Effect of Daily Light Integral on Cucumber Plug Seedlings in Artificial Light Plant Factory
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Growth Chamber Environment
2.3. Sole-Source Lighting Treatments
2.4. Morphology and Growth Measurement
2.5. Physiological Characteristics and Photosynthesis Pigment Content
2.6. Chlorophyll Fluorescence
2.7. Flowering in Cucumber Plant after Transplant
2.8. Statistical Analysis
3. Results
3.1. Experiment 1
3.1.1. Morphology and Growth of Cucumber Plug Seedlings
3.1.2. Physiological Characteristics and Photosynthesis Pigment Content of Cucumber Plug Seedlings
3.1.3. Flowering in Cucumber Plant after Transplant
3.2. Experiment 2
3.2.1. Morphology and Growth of Cucumber Plug Seedlings
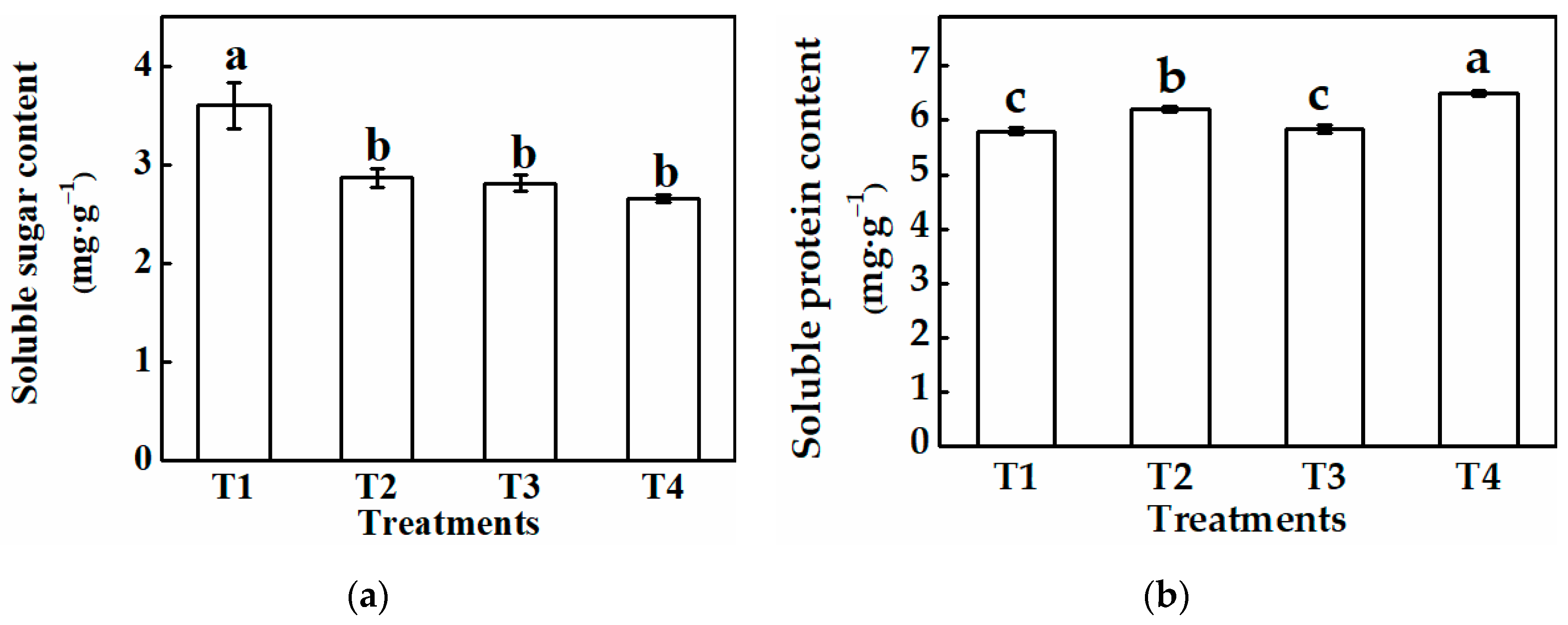
3.2.2. Physiological Characteristics of Cucumber Plug Seedlings
3.2.3. Photosynthesis Pigment Content and Chlorophyll Fluorescence of Cucumber Plug Seedlings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faust, J.E.; Logan, J. Daily Light Integral: A research review and high-resolution maps of the United States. HortScience 2018, 53, 1250–1257. [Google Scholar] [CrossRef]
- Christiaens, A.; Lootens, P.; Roldán-Ruiz, I.; Pauwels, E.; Gobin, B.; Van Labeke, M.C. Determining the minimum daily light integral for forcing of azalea (Rhododendron simsii). Sci. Hortic. 2014, 177, 1–9. [Google Scholar] [CrossRef]
- Lee, J.H. Vegetative Growth and Inflorescence Emergence of Phalaenopsis ‘Mantefon’ as Affected by Photoperiod, Light Intensity, and Daily Light Integral; Seoul National University: Seoul, Korea, 2018. [Google Scholar]
