxidants of Perennial Wall Rocket Crops Grown from Spring to Summer in Southern Italy. Plants 2020, 9, 933. [Google Scholar] [CrossRef] [PubMed]
- Cometti, N.N.; Martins, M.Q.; Bremenkamp, C.A.; Nunes, J.A. Nitrate concentration in lettuce leaves depending on photosynthetic photon flux and nitrate concentration in the nutrient solution. Hortic. Bras. 2011, 29, 548–553. [Google Scholar] [CrossRef][Green Version]
- Song, J.; Huang, H.; Hao, Y.; Song, S.; Zhang, Y.; Su, W.; Liu, H. Nutritional quality, mineral and antioxidant content in lettuce affected by interaction of light intensity and nutrient solution concentration. Sci. Rep. 2020, 10, 2796. [Google Scholar] [CrossRef] [PubMed]
- Limantara, L.; Dettling, M.; Indrawati, R.; Brotosudarmo, T.H.P. Analysis on the Chlorophyll Content of Commercial Green Leafy Vegetables. Procedia Chem. 2015, 14, 225–231. [Google Scholar] [CrossRef]
- Hedges, J.; Lister, C.E. Nutritional attributes of spinach, silver beet and eggplant. Crop & Food Research Confidential Report No. 1928. N. Zeal. J. Agric. Res. 2007, 29, 1–30. [Google Scholar]
- Jiang, H.; Yang, Y.; Wang, H.; Bai, Y.; Bai, Y. Surface Diffuse Solar Radiation Determined by Reanalysis and Satellite over East Asia: Evaluation and Comparison. Remote Sens. 2020, 12, 1387. [Google Scholar] [CrossRef]
- Marpaung, F.; Hirano, T. Environmental dependence and seasonal variation of diffuse solar radiation in tropical peatland. J. Agric. Meteorol. 2014, 70, 223–232. [Google Scholar] [CrossRef]
- Kanniah, K.D.; Beringer, J.; North, P.; Hutley, L. Control of atmospheric particles on diffuse radiation and terrestrial plant productivity: A review. Prog. Phys. Geog. 2012. [Google Scholar] [CrossRef]
- Department of Energy. Available online: https://www.energy.gov/eere/solar/solar-radiation-basics (accessed on 19 March 2021).
- Heuberger, H.; Preager, U.; Georgi, M.; Schirrmacher, G.; Graßmann, J.; Schnitzler, W.H. Precision stressing by UV-B radiation to improvequality of spinach under protected cultivation. Acta Hort. 2004, 659, 201–206. [Google Scholar] [CrossRef]
- Peet, M.M. Greenhouse crop stress management. Acta Hort. 1999, 481, 643–654. [Google Scholar] [CrossRef]
- Warren, W.J.; Hand, D.W.; Hannah, M.A. Light interception and photosynthetic efficiency in some glasshouse crops. J. Exp. Bot. 1992, 43, 363–373. [Google Scholar]
- Gruda, N. Impact of environmental factors on product quality of greenhouse vegetables for fresh consumption. CRC Crit. Rev. Plant Sci. 2005, 24, 227–247. [Google Scholar] [CrossRef]
- Cozzolino, E.; Di Mola, I.; Ottaiano, L.; El-Nakhel, C.; Mormile, P.; Rouphael, Y.; Mori, M. The potential of greenhouse diffusing cover material on yield and nutritive values of lamb’s lettuce grown under diverse nitrogen regimes. Italus Hortus 2020, 27, 55–67. [Google Scholar] [CrossRef]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Giordano, M.; Rouphael, Y.; Colla, G.; Mori, M. Effect of Vegetal- and Seaweed Extract-Based Biostimulants on Agronomical and Leaf Quality Traits of Plastic Tunnel-Grown Baby Lettuce under Four Regimes of Nitrogen Fertilization. Agronomy 2019, 9, 571. [Google Scholar] [CrossRef]
- Pellegrini, N.; Re, R.; Yang, M.; Rice-Evans, C. Screening of dietary carotenoids and carotenoid-rich-fruit extracts for antioxidant activities applying 2,2-Azinobis (3-ethylenebenzothiazoline-6-sulfonic acid radical cation decoloration assay. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 379–384. [Google Scholar]
