Sensory Profile, Shelf Life, and Dynamics of Bioactive Compounds during Cold Storage of 17 Edible Flowers
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Sensory Analysis
2.2.1. Panel Member Selection and Training
2.2.2. Sensory Evaluation Test
2.2.3. Quantitative Descriptive Analysis (QDA)
2.3. Shelf Life
2.4. Plant Extracts
2.5. Bioactive Compounds
2.5.1. Total Polyphenols
2.5.2. Total Anthocyanins
2.6. Antioxidant Activity
2.6.1. DPPH Assay
2.6.2. ABTS Assay
2.6.3. FRAP Assay
2.7. Statistical Analysis
3. Results
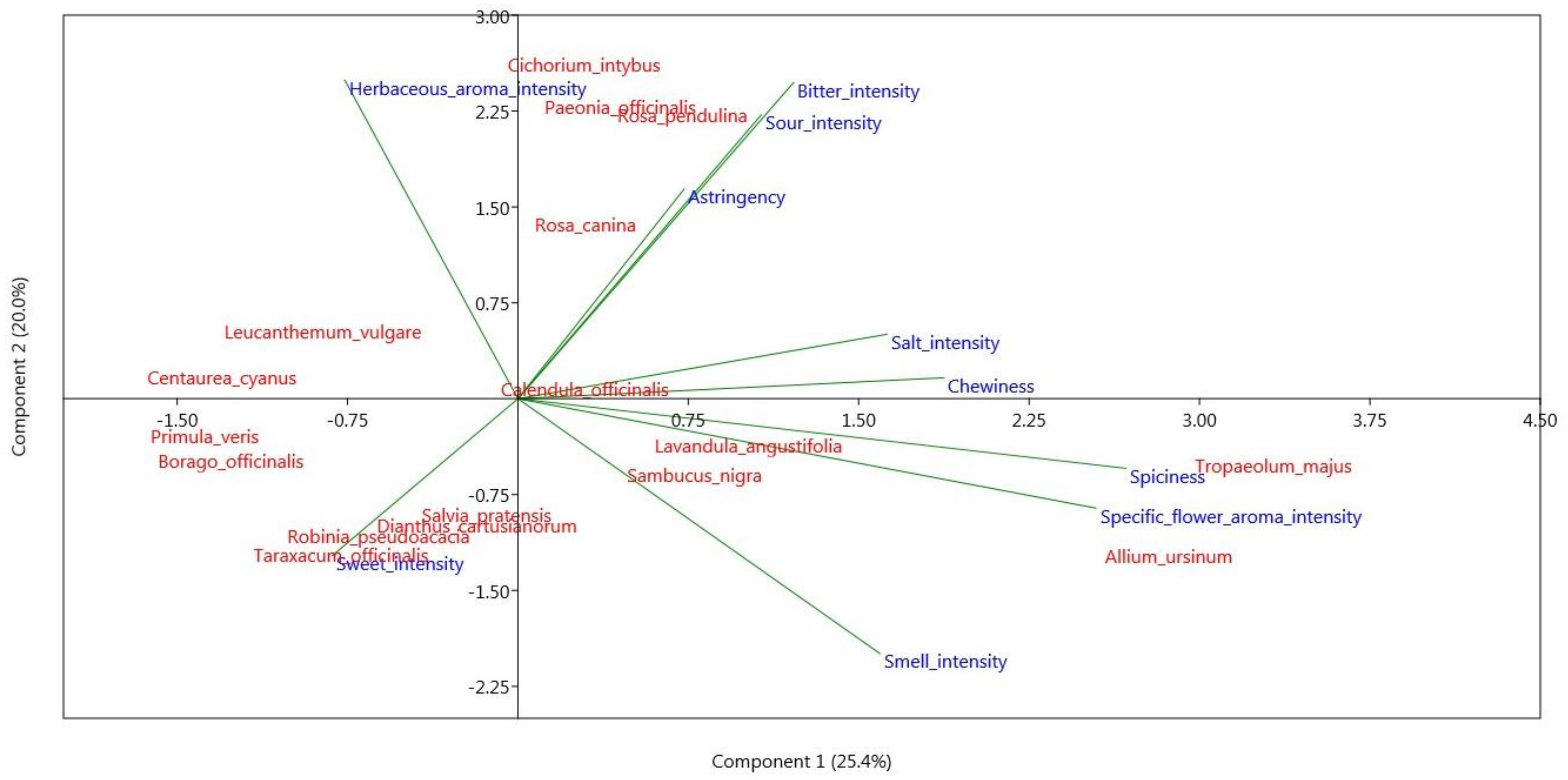
3.1. Sensory Analysis
3.1.1. Lexicon and QDA Sensory Sheet Definition
3.1.2. Sensory Profiles
3.1.3. Subjective Judgement
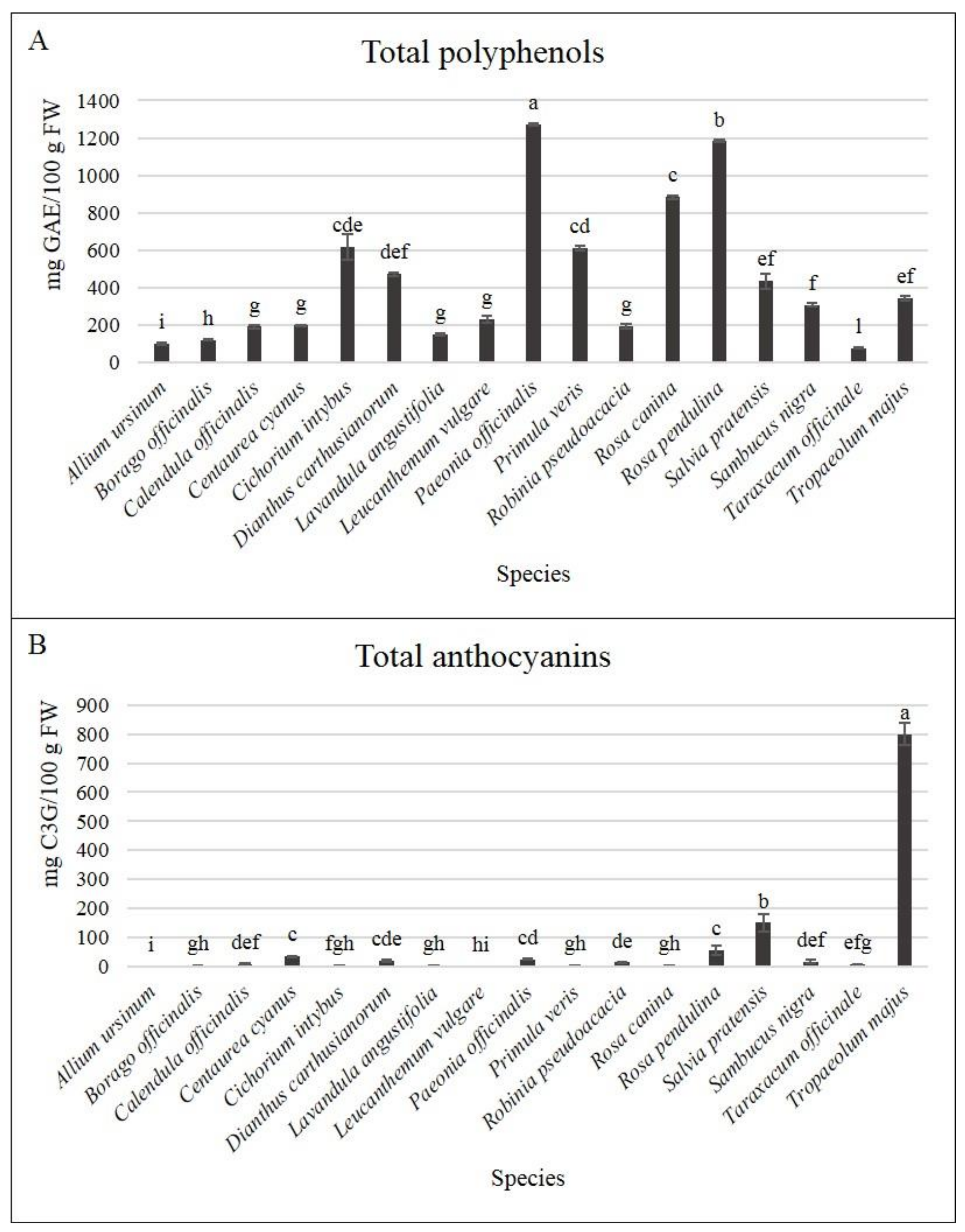
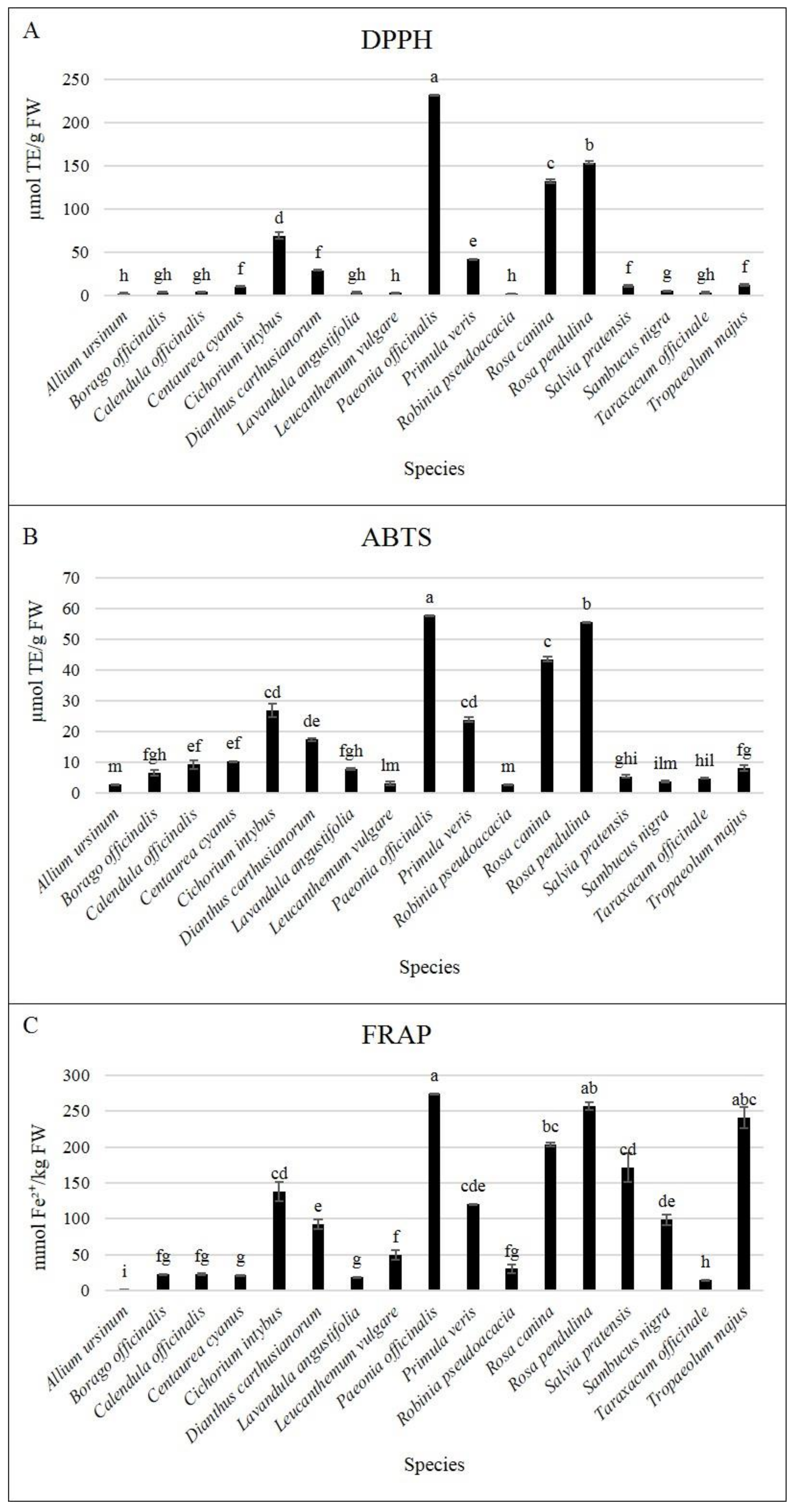
3.2. Bioactive Compounds and Antioxidant Activity at Harvest
3.3. Shelf Life and Dynamics of Bioactive Compounds
Antioxidant Activity during Postharvest
4. Discussion
4.1. Sensory Evaluation
4.2. Shelf Life
4.3. Bioactive Compounds and Antioxidant Activity Dynamics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mlcek, J.; Rop, O. Fresh edible flowers of ornamental plants—A new source of nutraceutical foods. Trends Food Sci. Technol. 2011, 22, 561–569. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. Edible flowers: A review of the nutritional, antioxidant, antimicrobial properties and effects on human health. J. Food Compos. Anal. 2017, 60, 38–50. [Google Scholar] [CrossRef]
- Scariot, V.; Gaino, W.; Demasi, S.; Caser, M.; Ruffoni, B. Flowers for edible gardens: Combinations of species and colours for northwestern Italy. Acta Hortic. 2018, 1215, 363–368. [Google Scholar] [CrossRef]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants. Volume 7, Flowers; Springer: Dordrecht, The Netherlands, 2014; ISBN 978-94-007-7394-3. [Google Scholar]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants. Volume 8, Flowers; Springer: Dordrecht, The Netherlands, 2014; ISBN 978-94-017-8747-5. [Google Scholar]
- Chitrakar, B.; Zhang, M.; Bhandari, B. Edible flowers with the common name “marigold”: Their therapeutic values and processing. Trends Food Sci. Technol. 2019, 89, 76–87. [Google Scholar] [CrossRef]
- Koike, A.; Barreira, J.C.M.; Barros, L.; Santos-Buelga, C.; Villavicencio, A.L.C.H.; Ferreira, I.C.F.R. Edible flowers of Viola tricolor L. as a new functional food: Antioxidant activity, individual phenolics and effects of gamma and electron-beam irradiation. Food Chem. 2015, 179, 6–14. [Google Scholar] [CrossRef]
- Chen, N.H.; Wei, S. Factors influencing consumers’ attitudes towards the consumption of edible flowers. Food Qual. Prefer. 2017, 56, 93–100. [Google Scholar] [CrossRef]
- Rop, O.; Mlcek, J.; Jurikova, T.; Neugebauerova, J.; Vabkova, J. Edible Flowers—A New Promising Source of Mineral Elements in Human Nutrition. Molecules 2012, 17, 6672–6683. [Google Scholar] [CrossRef]
- Cunningham, E. What nutritional contribution do edible flowers make? J. Acad. Nutr. Diet. 2015, 115, 856. [Google Scholar] [CrossRef]
- Fernandes, L.; Saraiva, J.A.; Pereira, J.A.; Casal, S.; Ramalhosa, E. Post-harvest technologies applied to edible flowers: A review. Food Rev. Int. 2019, 35, 132–154. [Google Scholar] [CrossRef]
- Falla, N.M.; Contu, S.; Demasi, S.; Caser, M.; Scariot, V. Environmental impact of edible flower production: A case study. Agronomy 2020, 10, 579. [Google Scholar] [CrossRef]
- Demasi, S.; Caser, M.; Donno, D.; Ravetto Enri, S.; Lonati, M.; Scariot, V. Exploring wild edible flowers as a source of bioactive compounds: New perspectives in horticulture. Folia Hortic. 2021, 33, 1–22. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible flowers: Emerging components in the diet. Trends Food Sci. Technol. 2019, 93, 244–258. [Google Scholar] [CrossRef]
- Grzeszczuk, M.; Stefaniak, A.; Pachlowska, A. Biological value of various edible flower species. Sci. Pol. 2016, 15, 109–119. [Google Scholar]
- González-Barrio, R.; Periago, M.J.; Luna-Recio, C.; Garcia-Alonso, F.J.; Navarro-González, I. Chemical composition of the edible flowers, pansy (Viola wittrockiana) and snapdragon (Antirrhinum majus) as new sources of bioactive compounds. Food Chem. 2018, 252, 373–380. [Google Scholar] [CrossRef]
- Kaisoon, O.; Konczak, I.; Siriamornpun, S. Potential health enhancing properties of edible flowers from Thailand. Food Res. Int. 2012, 46, 563–571. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; Tenuta, M.C.; Menichini, F.; Xiao, J.; Tundis, R. Edible flowers: A rich source of phytochemicals with antioxidant and hypoglycemic properties. J. Agric. Food Chem. 2016, 64, 2467–2474. [Google Scholar] [CrossRef]
- Ceccanti, C.; Landi, M.; Benvenuti, S.; Pardossi, A.; Guidi, L. Mediterranean wild edible plants: Weeds or “new functional crops”. Molecules 2018, 23, 2299. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phyther. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Landi, M.; Ruffoni, B.; Combournac, L.; Guidi, L. Nutraceutical value of edible flowers upon cold storage. Ital. J. Food Sci. 2018, 30, 336–347. [Google Scholar] [CrossRef]