- Lee, H.B.; Lee, J.H.; An, S.K.; Park, J.H.; Kim, K.S. Growth characteristics and flowering initiation of Phalaenopsis Queen Beer ‘Mantefon’ as affected by the daily light integral. Hortic. Environ. Biote. 2019, 60, 637–645. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, X.; Ma, L.; Zhang, Y. Influence of light on germination and seedling status of Centipeda herba. Seed 2016, 35, 91–93. [Google Scholar]
- Walters, K.J.; Curry, C.J. Effects of nutrient solution concentration and daily light integral on growth and nutrient concentration of several basil species in hydroponic production. HortScience 2018, 53, 1319–1325. [Google Scholar] [CrossRef]
- Currey, C.J.; Hutchinson, V.A.; Lopez, R.G. Growth, morphology, and quality of rooted cuttings of several herbaceous annual bedding plants are influenced by photosynthetic daily light integral during root development. HortScience 2012, 47, 25–30. [Google Scholar] [CrossRef]
- Warner, R.M.; Erwin, J.E. Effect of photoperiod and daily light integral on flowering of five Hibiscus sp. Sci. Hortic. 2003, 97, 341–351. [Google Scholar] [CrossRef]
- Solis-Toapanta, E.; Gómez, C. Growth and photosynthetic capacity of basil grown for indoor gardening under constant or increasing daily light integrals. HortTechnology 2019, 29, 880–888. [Google Scholar] [CrossRef]
- Baumbauer, D.A.; Schmidt, C.B.; Burgess, M.H. Leaf lettuce yield is more sensitive to low daily light integral than kale and spinach. HortScience 2019, 54, 2159–2162. [Google Scholar] [CrossRef]
- Currey, C.J.; Lopez, R.G. Biomass Accumulation and allocation, photosynthesis, and carbohydrate status of new guinea impatiens, geranium, and petunia cuttings are affected by photosynthetic daily light integral during root development. J. Amer. Soc. Hort. Sci. 2015, 140, 542–549. [Google Scholar] [CrossRef]