- Kampfenkel, K.; Van Montagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substratesand antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Lichtenhaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. In Proceedings of the Biochemical Society Transactions 603rd Meeting, Liverpool, UK, 1 October 1983; Volume 11, pp. 591–592. [Google Scholar] [CrossRef]
- Duek, T.; Janse, J.; Li, T.; Kempkes, F.; Eveleens, B. Influence of diffuse glass on the growth and production of tomato. Acta Hort. 2012, 956, 75–82. [Google Scholar] [CrossRef]
- Chun, H.; Yum, S.; Kang, Y.; Kim, H.; Lee, S. Environments and canopy productivity of green pepper (Capsicum annuum L.) in a greenhouse using light-diffused woven film. Korean J. Hortic. Sci. Technol. 2005, 23, 367–371. [Google Scholar]
- Roderick, M.L.; Farquhar, G.D.; Berry, S.L.; Noble, I.R. On the direct effect of clouds and atmospheric particles on the productivity and structure of vegetation. Oecologia 2001, 129, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Mola, I.D.; Conti, S.; Cozzolino, E.; Melchionna, G.; Ottaiano, L.; Testa, A.; Sabatino, L.; Rouphael, Y.; Mori, M. Plant-Based Protein Hydrolysate Improves Salinity Tolerance in Hemp: Agronomical and Physiological Aspects. Agronomy 2021, 11, 342. [Google Scholar] [CrossRef]
- Urban, O.; Klem, K.; Ač, A.; Havránková, K.; Holišová, P.; Navrátil, M.; Zitová, M.; Kozlová, K.; Pokorný, R.; Šprtová, M.; et al. Impact of clear and cloudy sky conditions on the vertical distribution of photosynthetic CO2 uptake within a spruce canopy. Funct. Ecol. 2012, 26, 46–55. [Google Scholar] [CrossRef]
- Steiner, A.L.; Chameides, W.L. Aerosol-induced thermal effects increase modelled terrestrial photosynthesis and transpiration. Tellus B 2005, 57, 404–411. [Google Scholar] [CrossRef]
- Riga, P.; Benedicto, L. Effects of light-diffusing plastic film on lettuce production and quality attributes. Span. J. Agric. Res. 2017, 15, 085. [Google Scholar] [CrossRef]
- Neugart, S.; Schreiner, M. UVB and UVA as eustressors in horticultural and agricultural crops. Sci. Hort. 2018, 234, 370–381. [Google Scholar] [CrossRef]
- Kittas, C.; Tchamitchian, M.; Katsoulas, N.; Karaiskou, P.; Papaioannou, C.H. Effect of two UV-absorbing greenhouse-covering films on growth and yield of an eggplant soilless crop. Sci. Hort. 2006, 110, 30–37. [Google Scholar] [CrossRef]
- Paul, N.D.; Moore, J.P.; McPherson, M.; Lambourne, C.; Croft, P.; Heaton, J.C.; Wargent, J.J. Ecological responses to UV radiation: Interactions between the biological effects of UV on plants and on associated organisms. Phys. Plant. 2012, 145, 565–581. [Google Scholar] [CrossRef]
- Kataria, S.; Guruprasad, K.N.; Ahuja, S.; Singh, B. Enhancement of growth, photosynthetic performance and yield by exclusion of ambient UV components in C3 and C4 plants. J. Phot. Phot. B Biol. 2013, 127, 140–152. [Google Scholar] [CrossRef]
- Sakalauskaite, J.; Viškelis, P.; Duchovskis, P.; Dambrauskiene, E.; Sakalauskiene, S.; Samuoliene, G.; Brazaityte, A. Supplementary UV-B irradiation effects on basil (Ocimum basilicum L.) growth and phytochemical properties. J. Food Agric. Environ. 2012, 10, 342–346. [Google Scholar]
- Pal, M.A.; Sharma, A.; Abrol, Y.P.; Sengupta, U.K. Exclusion of UV-B radiation from normal solar spectrum on growth of mung bean and maize. Agric. Ecosyst. Environ. 1997, 61, 29–34. [Google Scholar] [CrossRef]