- Li, A.-N.; Li, S.; Li, H.-B.; Xu, D.-P.; Xu, X.-R.; Chen, F. Total phenolic contents and antioxidant capacities of 51 edible and wild flowers. J. Funct. Foods 2014, 6, 319–330. [Google Scholar] [CrossRef]
- Lu, B.; Li, M.; Yin, R. Phytochemical content, health benefits, and toxicology of common edible flowers: A review (2000–2015). Crit. Rev. Food Sci. Nutr. 2016, 56, S130–S148. [Google Scholar] [CrossRef]
- Zheng, J.; Yu, X.; Maninder, M.; Xu, B. Total phenolics and antioxidants profiles of commonly consumed edible flowers in China. Int. J. Food Prop. 2018, 21, 1524–1540. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. An overview on the market of edible flowers. Food Rev. Int. 2020, 36, 258–275. [Google Scholar] [CrossRef]
- Bursać, D.; Vahčić, N.; Levaj, B.; Dragović-Uzelac, V.; Biško, A. The influence of cultivar on sensory profiles of fresh and processed strawberry fruits grown in Croatia. Flavour Fragr. J. 2007, 22, 512–520. [Google Scholar] [CrossRef]
- Rodrigues, H.; Cielo, D.P.; Goméz-Corona, C.; Silveira, A.A.S.; Marchesan, T.A.; Galmarini, M.V.; Richards, N.S.P.S. Eating flowers? Exploring attitudes and consumers’ representation of edible flowers. Food Res. Int. 2017, 100, 227–234. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food-Principles and Practices; Food Science Text Series; Springer: New York, NY, USA, 2010; Volume 24, ISBN 978-1-4419-6487-8. [Google Scholar]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Malheiro, R.; Rodrigues, N.; Saraiva, J.A.; Ramalhosa, E. Borage, calendula, cosmos, Johnny Jump up, and pansy flowers: Volatiles, bioactive compounds, and sensory perception. Eur. Food Res. Technol. 2019, 245, 593–606. [Google Scholar] [CrossRef]
- Sharif, M.K.; Butt, M.S.; Sharif, H.R.; Nasir, M. Sensory Evaluation and Consumer Acceptability. In Handbook of Food Science and Technology; University of Agriculture: Faisalabad, Pakistan, 2017; pp. 362–386. [Google Scholar]
- Benvenuti, S.; Bortolotti, E.; Maggini, R. Antioxidant power, anthocyanin content and organoleptic performance of edible flowers. Sci. Hortic. 2016, 199, 170–177. [Google Scholar] [CrossRef]
- Kelley, K.M.; Behe, B.K.; Biernbaum, J.A.; Poff, K.L. Consumer preference for edible-flower color, container size, and price. HortScience 2001, 36, 801–804. [Google Scholar] [CrossRef]
- Kelley, K.M.; Behe, B.K.; Biernbaum, J.A.; Poff, K.L. Consumer and professional chef perceptions of three edible-flower species. HortScience 2001, 36, 162–166. [Google Scholar] [CrossRef]
- Kelley, K.M.; Behe, B.K.; Biernbaum, J.A.; Poff, K.L. Combinations of colors and species of containerized edible flowers: Effect on consumer preferences. HortScience 2002, 37, 218–221. [Google Scholar] [CrossRef]
- Simoni, N.K.; Santos, F.F.; Andrade, T.A.; Villavicencio, A.L.C.H.; Pinto-e-Silva, M.E.M. The use of edible flowers in human food: Sensory analysis of preparations. ETP Int. J. Food Eng. 2018, 140–143. [Google Scholar] [CrossRef][Green Version]
- D’Antuono, L.F.; Manco, M.A. Preliminary sensory evaluation of edible flowers from wild Allium species. J. Sci. Food Agric. 2013, 93, 3520–3523. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Turner, E.R.; Luo, Y. Extending the shelf life of edible flowers with controlled release of 1-Methylcyclopropene and modified atmosphere packaging. J. Food Sci. 2012, 77, S188–S193. [Google Scholar] [CrossRef] [PubMed]
- Kelley, K.M.; Cameron, A.C.; Biernbaum, J.A.; Poff, K.L. Effect of storage temperature on the quality of edible flowers. Postharvest Biol. Technol. 2003, 27, 341–344. [Google Scholar] [CrossRef]
- Flores-López, M.L.; Cerqueira, M.A.; de Rodríguez, D.J.; Vicente, A.A. Perspectives on utilization of edible coatings and nano-laminate coatings for extension of postharvest storage of fruits and vegetables. Food Eng. Rev. 2016, 8, 292–305. [Google Scholar] [CrossRef]
- Edelman, N.F.; Jones, M.L. Evaluating ethylene sensitivity within the family Solanaceae at different developmental stages. HortScience 2014, 49, 628–636. [Google Scholar] [CrossRef]
- Edelman, N.F.; Kaufman, B.A.; Jones, M.L. Comparative evaluation of seedling hypocotyl elongation and mature plant assays for determining ethylene sensitivity in bedding plants. HortScience 2014, 49, 472–480. [Google Scholar] [CrossRef]
- Macnish, A.J.; Jiang, C.-Z.; Negre-Zakharov, F.; Reid, M.S. Physiological and molecular changes during opening and senescence of Nicotiana mutabilis flowers. Plant Sci. 2010, 179, 267–272. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Zhang, Q.; Shan, Y. Effects of postharvest chilling and heating treatments on the sensory quality and antioxidant system of daylily flowers. Hortic. Environ. Biotechnol. 2018, 59, 671–685. [Google Scholar] [CrossRef]
- Aquino-Bolaños, E.N.; Urrutia-Hernández, T.A.; López Del Castillo-Lozano, M.; Chavéz-Servia, J.L.; Verdalet-Guzmán, I. Physicochemical parameters and antioxidant compounds in edible squash (Cucurbita pepo) flower stored under controlled atmospheres. J. Food Qual. 2013, 36, 302–308. [Google Scholar] [CrossRef]