- Kelly, N.; Choe, D.; Meng, Q.; Runkle, E.S. Promotion of lettuce growth under an increasing daily light integral depends on the combination of the photosynthetic photon flux density and photoperiod. Sci. Hortic. 2020, 272, 1–8. [Google Scholar] [CrossRef]
- Graver, J.K.; Boldt, J.K.; Lopez, R.G. Radiation intensity and quality from sole-source light-emitting diodes affect seedling quality and subsequent flowering of long-day bedding plant species. HortScience 2018, 53, 1407–1415. [Google Scholar]
- Owen, W.G.; Meng, Q.W.; Lopez, R.G. Promotion of flowering from far red radiation depends on the photosynthetic daily light integral. HortScience 2018, 53, 1–7. [Google Scholar] [CrossRef]
- Randall, W.C.; Lopez, R.G. Comparison of supplemental lighting from high-pressure sodiumlamps and light-emitting diodes during bedding plant seedling production. HortScience 2014, 49, 589–595. [Google Scholar] [CrossRef]
- Torres, A.P.; Lopez, R.G. Photosynthetic daily light integral during propagation of Tecoma stans influences seedling rooting and growth. HortScience 2011, 46, 282–286. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, H.; Song, S.; Su, W.; Liu, H. Morphological and physiological responses of cucumber seedlings to supplemental led light under extremely low irradiance. Agronomy 2020, 10, 1698. [Google Scholar] [CrossRef]
- Elkins, C.; van Iersel, M.W. Longer photoperiods with the same daily light integral improve growth of rudbeckia seedlings in a greenhouse. HortScience 2020, 55, 1676–1682. [Google Scholar] [CrossRef]
- Hernández, R.; Kubota, C. Growth and morphological response of cucumber seedlings to supplemental red and blue photon flux ratios under varied solar daily light integrals. Sci. Hortic. 2014, 173, 92–99. [Google Scholar] [CrossRef]