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Senatore, M.; Giordano, M.; El-Nakhel, C.; Sacco, A.; Rouphael, Y.; Colla, G.; Mori, M. Plant-based biostimulants influence the agronomical, physiological, and qualitative responses of baby rocket leaves under diverse nitrogen conditions. Plants 2019, 8, 522. [Google Scholar] [CrossRef] [PubMed]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Giordano, M.; Rouphael, Y.; El Nakhel, C.; Leone, V.; Mori, M. Effect of seaweed (Ecklonia maxima) extract and legume-derived protein hydrolysate biostimulants on baby leaf lettuce grown on optimal doses of nitrogen under greenhouse conditions. Aust. J. Crop Sci. 2020, 14, 1456–1464. [Google Scholar] [CrossRef]
- Colonna, E.; Rouphael, Y.; Barbieri, G.; De Pascale, S. Nutritional quality of leafy vegetables harvested at two light intensities. Food Chem. 2020, 199, 702–710. [Google Scholar] [CrossRef]
- Nitz, G.M.; Schnitzler, W.H. Effect of PAR and UV-B radiation on the quality and quantity of the essential oil in sweet basil (Ocimum basilicum L.). Acta Hort. 2004, 659, 375–381. [Google Scholar] [CrossRef]
- Jansen, M.A.K.; Gaba, V.; Greenberg, B.M. Higher plants and UV-B radiation: Balancing damage, repair and acclimation. Trends Plant Sci. 1998, 3, 131–135. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Z.; Gerendás, J. Effects of nitrogen and sulfur on total phenolics and antioxidant activity in two genotypes of leaf mustard. J. Plant Nutr. 2008, 31, 1642–1655. [Google Scholar] [CrossRef]
- Weerakkody, W.A.P. Nutritional value of fresh leafy vegetables as affected by pre-harvest factors. Acta Hort. 2003, 604, 511–515. [Google Scholar] [CrossRef]
- Oyama, H.; Shinohara, Y.; Ito, T. Effects of air temperature and light intensity onβ-carotene concentration in spinach and lettuce. J. Japan. Soc. Hort. Sci. 1999, 68, 414–420. [Google Scholar] [CrossRef]
- Bruning-Fann, C.S.; Kaneene, J.B. The effects of nitrate, nitrite and N-nitroso compounds on human health: A review. Vet. Hum. Toxicol. 1993, 35, 521–538. [Google Scholar]
- Milkowski, A.; Garg, H.K.; Coughlin, J.R.; Bryan, N.S. Nutritional epidemiology in the context of nitric oxide biology: A risk–benefit evaluation for dietary nitrite and nitrate. Nitric Oxide 2010, 22, 110–119. [Google Scholar] [CrossRef]
- Speijers, G.; Van den Brandt, P.A. Nitrite and potential endogenous formation of N-nitroso compounds; safety evaluation of certain food additives, JECFA. WHO Food Addit. Ser. 2003, 50, 49–74. [Google Scholar]
- European Community. Reg. n° 1258 of 2 December 2011. Off. J. Eur. Union 2011, L320, 15–17. [Google Scholar]
- Proietti, S.; Moscatello, S.; Giacomelli, G.A.; Battistelli, A. Influence of the interaction between light intensity and CO2 concentration on productivity and quality of spinach (Spinacia oleracea L.) grown in fully controlled environment. Adv. Space Res. 2013, 52, 1193–1200. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbate biosynthesis and function in photoprotection. Philos. T Roy. Soc. B 2000, 355, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.L.; Liu, W.K.; Yang, Q.C. Quality changes in hydroponic lettuce grown under pre-harvest short-duration continuous light of different intensities. J. Hort. Sci. Biotech. 2012, 87, 429–434. [Google Scholar] [CrossRef]
- Demšar, J.; Osvald, J.; Vodnik, D. The effect of light-dependent application of nitrate on the growth of aeroponically grown lettuce (Lactuca sativa L.). J. Am. Soc. Hortic. Sci. 2004, 129, 570–575. [Google Scholar] [CrossRef]