- Demasi, S.; Falla, N.M.; Caser, M.; Scariot, V. Postharvest aptitude of Begonia semperflorens and Viola cornuta edible flowers. Adv. Hortic. Sci. 2020, 34, 13–20. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Tzionis, A.; Xylia, P.; Tzortzakis, N. Effects of salinity on tagetes growth, physiology, and shelf life of edible flowers stored in passive modified atmosphere packaging or treated with ethanol. Front. Plant Sci. 2018, 871. [Google Scholar] [CrossRef]
- Pavlović, D.R.; Veljković, M.; Stojanović, N.M.; Gočmanac-Ignjatović, M.; Mihailov-Krstev, T.; Branković, S.; Sokolović, D.; Marčetić, M.; Radulović, N.; Radenković, M. Influence of different wild-garlic (Allium ursinum) extracts on the gastrointestinal system: Spasmolytic, antimicrobial and antioxidant properties. J. Pharm. Pharmacol. 2017, 69, 1208–1218. [Google Scholar] [CrossRef]
- Bombicz, M.; Priksz, D.; Varga, B.; Kurucz, A.; Kertész, A.; Takacs, A.; Posa, A.; Kiss, R.; Szilvassy, Z.; Juhasz, B. A novel therapeutic approach in the treatment of pulmonary arterial hypertension: Allium ursinum liophylisate alleviates symptoms comparably to Sildenafil. Int. J. Mol. Sci. 2017, 18, 1436. [Google Scholar] [CrossRef]
- Sobolewska, D.; Podolak, I.; Makowska-Wąs, J. Allium ursinum: Botanical, phytochemical and pharmacological overview. Phytochem. Rev. 2015, 14, 81–97. [Google Scholar] [CrossRef]
- Karimi, E.; Oskoueian, E.; Karimi, A.; Noura, R.; Ebrahimi, M. Borago officinalis L. flower: A comprehensive study on bioactive compounds and its health-promoting properties. J. Food Meas. Charact. 2018, 12, 826–838. [Google Scholar] [CrossRef]
- Ukiya, M.; Akihisa, T.; Yasukawa, K.; Tokuda, H.; Suzuki, T.; Kimura, Y. Anti-inflammatory, anti-tumor-promoting, and cytotoxic activities of constituents of marigold (Calendula officinalis) flowers. J. Nat. Prod. 2006, 69, 1692–1696. [Google Scholar] [CrossRef]
- Muley, B.P.; Khadabadi, S.S.; Banarase, N.B. Phytochemical constituents and pharmacological activities of Calendula officinalis L. (Asteraceae): A review. Trop. J. Pharm. Res. 2009, 8, 455–465. [Google Scholar] [CrossRef]
- Palma, L. Le Piante Medicinali d’Italia; Società Editrice Internazionale: Torino, Italy, 1964. [Google Scholar]
- Rashed, M.M.A.; Tong, Q.; Nagi, A.; Li, J.; Khan, N.U.; Chen, L.; Rotail, A.; Bakry, A.M. Isolation of essential oil from Lavandula angustifolia by using ultrasonic-microwave assisted method preceded by enzymolysis treatment, and assessment of its biological activities. Ind. Crops Prod. 2017, 100, 236–245. [Google Scholar] [CrossRef]
- Facciola, S. Cornucopia: A Source Book of Edible Plants; Kampong Publications: Vista, CA, USA, 1990; ISBN 0-9628087-0-9. [Google Scholar]
- Magharri, E.; Razavi, S.M.; Ghorbani, E.; Nahar, L.; Sarker, S.D. Chemical composition, some allelopathic aspects, free-radical-scavenging property and antifungal activity of the volatile oil of the flowering tops of Leucanthemum vulgare Lam. Rec. Nat. Prod. 2015, 9, 538–545. [Google Scholar]
- Chen, Z.; Li, X.-P.; Li, Z.-J.; Xu, L.; Li, X.-M. Reduced hepatotoxicity by total glucosides of paeony in combination treatment with leflunomide and methotrexate for patients with active rheumatoid arthritis. Int. Immunopharmacol. 2013, 15, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Ghédira, K.; Goetz, P. Pivoine Paeonia officinalis L. (Paeoniaceae). Phytothérapie 2015, 13, 328–331. [Google Scholar] [CrossRef]
- Tünde, J.; Eleonora, M.; Laura, V.; Neagu, O.; Annamária, P. Bioactive compounds and antioxidant capacity of Primula veris L. flower extracts. Ecotoxicologie Zooteh. Ind. 2015, 15B, 235–242. [Google Scholar]
- Lattanzio, F.; Greco, E.; Carretta, D.; Cervellati, R.; Govoni, P.; Speroni, E. In vivo anti-inflammatory effect of Rosa canina L. extract. J. Ethnopharmacol. 2011, 137, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Guarrera, P.M.; Savo, V. Wild food plants used in traditional vegetable mixtures in Italy. J. Ethnopharmacol. 2016, 185, 202–234. [Google Scholar] [CrossRef]
- Schütz, K.; Carle, R.; Schieber, A. Taraxacum—A review on its phytochemical and pharmacological profile. J. Ethnopharmacol. 2006, 107, 313–323. [Google Scholar] [CrossRef]
- Martinez, M.; Poirrier, P.; Chamy, R.; Prüfer, D.; Schulze-Gronover, C.; Jorquera, L.; Ruiz, G. Taraxacum officinale and related species—An ethnopharmacological review and its potential as a commercial medicinal plant. J. Ethnopharmacol. 2015, 169, 244–262. [Google Scholar] [CrossRef]
- Garzón, G.A.; Wrolstad, R.E. Major anthocyanins and antioxidant activity of Nasturtium flowers (Tropaeolum majus). Food Chem. 2009, 114, 44–49. [Google Scholar] [CrossRef]
- Bazylko, A.; Granica, S.; Filipek, A.; Piwowarski, J.; Stefańska, J.; Osińska, E.; Kiss, A.K. Comparison of antioxidant, anti-inflammatory, antimicrobial activity and chemical composition of aqueous and hydroethanolic extracts of the herb of Tropaeolum majus L. Ind. Crops Prod. 2013, 50, 88–94. [Google Scholar] [CrossRef]