- Ji, F.; Wei, S.; Liu, N.; Xu, L.; Yang, P. Growth of cucumber seedlings in different varieties as affected by light environment. Int. J. Agric. Biol. Eng. 2020, 13, 73–78. [Google Scholar] [CrossRef]
- Oh, W.; Runkle, E.S.; Warner, R.M. Timing and duration of supplemental lighting during the seedling stage influence quality and flowering in petunia and pansy. HortScience 2010, 45, 1332–1337. [Google Scholar] [CrossRef]
- Pramuk, L.A.; Runkle, E.S. Photosynthetic daily light integral during the seedling stage influences subsequent growth and flowering of Celosia, Impatiens, Salvia, Tagetes, and Viola. HortScience 2005, 40, 1336–1339. [Google Scholar] [CrossRef]
- Wang, R.; Gui, Y.; Zhao, T.; Ishii, M.; Eguchi, M.; Xu, H.; Li, T.; Iwasaki, Y. Determining the relationship between floral initiation and source–sink dynamics of tomato seedlings affected by changes in shading and nutrients. HortScience 2020, 55, 457–464. [Google Scholar] [CrossRef]
- Velez-Ramirez, A.I.; Dünner-Planella, G.; Vreugdenhil, D.; Millenaar, F.F.; van Ieperen, W. On the induction of injury in tomato under continuous light: Circadian asynchrony as the main triggering factor. Funct. Plant Biol. 2017, 44, 597–611. [Google Scholar] [CrossRef]
- Fan, X.; Xu, Z.; Liu, X.; Tang, C.; Wang, L.; Han, X. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Fu, W.; Li, P.; Wu, Y. Effects of different light intensities on chlorophyll fluorescence characteristics and yield in lettuce. Sci. Hortic. 2012, 135, 45–51. [Google Scholar] [CrossRef]
- Gao, W.; He, D.; Ji, F.; Zhang, S.; Zheng, J. Effects of daily light integral and LED spectrum on growth and nutritional quality of hydroponic spinach. Agronomy 2020, 10, 1082. [Google Scholar] [CrossRef]