| Treatments | L* | a* | b* |
|---|---|---|---|
| Fertilization | |||
| N0% | 38.99 ± 0.63 a | −14.33 ± 0493 | 19.87 ± 0.65 |
| N50% | 37.48 ± 0.55 b | −13.52 ± 0.35 | 18.44 ± 0.44 |
| N100% | 37.31 ± 0.76 b | −13.40 ± 0.67 | 18.60 ± 0.82 |
| Film | |||
| Film A | 38.53 ± 0.67 a | −13.92 ± 0.47 | 19.42 ± 0.82 |
| Film B | 37.32 ± 0.51 b | −13.58 ± 0.42 | 18.51 ± 0.69 |
| Significance | |||
| Fertilization (N) | * | ns | ns |
| Film (F) | * | ns | ns |
| N × F | ns | ns | ns |
| Treatments | ABTS | Total Phenols | AsA | DM |
|---|---|---|---|---|
| mM Trolox eq. 100g−1 dw | mg Gallic Acid g−1 dw | mg 100 g−1 fw | % | |
| Fertilization | ||||
| N0% | 22.77 ± 1.70 | 3.22 ± 0.18 a | 33.49 ± 4.93 | 9.9 ± 0.6 a |
| N50% | 21.90 ± 1.29 | 2.88 ± 0.17 a | 27.45 ± 7.33 | 8.8 ± 0.2 b |
| N100% | 20.55 ± 1.72 | 2.48 ± 0.15 b | 23.62 ± 3.81 | 8.3 ± 0.3 b |
| Film | ||||
| Film A | 22.10 ± 1.91 | 2.95 ± 0.17 | 40.62 ± 6.71 a | 9.4 ± 0.5 a |
| Film B | 21.42 ± 1.23 | 2.78 ± 0.16 | 15.75 ± 5.33 b | 8.6 ± 0.2 b |
| Significance | ||||
| Fertilization (N) | ns | ** | ns | * |
| Film (F) | ns | ns | ** | * |
| N × F | ns | ns | ns | ns |
| Treatments | Chlorophyll a | Chlorophyll b | Total Chlorophylls | Nitrate |
|---|---|---|---|---|
| mg g−1 fw | mg g−1 fw | mg g−1 fw | mg kg−1 fw | |
| Fertilization | ||||
| N0% | 0.905 ± 0.034 b | 0.547 ± 0.045 b | 1.452 ± 0.079 b | 52.3 ± 20.8 c |
| N50% | 0.976 ± 0.044 ab | 0.716 ± 0.105 a | 1.692 ± 0.146 a | 1968.4 ± 650.7 b |
| N100% | 1.015 ± 0.025 a | 0.786 ± 0.075 a | 1.800 ± 0.099 a | 3205.5 ± 537.5 a |
| Film | ||||
| Film A | 0.976 ± 0.029 | 0.689 ± 0.075 | 1.665 ± 0.103 | 476.4 ± 134.4 b |
| Film B | 0.954 ± 0.040 | 0.677 ± 0.076 | 1.632 ± 0.114 | 3007.7 ± 671.6 a |
| Significance | ||||
| Fertilization (N) | * | * | * | ** |
| Film (F) | ns | ns | ns | ** |
| N x F | ns | ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mola, I.D.; Ottaiano, L.; Cozzolino, E.; Sabatino, L.; Sifola, M.I.; Mormile, P.; El-Nakhel, C.; Rouphael, Y.; Mori, M. Optical Characteristics of Greenhouse Plastic Films Affect Yield and Some Quality Traits of Spinach (Spinacia oleracea L.) Subjected to Different Nitrogen Doses. Horticulturae 2021, 7, 200. https://doi.org/10.3390/horticulturae7070200
Mola ID, Ottaiano L, Cozzolino E, Sabatino L, Sifola MI, Mormile P, El-Nakhel C, Rouphael Y, Mori M. Optical Characteristics of Greenhouse Plastic Films Affect Yield and Some Quality Traits of Spinach (Spinacia oleracea L.) Subjected to Different Nitrogen Doses. Horticulturae. 2021; 7(7):200. https://doi.org/10.3390/horticulturae7070200
Chicago/Turabian StyleMola, Ida Di, Lucia Ottaiano, Eugenio Cozzolino, Leo Sabatino, Maria Isabella Sifola, Pasquale Mormile, Christophe El-Nakhel, Youssef Rouphael, and Mauro Mori. 2021. "Optical Characteristics of Greenhouse Plastic Films Affect Yield and Some Quality Traits of Spinach (Spinacia oleracea L.) Subjected to Different Nitrogen Doses" Horticulturae 7, no. 7: 200. https://doi.org/10.3390/horticulturae7070200
APA StyleMola, I. D., Ottaiano, L., Cozzolino, E., Sabatino, L., Sifola, M. I., Mormile, P., El-Nakhel, C., Rouphael, Y., & Mori, M. (2021). Optical Characteristics of Greenhouse Plastic Films Affect Yield and Some Quality Traits of Spinach (Spinacia oleracea L.) Subjected to Different Nitrogen Doses. Horticulturae, 7(7), 200. https://doi.org/10.3390/horticulturae7070200