- Canterino, S.; Donno, D.; Mellano, M.G.; Beccaro, G.L.; Bounous, G. Nutritional and sensory survey of Citrus sinensis (L.) cultivars grown at the most northern limit of the mediterranean latitude. J. Food Qual. 2012, 35, 108–118. [Google Scholar] [CrossRef]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Torello Marinoni, D.; Cerutti, A.K.; Canterino, S.; Bounous, G. Application of sensory, nutraceutical and genetic techniques to create a quality profile of ancient apple cultivars. J. Food Qual. 2012, 35, 169–181. [Google Scholar] [CrossRef]
- Kemp, S.E.; Hollowood, T.; Hort, J. Sensory Evaluation: A Practical Handbook; John Wiley & Sons, Ltd.: Chichester, UK, 2009; ISBN 978-1-405-16210-4. [Google Scholar]
- Meilgaard, M.; Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques; CRC Press: Boca Raton, FL, USA, 1999; ISBN 9780849302763. [Google Scholar]
- Stone, H.; Sidel, J.; Oliver, S.; Woolsey, A.; Singleton, R.C. Sensory evaluation by Quantitative Descriptive Analysis. In Descriptive Sensory Analysis in Practice; Food & Nutrition Press, Inc.: Trumbull, CT, USA, 2008; pp. 23–34. [Google Scholar]
- De Biaggi, M.; Rapalino, S.; Donno, D.; Mellano, M.G.; Beccaro, G.L. Genotype influence on chemical composition and sensory traits of chestnut in 18 cultivars grown on the same rootstock and at the same agronomic conditions. Acta Hortic. 2018, 215–220. [Google Scholar] [CrossRef]
- Mellano, M.G.; Carli, C.; Folini, L.; Draicchio, P.; Beccaro, G. Training of two groups of tasters for the creation of sensory profiles of highbush blueberry cultivars grown in Northern Italy. Acta Hortic. 2009, 810, 835–840. [Google Scholar] [CrossRef]
- Mellano, M.G.; Rapalino, S.; Donno, D. Profili sensoriali di cultivar di Castanea sativa e ibridi eurogiapponesi (in Italian). Castanea 2017, 9, 8–9. [Google Scholar]
- Montevecchi, G.; Mellano, M.G.; Simone, G.V.; Masino, F.; Bignami, C.; Antonelli, A. Physico-chemical and sensory characterization of pescabivona P.G.I., A sicilian white flesh peach cultivar [Prunus persica (L.) batsch]: A case study. In Apricots and Peaches: Nutritional Properties, Post-Harvest Management and Potential Health Benefits; Nova Science Publishers, Inc.: New York, NY, USA, 2016; pp. 93–124. ISBN 9781634846851. [Google Scholar]
- Tobin, R.; Moane, S.; Larkin, T. Sensory evaluation of organic and conventional fruits and vegetables available to Irish consumers. Int. J. Food Sci. Technol. 2013, 48, 157–162. [Google Scholar] [CrossRef]
- Zhao, X.; Chambers, E., IV; Matta, Z.; Loughin, T.M.; Carey, E.E. Consumer sensory analysis of organically and conventionally grown vegetables. J. Food Sci. 2007, 72, S87–S91. [Google Scholar] [CrossRef]
- Caser, M.; Demasi, S.; Stelluti, S.; Donno, D.; Scariot, V. Crocus sativus L. cultivation in alpine environments: Stigmas and tepals as source of bioactive compounds. Agronomy 2020, 10, 1473. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Caser, M.; Victorino, Í.M.M.; Demasi, S.; Berruti, A.; Donno, D.; Lumini, E.; Bianciotto, V.; Scariot, V. Saffron cultivation in marginal alpine environments: How AMF inoculation modulates yield and bioactive compounds. Agronomy 2019, 9, 12. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Eisele, T.; Giusti, M.M.; Hach, J.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Kupina, S.; et al. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Wong, S.P.; Leong, L.P.; William Koh, J.H. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006, 99, 775–783. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1998, 299, 15–27. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Bachmanov, A.A. Genetic Architecture of Sweet Taste. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2008; Volume 979, pp. 18–47. ISBN 978-0-84127-432-7. [Google Scholar]
- Beauchamp, G.K. Basic taste: A perceptual concept. J. Agric. Food Chem. 2019, 67, 13860–13869. [Google Scholar] [CrossRef] [PubMed]
- Shallenberger, R.S. Sugar structure and taste. In Advances in Chemistry; ACS Publications: Washington, DC, USA, 1973; pp. 256–263. ISBN 978-0-84120-178-1. [Google Scholar]
- Zarzo, M. A sensory 3D map of the odor description space derived from a comparison of numeric odor profile databases. Chem. Senses 2015, 40, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Forde, C.G.; van Kuijk, N.; Thaler, T.; de Graaf, C.; Martin, N. Oral processing characteristics of solid savoury meal components, and relationship with food composition, sensory attributes and expected satiation. Appetite 2013, 60, 208–219. [Google Scholar] [CrossRef]
- Drewnowski, A. The science and complexity of bitter taste. Nutr. Rev. 2009, 59, 163–169. [Google Scholar] [CrossRef]
- Simat, T. Panel Training on Odour and Aroma Perception for Sensory Analysis. Available online: https://www.dlg.org/en/food/topics/dlg-expert-reports/sensory-technology/dlg-expert-report-1-2017/ (accessed on 27 October 2017).