- Lopez, R.G.; Runkle, E.S. Photosynthetic daily light integral during propagation influences rooting and growth of cuttings and subsequent development of new guinea impatiens and petunia. HortScience 2008, 43, 2052–2059. [Google Scholar] [CrossRef]
- Blanchard M, G. Manipulating Light and Temperature for Energy-Efficient Greenhouse Production of Ornamental Crops; Michigan State University: East Lansing, MI, USA, 2009. [Google Scholar]
- Hutchinson, V.A.; Currey, C.J.; Lopez, R.G. Photosynthetic daily light integral during root development influences subsequent growth and development of several herbaceous annual bedding plants. HortScience 2012, 47, 856–860. [Google Scholar] [CrossRef]
- Palmer, S.; van Iersel, M.W. Increasing growth of lettuce and mizuna under sole-source LED lighting using longer photoperiods with the same daily light integral. Agronomy 2020, 10, 1659. [Google Scholar] [CrossRef]
- Mao, H.; Hang, T.; Zhang, X.; Lu, N. Both multi-segment light intensity and extended photoperiod lighting strategies, with the same daily light integral, promoted Lactuca sativa L. growth and photosynthesis. Agronomy 2019, 9, 857. [Google Scholar] [CrossRef]
- Bai, Y.; Shi, W.; Xing, X.; Wang, Y.; Jin, Y.; Zhang, L.; Song, Y.; Dong, L.; Liu, H. Study on tobacco vigorous seedling indexes model. Sci. Agri. Sin. 2014, 47, 1086–1098. [Google Scholar]
- Faust, J.E.; Holcombe, V.; Rajapakse, N.C.; Layne, D.R. The effect of daily light integral on bedding plant growth and flowering. HortScience 2005, 40, 645–649. [Google Scholar] [CrossRef]
- Li, H.S. Principles and Techniques of Plant Physiological and Biochemical Experiments; Higher Education Press: Beijing, China, 2000. [Google Scholar]