- Vieira, P.M. Avaliação da Composição Química, dos Compostos Bioativos e da Atividade Antioxidante Em Seis Espécies de Flores Comestíveis, Universidade Estadual Paulista “Júlio De Mesquita Filho”. 2013. Available online: http://hdl.handle.net/11449/100866 (accessed on 27 October 2017).
- Byrnes, N.K. The Influence of Experience and Personality on the Perception, Liking, and Intake of Spicy Foods; Pennsylvania State University: State College, PA, USA, 2014. [Google Scholar]
- Yang, P.; Song, H.; Wang, L.; Jing, H. Characterization of key Aroma-active compounds in black garlic by sensory-directed flavor analysis. J. Agric. Food Chem. 2019, 67, 7926–7934. [Google Scholar] [CrossRef]
- Ashok, P.K.; Upadhyaya, K. Tannins are astringent. J. Pharmacogn. Phytochem. 2012, 1, 45–50. [Google Scholar]
- Noble, A.C. Astringency and Bitterness of Flavonoid Phenols. In Chemistry of Taste; ACS Symposium Series; ACS Publications: Washington, DC, USA, 2002; Volume 825, pp. 192–201. [Google Scholar]
- Ma, W.; Guo, A.; Zhang, Y.; Wang, H.; Liu, Y.; Li, H. A review on astringency and bitterness perception of tannins in wine. Trends Food Sci. Technol. 2014, 40, 6–19. [Google Scholar] [CrossRef]
- Dinnella, C.; Recchia, A.; Tuorila, H.; Monteleone, E. Individual astringency responsiveness affects the acceptance of phenol-rich foods. Appetite 2011, 56, 633–642. [Google Scholar] [CrossRef]
- Neta, E.R.D.C.; Johanningsmeier, S.D.; McFeeters, R.F. The chemistry and physiology of sour taste—A review. J. Food Sci. 2007, 72, R33–R38. [Google Scholar] [CrossRef]
- Rocha, I.F.D.O.; Bolini, H.M.A. Passion fruit juice with different sweeteners: Sensory profile by descriptive analysis and acceptance. Food Sci. Nutr. 2015, 3, 129–139. [Google Scholar] [CrossRef]
- Melgarejo, P.; Calín-Sánchez, Á.; Carbonell-Barrachina, Á.A.; Martínez-Nicolás, J.J.; Legua, P.; Martínez, R.; Hernández, F. Antioxidant activity, volatile composition and sensory profile of four new very-early apricots (Prunus armeniaca L.). J. Sci. Food Agric. 2014, 94, 85–94. [Google Scholar] [CrossRef]
- Hernández, F.; Noguera-Artiaga, L.; Burló, F.; Wojdyło, A.; Carbonell-Barrachina, Á.A.; Legua, P. Physico-chemical, nutritional, and volatile composition and sensory profile of Spanish jujube (Ziziphus jujuba Mill.) fruits. J. Sci. Food Agric. 2016, 96, 2682–2691. [Google Scholar] [CrossRef]
- Kirsanov, D.; Mednova, O.; Vietoris, V.; Kilmartin, P.A.; Legin, A. Towards reliable estimation of an “electronic tongue” predictive ability from PLS regression models in wine analysis. Talanta 2012, 90, 109–116. [Google Scholar] [CrossRef]
- Cheong, M.W.; Liu, S.Q.; Zhou, W.; Curran, P.; Yu, B. Chemical composition and sensory profile of pomelo (Citrus grandis (L.) Osbeck) juice. Food Chem. 2012, 135, 2505–2513. [Google Scholar] [CrossRef]
- Pinto, T.; Vilela, A.; Pinto, A.; Nunes, F.M.; Cosme, F.; Anjos, R. Influence of cultivar and of conventional and organic agricultural practices on phenolic and sensory profile of blackberries (Rubus fruticosus). J. Sci. Food Agric. 2018, 98, 4616–4624. [Google Scholar] [CrossRef]
- Najar, B.; Demasi, S.; Caser, M.; Gaino, W.; Cioni, P.L.; Pistelli, L.; Scariot, V. Cultivation substrate composition influences morphology, volatilome and essential oil of Lavandula angustifolia Mill. Agronomy 2019, 9, 411. [Google Scholar] [CrossRef]
- Caser, M.; Demasi, S.; Victorino, Í.M.M.; Donno, D.; Faccio, A.; Lumini, E.; Bianciotto, V.; Scariot, V. Arbuscular mycorrhizal fungi modulate the crop performance and metabolic profile of saffron in soilless cultivation. Agronomy 2019, 9, 232. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Pereira, E.L.; Ramalhosa, E.; Saraiva, J.A. Effect of high hydrostatic pressure on the quality of four edible flowers: Viola × wittrockiana, Centaurea cyanus, Borago officinalis and Camellia japonica. Int. J. Food Sci. Technol. 2017, 52, 2455–2462. [Google Scholar] [CrossRef]
- Landi, M.; Ruffoni, B.; Salvi, D.; Savona, M.; Guidi, L. Cold storage does not affect ascorbic acid and polyphenolc content of edible flowers of a new hybrid of sage. Agrochimica 2015, 59, 348–357. [Google Scholar] [CrossRef]
- Stewart-Wade, S.M.; Neumann, S.; Collins, L.L.; Boland, G.J. The biology of Canadian weeds. 117 Taraxacum officinale G. H. Weber ex Wiggers. Can. J. Plant Sci. 2002, 82, 825–853. [Google Scholar] [CrossRef]
- Sadeghi, H.; Ghanaatiyan, K. Probing the responses of four chicory ecotypes by ethylene accumulation and growth characteristics under drought stress. Ital. J. Agron. 2017, 12, 177–182. [Google Scholar] [CrossRef]
- Saltveit, M.E. Effect of ethylene on quality of fresh fruits and vegetables. Postharvest Biol. Technol. 1999, 15, 279–292. [Google Scholar] [CrossRef]
- Toivonen, P.M.A.; Brummell, D.A. Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetables. Postharvest Biol. Technol. 2008, 48, 1–14. [Google Scholar] [CrossRef]
- Kumar, N.; Bhandari, P.; Singh, B.; Bari, S.S. Antioxidant activity and ultra-performance LC-electrospray ionization-quadrupole time-of-flight mass spectrometry for phenolics-based fingerprinting of Rose species: Rosa damascena, Rosa bourboniana and Rosa brunonii. Food Chem. Toxicol. 2009, 47, 361–367. [Google Scholar] [CrossRef]
- Fan, J.; Zhu, W.; Kang, H.; Ma, H.; Tao, G. Flavonoid constituents and antioxidant capacity in flowers of different Zhongyuan tree penoy cultivars. J. Funct. Foods 2012, 4, 147–157. [Google Scholar] [CrossRef]
- Xiong, L.; Yang, J.; Jiang, Y.; Lu, B.; Hu, Y.; Zhou, F.; Mao, S.; Shen, C. Phenolic compounds and antioxidant capacities of 10 common edible flowers from China. J. Food Sci. 2014, 79, C517–C525. [Google Scholar] [CrossRef]
- Petrova, I.; Petkova, N.; Ivanov, I. Five edible flowers—Valuable source of antioxidants in human nutrition. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 604–610. [Google Scholar]
- Aliakbarlu, J.; Tajik, H. Antioxidant and antibacterial activities of various extracts of Borago officinalis flowers. J. Food Process. Preserv. 2012, 36, 539–544. [Google Scholar] [CrossRef]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive properties of Sambucus nigra L. as a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Butnariu, M.; Coradini, C.Z. Evaluation of biologically active compounds from Calendula officinalis flowers using spectrophotometry. Chem. Cent. J. 2012, 6, 35. [Google Scholar] [CrossRef]
- Ji, H.F.; Du, A.L.; Zhang, L.W.; Xu, C.Y.; Yang, M.D.; Li, F.F. Effects of drying methods on antioxidant properties in Robinia pseudoacacia L. flowers. J. Med. Plants Res. 2012, 6. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Friedman, H.; Rot, I.; Agami, O.; Vinokur, Y.; Rodov, V.; Reznick, N.; Umiel, N.; Dori, I.; Ganot, L.; Shmuel, D.; et al. Edible flowers: New crops with potential health benefits. Acta Hortic. 2007, 755, 283–290. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the Polyphenols: Status and Controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Pun, U.K.; Yamada, T.; Tanase, K.; Shimizu-Yumoto, H.; Satoh, S.; Ichimura, K. Effect of ethanol on ethylene biosynthesis and sensitivity in cut carnation flowers. Postharvest Biol. Technol. 2014, 98, 30–33. [Google Scholar] [CrossRef]
- Khatami, F.; Najafi, F.; Yari, F.; Khavari-Nejad, R.A. Expression of etr1-1 gene in transgenic Rosa hybrida L. increased postharvest longevity through reduced ethylene biosynthesis and perception. Sci. Hortic. 2020, 263, 109103. [Google Scholar] [CrossRef]
- Singh, P.; Bharti, N.; Singh, A.P.; Tripathi, S.K.; Pandey, S.P.; Chauhan, A.S.; Kulkarni, A.; Sane, A.P. Petal abscission in fragrant roses is associated with large scale differential regulation of the abscission zone transcriptome. Sci. Rep. 2020, 10, 17196. [Google Scholar] [CrossRef]
- Gong, T.; Li, C.; Bian, B.; Wu, Y.; Dawuda, M.M.; Liao, W. Advances in application of small molecule compounds for extending the shelf life of perishable horticultural products: A review. Sci. Hortic. 2018, 230, 25–34. [Google Scholar] [CrossRef]

| Species (Common Name) | Flower Properties | Eaten in/as | References |
|---|---|---|---|
| Allium ursinum L. (wild garlic) | Antioxidant, anti-inflammatory, antimycotic, cardioprotective. | Garlic substitute. | [47,48,49] |
| Borago officinalis L. (borage) | Purifying, emollient, antitussive, diuretic, sudorific, anti-inflammatory | Salads, soups, desserts, syrups and drinks. Cucumber taste. | [2,15,50] |
| Calendula officinalis L. (calendula) | Anti-inflammatory, antispasmodic, antiseptic, hepatoprotective, emollient, refreshing, cicatrizing. | Flavoring and decoration of salted dishes, bakery products and herb teas. Food coloring. | [2,51,52] |
| Centaurea cyanus L. (cornflower) | Diuretic, anti-inflammatory, disinfectant. | Garnishing dishes, syrups, teas | [2] |
| Cichorium intybus L. (chicory) | Laxative, diuretic, hypoglycemic, depurative, disinfectant, hepatoprotective. | Salads, soups. | [18] |
| Dianthus carthusianorum L. (Carthusian pink) | Diuretic, sudorific, nervine stimulant, febrifuge, sedative. | Infusions, liquors. | [53] |
| Lavandula angustifolia Mill. (lavender) | Antispasmodic, antiseptic, sedative, carminative, cicatrizing. | Flavoring and decoration of cakes, soups, salads, jellies. Essential oil to flavor food. | [15,54] |
| Leucanthemum vulgare Lam. (ox-eye daisy) | Antispasmodic, diuretic, tonic, antifungal, antibacterial. | Tea, salads | [55,56] |
| Paeonia officinalis L. (common peony) | Antirheumatic, antispasmodic, anti-inflammatory, analgesic, hepatoprotective. | Infusions. | [57,58] |
| Primula veris L. (cowslip) | Anti-inflammatory, anti-viral, anti-asthmatic. | Garnishing dishes, conserves, salads | [55,59] |
| Robinia pseudoacacia L. (black locust) | Antispasmodic, antiviral, diuretic, emollient, febrifuge, laxative, purgative, tonic. | Flavoring liquors, jams, honey, pancakes. | [18,55] |
| Rosa canina L. (dog rose) | Anticancer, diuretic, laxative, anti-rheumatic, anti-inflammatory. | Salads, jellies, syrups, teas. | [2,60] |
| Rosa pendulina L. (Alpine rose) | Anticancer, diuretic, laxative, anti-rheumatic. | Salads, jellies. | [2] |
| Salvia pratensis L. (meadow sage) | Anti-inflammatory, antibacterial, antiseptic, eupeptic. | Flavoring of butter, vinegar, oil, salads and creams, soups. Essential oil to flavor food. | [61] |
| Sambucus nigra L. (elder) | Antioxidant, anti-inflammatory, antibacterial, diuretic, emollient, sudorific, laxative, cardioprotective. | Herb teas and drinks. Flavoring honey, jellies and jams. Salads. | [18] |
| Taraxacum officinale Weber (dandelion) | Antioxidant, anti-inflammatory, hepatoprotective, diuretic, laxative, depurative, analgesic. | Salads and soups. | [62,63] |
| Tropaeolum majus L. (nasturtium) | Disinfectant, antimicrobial, expectorant, diuretic, anti-inflammatory. | Salads, flavoring of soups, meat, pasta, cheese, vinegar. Peppery flavor. | [2,64,65] |
| Sensory Descriptor | Definition | References |
|---|---|---|
| Sweet intensity | Taste of sucrose | [84,85,86] |
| Sour intensity | Taste of citric acid | [85,87,88] |
| Bitter intensity | Taste of caffeine | [85,89] |
| Salt intensity | Taste of sodium chloride | [85,88] |
| Smell intensity | Odor’s intensity of edible flower in evaluation | [87,90] |
| Specific flower aroma intensity | Aroma’s intensity of edible flower in evaluation | [2,9,87,90,91] |
| Herbaceous aroma intensity | Intensity of herbaceous and cut grass aroma | [87] |
| Spiciness | Intensity of spice aroma, hot and pungent taste | [85,87,92,93] |
| Chewiness | The amount of chewing required to break down the sample so that it can be swallowed | [88] |
| Astringency | The tactile sensation described as dryness, tightening, tannic and puckering sensations perceived in the oral cavity. | [94,95,96] |
| Smell | Sweet | Sour | Bitter | Salt | Specific Flower Aroma | Herbaceous Aroma | Spiciness | Chewiness | Astringency | |
|---|---|---|---|---|---|---|---|---|---|---|
| Allium ursinum | 8.3 | 2.4 | 1.8 | 2.1 | 2.7 | 8.8 | 1.4 | 6.1 | 7.4 | 0.3 |
| Borago officinalis | 4.3 | 3.6 | 0.8 | 1.2 | 0.8 | 3.9 | 2.4 | 0.1 | 6.1 | 0.4 |