- Hao, J.J.; Kang, Z.L.; Yu, Y. Experimental Techniques for Plant Physiology; Chemical Industrial Press: Beijing, China, 2007. [Google Scholar]
- Currey, C.J.; Lopez, R.G. Biomass accumulation, allocation and leaf morphology of impatiens hawker ‘Magnum Salmon’ cuttings is affected by photosynthetic daily light integral in propagation. Acta Hortic. 2012, 956, 349–356. [Google Scholar] [CrossRef]
- Hurt, A.; Lopez, R.G.; Craver, J.K. Supplemental but not photoperiodic lighting increased seedling quality and reduced production time of annual bedding Plants. HortScience 2019, 54, 289–296. [Google Scholar] [CrossRef]
- Garland, K.F.; Burnett, S.E.; Day, M.E.; van Iersel, M.W. Influence of substrate water content and daily light integral on photosynthesis, water use efficiency, and morphology of Heuchera americana. J. Amer. Soc. Hort. Sci. 2012, 137, 57–67. [Google Scholar] [CrossRef]
- Gent, M.P.N. Effect of daily light integral on composition of hydroponic lettuce. HortScience 2014, 49, 173–179. [Google Scholar] [CrossRef]
- Gerovac, J.R.; Craver, J.K.; Boldt, J.K.; Lopez, R.G. Light intensity and quality from sole-source light-emitting diodes impact growth, morphology, and nutrient content of Brassica microgreens. HortScience 2016, 51, 497–503. [Google Scholar] [CrossRef]
- Kjaer, K.H.; Ottosen, C.; Jørgensen, B.N. Timing growth and development of Campanula by daily light integral and supplemental light level in a cost-efficient light control system. Sci. Hortic. 2012, 143, 189–196. [Google Scholar] [CrossRef]
- Hutchinson, V.A. Photosynthetic Daily Light Integral During Vegetative Propagation and Finish Stages Influence Growth and Development of Annual Bedding Plant Species; Purdue University: West Lafayette, IN, USA, 2012. [Google Scholar]