| Calendula officinalis | 6.7 | 2.6 | 1.4 | 3.0 | 0.8 | 5.2 | 2.2 | 0.6 | 7.2 | 2.1 |
| Centaurea cyanus | 5.1 | 2.2 | 1.1 | 2.2 | 0.9 | 4.1 | 3.4 | 0.2 | 3.8 | 0.7 |
| Cichorium intybus | 3.1 | 0.9 | 2.8 | 7.2 | 0.7 | 5.5 | 4.1 | 1.3 | 5.8 | 1.3 |
| Dianthus carthusianorum | 6.7 | 1.9 | 0.4 | 2.9 | 0.5 | 6.0 | 1.3 | 1.4 | 4.6 | 0.5 |
| Lavandula angustifolia | 9.0 | 2.8 | 1.7 | 5.0 | 0.5 | 8.2 | 2.5 | 1.8 | 4.7 | 0.7 |
| Leucanthemum vulgare | 7.4 | 2.4 | 0.9 | 2.7 | 0.9 | 3.1 | 5.3 | 0.7 | 5.2 | 1.5 |
| Paeonia officinalis | 4.1 | 3.9 | 2.9 | 6.1 | 0.6 | 5.1 | 5.1 | 1.6 | 7.9 | 1.9 |
| Primula veris | 4.1 | 2.8 | 0.8 | 1.2 | 0.4 | 2.6 | 1.6 | 0.3 | 6.2 | 1.4 |
| Robina pseudoacacia | 7.1 | 6.9 | 1.0 | 1.4 | 0.9 | 5.9 | 3.1 | - | 6.3 | 1.2 |
| Rosa canina | 6.3 | 2.0 | 2.0 | 5.2 | 0.5 | 6.1 | 4.2 | 0.1 | 6.3 | 2.0 |
| Rosa pendulina | 5.5 | 1.3 | 2.1 | 7.3 | 0.3 | 5.0 | 2.5 | 0.6 | 6.5 | 4.4 |
| Salvia pratensis | 7.3 | 3.9 | 1.1 | 2.0 | 0.6 | 5.3 | 2.0 | 0.8 | 6.6 | 0.8 |
| Sambucus nigra | 7.8 | 3.5 | 0.7 | 3.5 | 1.3 | 6.7 | 2.8 | 1.3 | 7.4 | 0.9 |
| Taraxacum officinale | 6.4 | 3.7 | 0.6 | 1.4 | 0.8 | 4.5 | 1.4 | 0.4 | 5.7 | 0.4 |
| Tropaeolum majus | 8.3 | 0.8 | 0.8 | 5.4 | 1.6 | 7.3 | 0.6 | 7.4 | 8.2 | 1.9 |
| Range of variation | 5.9 | 6.1 | 2.5 | 6.1 | 2.4 | 6.2 | 4.7 | 7.4 | 4.4 | 4.1 |
| Species | Overall | Taste | Appearance |
|---|---|---|---|
| Allium ursinum | 7.07 ± 0.93 a 1 | 6.57 ± 0.79 a | 7.21 ± 0.91 a |
| Borago officinalis | 5.60 ± 0.55 ab | 4.60 ± 0.55 abcde | 6.60 ± 0.55 a |
| Centaurea cyanus | 5.64 ± 0.84 ab | 4.25 ± 0.94 bcde | 7.32 ± 0.87 a |
| Cichorium intybus | 4.63 ± 0.75 b | 4.00 ± 0.82 cde | 3.25 ± 0.96 c |
| Dianthus carthusianorum | 4.80 ± 0.45 b | 4.20 ± 0.84 bcde | 4.60 ± 0.89 bc |
| Lavandula angustifolia | 6.30 ± 0.84 ab | 5.30 ± 0.97 abcd | 7.60 ± 0.55 a |
| Paeonia officinalis | 6.93 ± 0.93 a | 6.21 ± 0.99 ab | 7.64 ± 0.99 a |
| Primula veris | 5.43 ± 0.98 ab | 3.29 ± 0.95 de | 6.36 ± 0.99 ab |
| Rosa canina | 5.57 ± 0.98 ab | 4.43 ± 0.79 bcde | 7.36 ± 0.63 a |
| Rosa pendulina | 5.25 ± 0.50 ab | 3.25 ± 0.96 e | 7.50 ± 0.71 a |
| Salvia pratensis | 6.00 ± 0.82 ab | 5.25 ± 0.50 abcde | 6.50 ± 0.58 ab |
| Taraxacum officinale | 6.00 ± 0.99 ab | 5.86 ± 0.90 abc | 6.21 ± 0.99 ab |
| Sensory Descriptors | Overall Subjective Judgement | Pearson Correlation Significance |
|---|---|---|
| Smell intensity | 0.342 | ** 1 |
| Sweet intensity | 0.421 | ** |
| Sour intensity | −0.009 | ns |
| Bitter intensity | −0.135 | ns |
| Salt intensity | −0.234 | ** |
| Specific flower aroma intensity | 0.272 | ** |
| Herbaceous aroma intensity | 0.179 | * |
| Spicy | 0.510 | ns |
| Chewiness | 0.022 | ns |
| Astringency | −0.171 | * |
| Polyphenols | Anthocyanins | DPPH | ABTS | FRAP | ||
|---|---|---|---|---|---|---|
| Polyphenols | r | 1 | 0.270 | 0.813 | 0.649 | 0.895 |
| Sign. | 0.032 | 0.000 | 0.000 | 0.000 | ||
| Anthocyanins | r | 1 | 0.386 | 0.214 | 0.444 | |
| Sign. | 0.002 | 0.091 | 0.000 | |||
| DPPH | r | 1 | 0.837 | 0.793 | ||
| Sign. | 0.000 | 0.000 | ||||
| ABTS | r | 1 | 0.557 | |||
| Sign. | 0.000 | |||||
| FRAP | r | 1 | ||||
| Sign. |
| Days | A. ursinum | D. carthusianorum | P. veris | S. pratensis | ||||
| 0 | 10 | a 1 | 10 | a | 10 | a | 10 | a |
| 3 | 8.5 | b | 8.3 | b | 6.5 | b | 9 | b |
| 7 | 6.5 | c | 7.2 | c | 5.6 | bc | 8 | c |
| 10 | 5.8 | d | 5 | d | 5 | c | 4.2 | d |
| 14 | 5 | e | 3 | e | 4.8 | c | 3.7 | e |
| *** | *** | *** | *** | |||||
| B. officinalis | L. angustifolia | R. pseudoacacia | S. nigra | |||||
| 0 | 10 | a | 10 | a | 10 | a | 10 | a |
| 3 | 9 | ab | 8 | b | 7.3 | b | 9 | b |
| 7 | 8.2 | b | 7.2 | c | 7.3 | b | 8.8 | b |
| 10 | 5.4 | c | 5.2 | d | 4.8 | c | 4 | c |
| 14 | 4.6 | c | 4 | e | 3.8 | d | 2.5 | d |
| *** | *** | *** | *** | |||||
| C. officinalis | L. vulgare | R. canina | T. officinale | |||||
| 0 | 10 | a | 10 | a | 10 | a | 10 | a |
| 3 | 8.6 | b | 8.1 | b | 8.8 | b | 3.3 | b |
| 7 | 7 | c | 7.7 | b | 8.3 | b | 2 | c |
| 10 | 4.5 | d | 5.9 | c | 7.3 | c | 2 | c |
| 14 | 1.7 | e | 5.3 | c | 5.8 | d | 1 | d |
| *** | *** | *** | *** | |||||
| C. cyanus | P. officinalis | R. pendulina | T. majus | |||||
| 0 | 10 | a | 10 | a | 10 | a | 10 | a |
| 3 | 9 | b | 8.4 | b | 9 | b | 8.6 | b |
| 7 | 8.8 | b | 7.8 | b | 6.8 | c | 6 | c |
| 10 | 5.6 | c | 5.7 | c | 6.7 | cd | 4.7 | d |
| 14 | 4.6 | d | 4.7 | c | 5.8 | d | 1 | d |
| *** | *** | *** | *** |
| Days | Total Polyphenols | Total Anthocyanins | Total Polyphenols | Total Anthocyanins | Total Polyphenols | Total Anthocyanins | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| mgGAE/100 g FW | mg C3G/100 g FW | mg GAE/100 g FW | mg C3G/100 g FW | mg GAE/100 g FW | mg C3G/100 g FW | |||||
| A. ursinum | L. vulgare | R. pendulina | ||||||||
| 0 | 99.26 | ab 1 | 0.58 | 230.76 | b | 0.83 | 1181.9 | a | 55.93 | |
| 3 | 109.2 | ab | 0.77 | 197.91 | b | 4.3 | 1043.5 | bc | 47.22 | |
| 7 | 138.7 | a | 1.4 | 338.45 | a | 6.67 | 1151.4 | ab | 60.13 | |
| 10 | 81.51 | b | 0.23 | 313.52 | ab | 4.32 | 1033.9 | c | 31.03 | |
| 14 | 47.96 | c | 0.92 | 343.48 | a | 13.93 | 1139.8 | abc | 39.48 | |
| * | ns | * | ns | * | ns | |||||
| B. officinalis | P. officinalis | S. pratensis | ||||||||
| 0 | 118.1 | b | 38.43 | 1270.7 | a | 25.96 | 433.93 | 150.7 | ||
| 3 | 100.8 | b | 40.27 | 1210.9 | b | 28.76 | 425.35 | 135.5 | ||
| 7 | 100.6 | b | 3.89 | 1208.6 | b | 25.58 | 396.64 | 107.4 | ||
| 10 | 115.6 | b | 45.43 | 1219.8 | b | 26.68 | 349.52 | 141.8 | ||
| 14 | 157.2 | a | 44.73 | 1161.1 | c | 26.2 | 457.66 | 118.7 | ||
| *** | ns | *** | ns | ns | ns | |||||
| C. officinalis | P. veris | S. nigra | ||||||||
| 0 | 189.6 | a | 9.9 | 609.16 | b | 3.99 | 307.64 | ab | 17.29 | |
| 3 | 156 | ab | 35.66 | 770.23 | a | 4.24 | 365.14 | a | 29.46 | |
| 7 | 149.2 | ab | 26.46 | 610.58 | b | 4.86 | 284.33 | b | 16.03 | |
| 10 | 136.2 | b | 20.11 | 638.24 | b | 4.96 | 315.84 | ab | 15.22 | |
| 14 | 135.7 | b | 19.61 | 731.4 | a | 3.52 | 292.16 | b | 11.72 | |
| * | ns | *** | ns | ** | ns | |||||