- Vaid, T.M. Improving the Scheduling and Profitability of Annual Bedding Plant Production by Manipulating Temperature, Daily Light Integral, Photoperiod, and Transplant Size; Michigan State University: East Lansing, MI, USA, 2012. [Google Scholar]
- Zhen, S.; van Iersel, M.W. Photochemical acclimation of three contrasting species to different light levels: Implications for optimizing supplemental lighting. J. Amer. Soc. Hort. Sci. 2017, 142, 346–354. [Google Scholar] [CrossRef]
- Elkins, C.; Van Iersel, M.W. Longer photoperiods with the same daily light integral increase daily electron transport through photosystem II in lettuce. Plants 2020, 9, 1172. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Responses of sweet basil to different daily light integrals in photosynthesis, morphology, yield, and nutritional quality. HortScience 2018, 53, 496–503. [Google Scholar] [CrossRef]
- Dai, Y.; Shen, Z.; Liu, Y.; Wang, L.; Hannaway, D.; Lu, H. Effects of shade treatments on the photosynthetic capacity, chlorophyll fluorescence, and chlorophyll content of Tetrastigma hemsleyanum Diels et Gilg. Enviro. Exp. Bot. 2009, 65, 177–182. [Google Scholar] [CrossRef]
- Pettersen, R.I.; Torreand, S.; Gislerød, H.R. Effects of leaf aging and light duration on photosynthetic characteristics in a cucumber canopy. Sci. Hortic. 2010, 125, 82–87. [Google Scholar] [CrossRef]
- Du, X.; Huang, P.; Yang, M. Phosphorus fertilizers on the growth and resistance physiology of Eucalyptus urophylla×Eucalyptus grandis DH3229 seedlings. J. For. Environ. 2020, 40, 526–533. [Google Scholar]
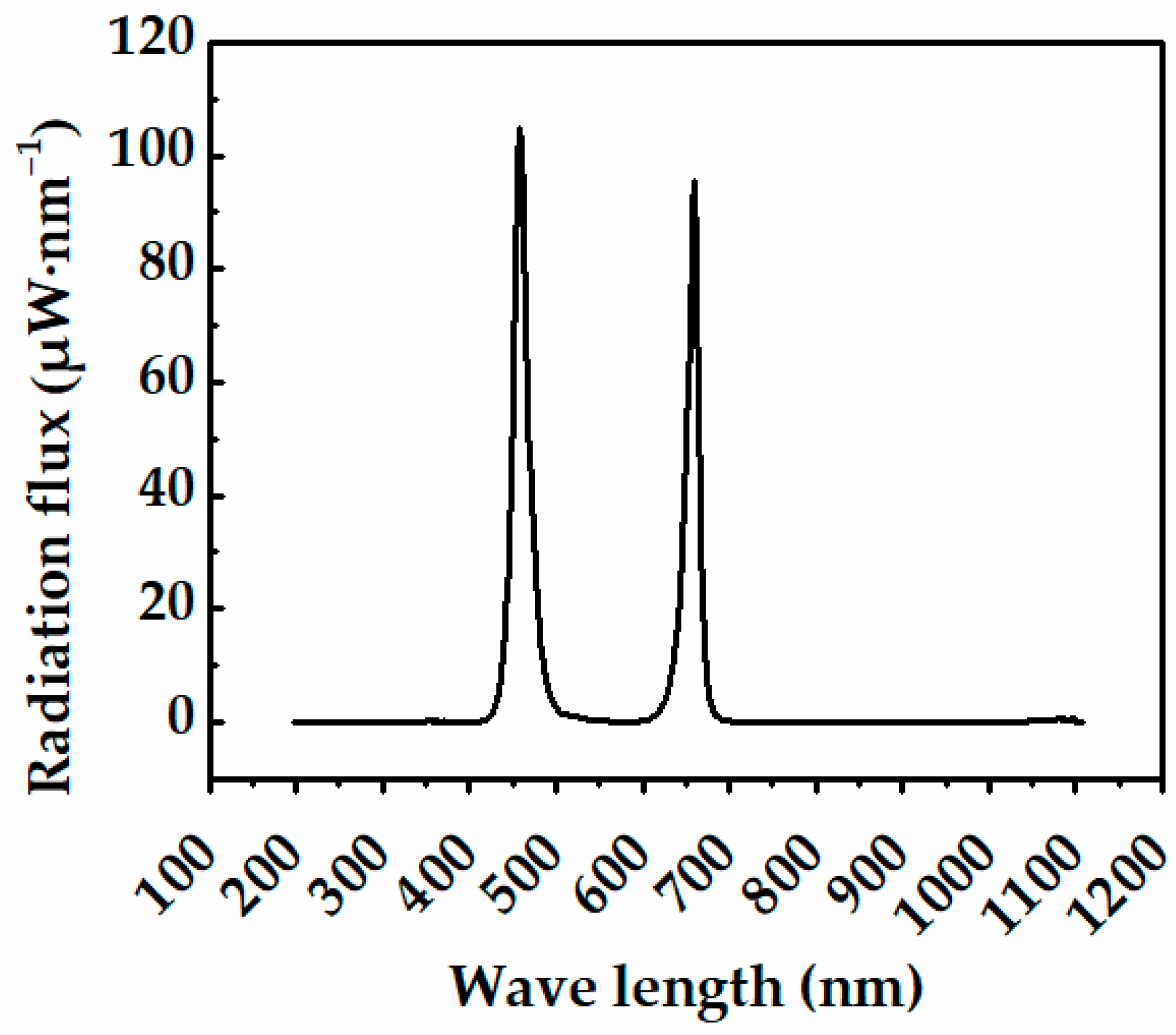

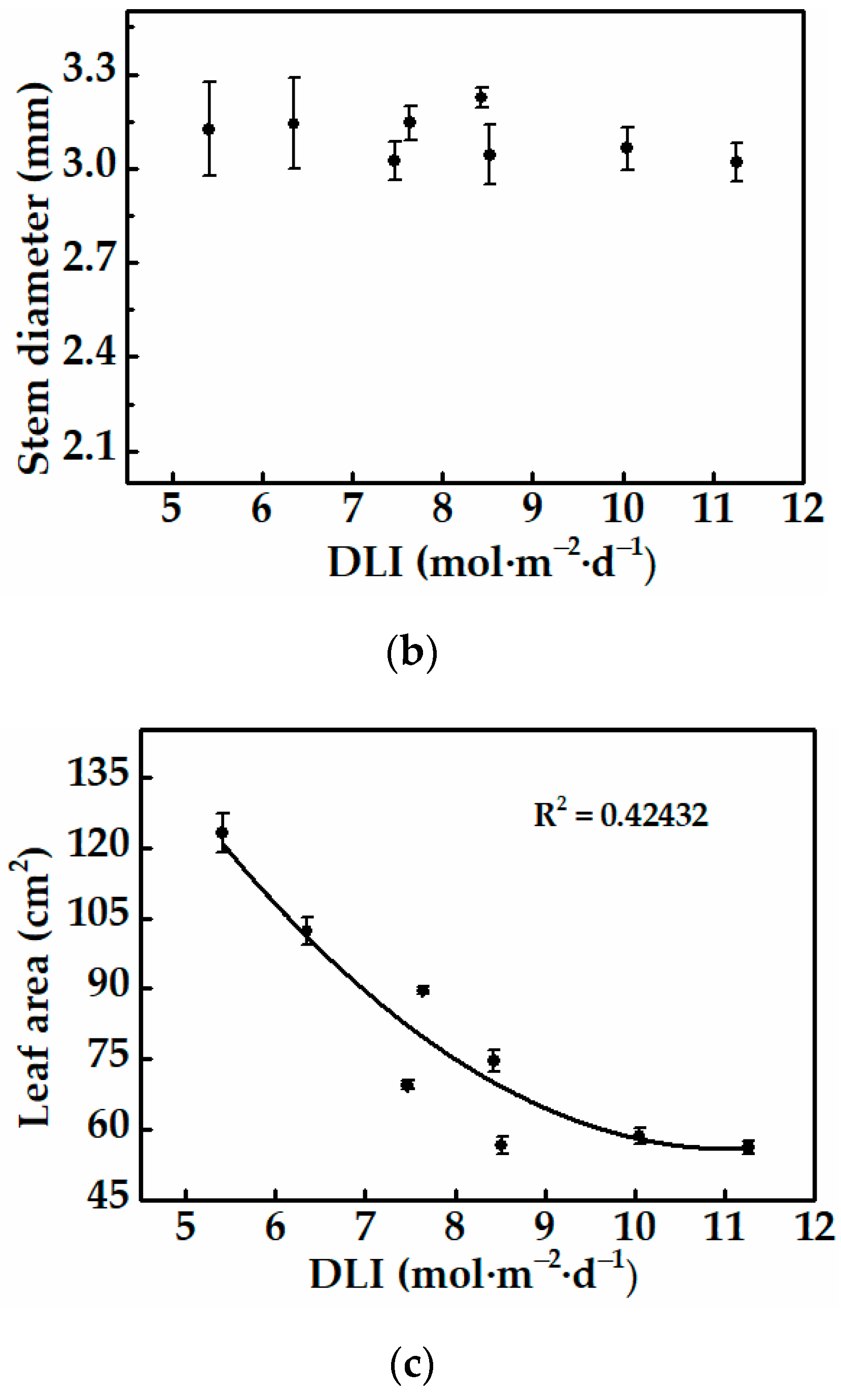
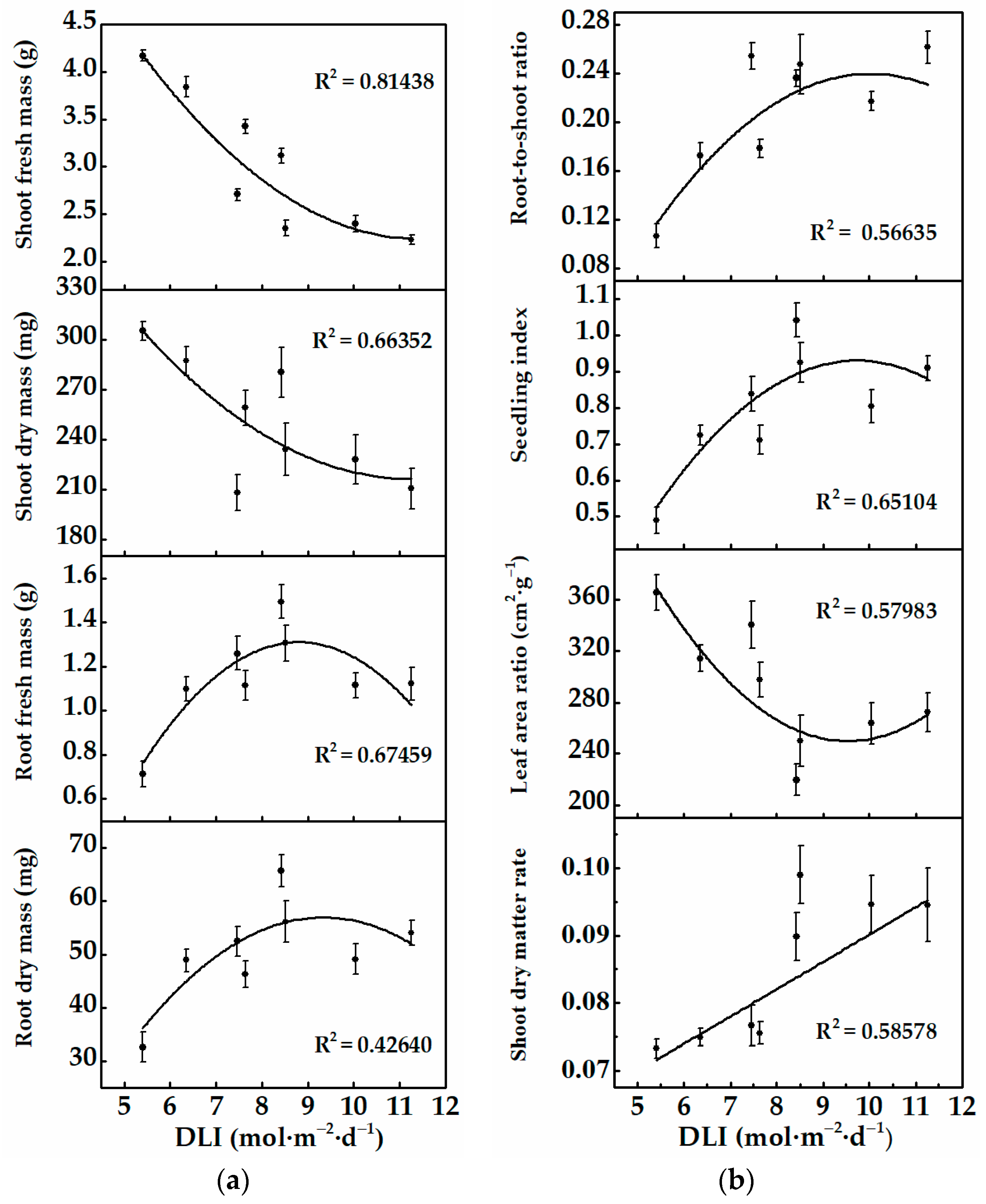