| C. cyanus | R. pseudoacacia | T. officinale | ||||||||
| 0 | 196.8 | d | 34.67 | 191.45 | 14.45 | 76.41 | d | 8.84 | ab | |
| 3 | 171.5 | e | 50.12 | 204.08 | 9.84 | 157.33 | a | 6.05 | b | |
| 7 | 276 | b | 28.57 | 243.17 | 10.83 | 100.35 | c | 10.62 | a | |
| 10 | 213.5 | c | 23.11 | 187.53 | 15.49 | 117.89 | b | 8.68 | ab | |
| 14 | 317.9 | a | 38.04 | 205.6 | 11.95 | 98.62 | c | 8.57 | ab | |
| *** | ns | ns | ns | *** | ** | |||||
| D. carthusianorum | R. canina | T. majus | ||||||||
| 0 | 470.5 | c | 19.16 | 884.44 | d | 4.39 | 341.33 | a | 800.2 | a |
| 3 | 446.8 | c | 15.92 | 1009.6 | c | 5.04 | 343.64 | a | 414.2 | b |
| 7 | 675.9 | a | 17.25 | 1204.2 | a | 4.96 | 353.95 | a | 327.5 | b |
| 10 | 635.9 | b | 16.49 | 1155 | ab | 4.5 | 271.93 | a | 335.8 | b |
| 14 | 697.7 | a | 18.97 | 1104.6 | b | 5.2 | 48.74 | b | 322 | b |
| *** | ns | *** | ns | *** | ** | |||||
| L. angustifolia | ||||||||||
| 0 | 148.2 | c | 4.27 | |||||||
| 3 | 198.7 | bc | 4.94 | |||||||
| 7 | 202.9 | bc | 5.03 | |||||||
| 10 | 212.3 | b | 4.98 | |||||||
| 14 | 387.5 | a | 5 | |||||||
| * | ns | |||||||||
| Days | DPPH | ABTS | FRAP | DPPH | ABTS | FRAP | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µmol TE/g FW | µmol TE/g FW | mmol Fe2+/kg FW | µmol TE/g FW | µmol TE/g FW | mmol Fe2+/kg FW | |||||||
| A. ursinum | P. veris | |||||||||||
| 0 | 2.38 | c 1 | 2.7 | c | 1.45 | b | 41.91 | abc | 23.83 | bc | 120.14 | b |
| 3 | 6.76 | a | 7.41 | a | 4.01 | a | 42.63 | ab | 24.7 | bc | 115.17 | b |
| 7 | 4.75 | b | 3.32 | b | 5.04 | a | 53.29 | a | 22.96 | c | 114.25 | b |
| 10 | 4.73 | b | 5.56 | ab | 3.77 | a | 28.68 | bc | 27.67 | a | 118.84 | b |
| 14 | 4.79 | b | 4.65 | ab | 4.22 | a | 29.96 | c | 25.43 | ab | 131.17 | a |
| *** | * | *** | * | *** | ** | |||||||
| B. officinalis | R. pseudoacacia | |||||||||||
| 0 | 3.47 | a | 6.53 | 22.74 | b | 2.08 | ab | 2.66 | ab | 30.35 | ab | |
| 3 | 1.81 | b | 4.61 | 15.63 | d | 2.53 | a | 3.43 | a | 40.44 | a | |
| 7 | 1.28 | b | 5.34 | 18.06 | c | 1.86 | ab | 4.16 | a | 47.1 | a | |
| 10 | 4.59 | a | 7.92 | 24.19 | b | 1.82 | b | 3.4 | ab | 24.66 | b | |
| 14 | 4.8 | a | 8.16 | 33.56 | a | 1.4 | b | 2.56 | b | 18.88 | c | |
| *** | ns | *** | ** | * | ** | |||||||
| C. officinalis | R. canina | |||||||||||
| 0 | 3.62 | a | 9.21 | 22.55 | b | 132.3 | b | 43.45 | c | 203.72 | b | |
| 3 | 1.29 | bc | 2.39 | 34.16 | a | 137.7 | b | 52.86 | b | 227.37 | ab | |
| 7 | 1.06 | bc | 2.3 | 32.56 | a | 137.4 | b | 50.91 | bc | 239.1 | ab | |
| 10 | 0.97 | c | 2.34 | 31.34 | a | 177.6 | a | 57.39 | a | 265.09 | a | |
| 14 | 1.89 | b | 1.97 | 34.87 | a | 115.8 | c | 49.98 | bc | 248 | ab | |
| *** | ns | *** | *** | * | * | |||||||
| C. cyanus | R. pendulina | |||||||||||
| 0 | 10.08 | b | 10.3 | b | 21.19 | c | 153.9 | b | 55.44 | ab | 257.04 | |
| 3 | 10.77 | b | 13.4 | ab | 34.31 | bc | 104.8 | c | 55.35 | ab | 248.22 | |
| 7 | 16.04 | a | 16.9 | a | 40.29 | b | 170.6 | a | 56.29 | a | 246.11 | |
| 10 | 11.59 | b | 14 | ab | 26.57 | bc | 99.85 | d | 54.62 | b | 251.63 | |
| 14 | 12.67 | ab | 13.7 | ab | 44.65 | a | 89.77 | e | 56.36 | a | 224.1 | |
| ** | * | * | ** | ** | ns | |||||||
| D. carthusianorum | S. pratensis | |||||||||||
| 0 | 29.13 | c | 17.4 | b | 92.51 | b | 11.02 | 5.39 | 171.09 | |||
| 3 | 17.19 | d | 12.8 | c | 69.6 | b | 10.78 | 5.23 | 151.51 | |||
| 7 | 37.59 | b | 20 | b | 121.3 | a | 10.3 | 5.64 | 144.54 | |||
| 10 | 34.87 | bc | 27.1 | a | 127.8 | a | 10.62 | 5.26 | 170.26 | |||
| 14 | 58.3 | a | 25.5 | a | 131.4 | a | 11.76 | 5.65 | 194.11 | |||
| *** | *** | *** | ns | ns | ns | |||||||
| L. angustifolia | S. nigra | |||||||||||
| 0 | 2.78 | bc | 7.77 | b | 19.25 | c | 5.14 | ab | 3.74 | b | 98.79 | |
| 3 | 9.72 | b | 8.75 | ab | 32.97 | abc | 6.64 | a | 5.4 | a | 109.38 | |
| 7 | 4.19 | bc | 8.9 | ab | 36.12 | ab | 4.34 | b | 4.35 | ab | 93.79 | |
| 10 | 3.3 | c | 8.97 | ab | 31.14 | bc | 5.35 | ab | 4.24 | ab | 92.91 | |
| 14 | 25.05 | a | 16.7 | a | 71.61 | a | 4.14 | b | 3.76 | b | 83.46 | |
| * | * | * | ** | * | ns | |||||||
| L. vulgare | T. officinale | |||||||||||
| 0 | 2.49 | cd | 3.08 | 50.08 | b | 3.18 | b | 4.78 | 14.41 | bc | ||
| 3 | 1.85 | d | 2.35 | 49.49 | b | 6.87 | a | 7.06 | 18.46 | ab | ||
| 7 | 3.86 | bc | 3.4 | 77.88 | a | 2.8 | b | 6.39 | 12.15 | c | ||
| 10 | 5.54 | ab | 3.18 | 89.05 | a | 9.59 | a | 6.16 | 22.29 | a | ||
| 14 | 5.74 | a | 3.51 | 96.72 | a | 2.53 | b | 6.53 | 15.59 | bc | ||
| *** | ns | *** | *** | ns | *** | |||||||
| P. officinalis | T. majus | |||||||||||
| 0 | 232.4 | a | 57.6 | b | 274.2 | a | 11.51 | a | 8.05 | 241.12 | a | |
| 3 | 227.3 | b | 57.9 | a | 265.8 | b | 5.85 | b | 4.73 | 129.15 | b | |
| 7 | 190.9 | c | 57.5 | b | 275 | a | 5.62 | b | 5.02 | 113.86 | b | |
| 10 | 221 | c | 57.6 | b | 274.3 | a | 4.53 | b | 5.69 | 119.76 | b | |
| 14 | 187.9 | c | 55.7 | c | 261.1 | b | 6.79 | ab | 5.88 | 83.47 | b | |
| * | * | *** | * | ns | *** | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demasi, S.; Mellano, M.G.; Falla, N.M.; Caser, M.; Scariot, V. Sensory Profile, Shelf Life, and Dynamics of Bioactive Compounds during Cold Storage of 17 Edible Flowers. Horticulturae 2021, 7, 166. https://doi.org/10.3390/horticulturae7070166
Demasi S, Mellano MG, Falla NM, Caser M, Scariot V. Sensory Profile, Shelf Life, and Dynamics of Bioactive Compounds during Cold Storage of 17 Edible Flowers. Horticulturae. 2021; 7(7):166. https://doi.org/10.3390/horticulturae7070166
Chicago/Turabian StyleDemasi, Sonia, Maria Gabriella Mellano, Nicole Mélanie Falla, Matteo Caser, and Valentina Scariot. 2021. "Sensory Profile, Shelf Life, and Dynamics of Bioactive Compounds during Cold Storage of 17 Edible Flowers" Horticulturae 7, no. 7: 166. https://doi.org/10.3390/horticulturae7070166
APA StyleDemasi, S., Mellano, M. G., Falla, N. M., Caser, M., & Scariot, V. (2021). Sensory Profile, Shelf Life, and Dynamics of Bioactive Compounds during Cold Storage of 17 Edible Flowers. Horticulturae, 7(7), 166. https://doi.org/10.3390/horticulturae7070166