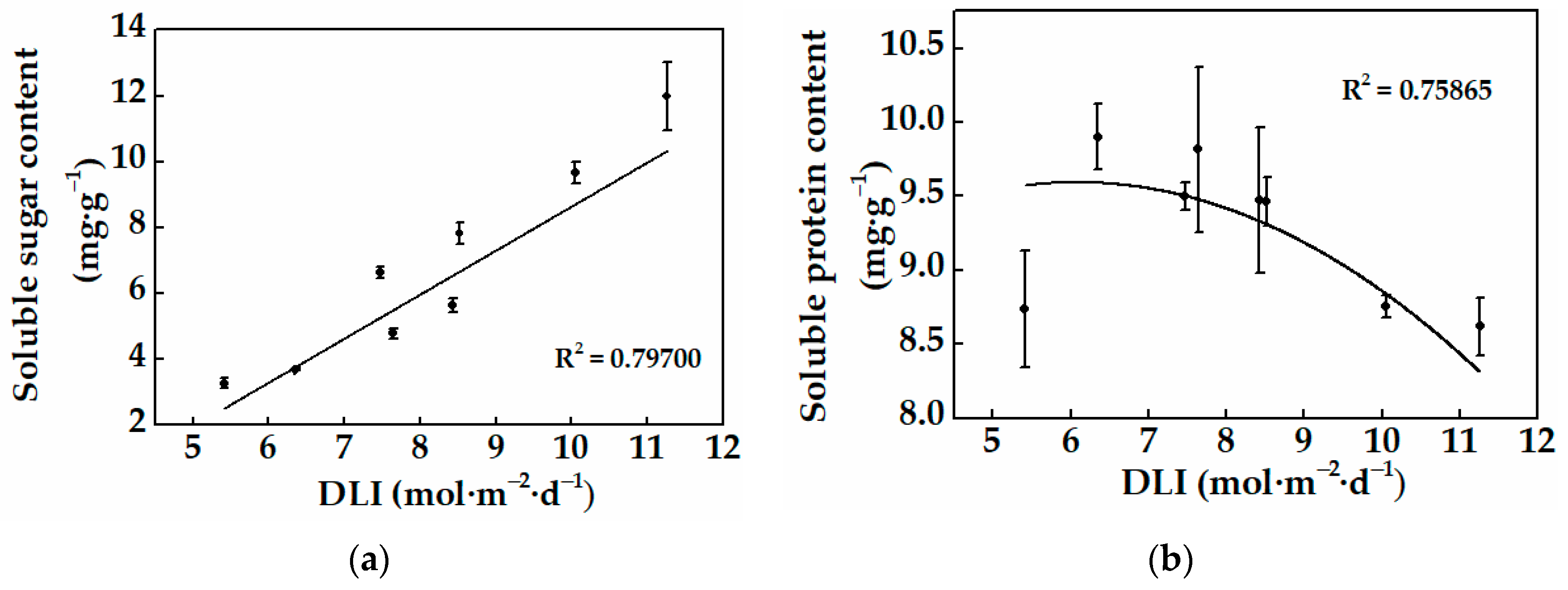


| Treatments | Light Intensity * (μmol·m−2·s−1) | Photoperiod (h) | Average DLI * (mol·m−2·d−1) |
|---|---|---|---|
| Run 1 | 148.17 ± 2.75 | 14 | 7.47 ± 0.14 |
| 148.00 ± 2.22 | 16 | 8.52 ± 0.13 | |
| 199.33 ± 2.50 | 14 | 10.05 ± 0.13 | |
| 195.43 ± 1.72 | 16 | 11.26 ± 0.10 | |
| Run 2 | 125.14 ± 1.06 | 12 | 5.41 ± 0.05 |
| 126.00 ± 1.69 | 14 | 6.35 ± 0.09 | |
| 151.57 ± 1.13 | 14 | 7.64 ± 0.06 | |
| 146.43 ± 0.87 | 16 | 8.43 ± 0.05 |
| Treatments | Light Intensity * (μmol·m−2·s−1) | Photoperiod (h) | Average DLI * (mol·m−2·d−1) |
|---|---|---|---|
| T1 | 111.17 ± 0.48 | 16 | 6.40 ± 0.03 |
| T2 | 125.17 ± 1.90 | 14 | 6.31 ± 0.10 |
| T3 | 146.00 ± 1.32 | 12 | 6.31 ± 0.06 |
| T4 | 176.67 ± 1.33 | 10 | 6.36 ± 0.05 |
| DLI/mol·m−2·d−1 | Days to First Flower Bloom from Transplant | Male Flower Number | Female Flower Number of Main Stem | Number of Lateral Branches | Total Female Flower Number |
|---|---|---|---|---|---|
| 5.41 | 39.83 ± 0.75 a | 46.00 ± 4.47 b | 1.17 ± 0.17 b | 1.00 ± 0.26 b | 2.17 ± 0.31 b |
| 6.35 | 39.00 ± 0.26 a | 57.33 ± 2.60 a | 1.17 ± 0.17 b | 2.50 ± 0.43 a | 3.67 ± 0.56 a |
| 7.64 | 38.83 ± 0.31 a | 50.33 ± 3.87 a,b | 1.33 ± 0.21 a | 2.17 ± 0.54 a,b | 3.50 ± 0.43 a,b |
| 8.43 | 40.00 ± 0.45 a | 39.83 ± 2.24 b | 1.33 ± 0.21 a | 1.33 ± 0.42 a,b | 2.67 ± 0.42 a,b |
| Treatment | Photochemical Quenching | Non-Photochemical Quenching | Y(NO) | Y(NPQ) | ΦPSII | Fv/Fm | ||
|---|---|---|---|---|---|---|---|---|
| qP | qL | qN | NPQ | |||||
| T1 | 0.63 ± 0.01 a | 0.39 ± 0.01 a | 0.62 ± 0.01 b | 1.11 ± 0.04 b | 0.29 ± 0.00 c | 0.32 ± 0.01 c | 0.39 ± 0.01 a | 0.78 ± 0.00 a |
| T2 | 0.58 ± 0.01 b | 0.35 ± 0.00 b | 0.65 ± 0.01 a,b | 1.23 ± 0.05 a,b | 0.29 ± 0.00 c | 0.36 ± 0.01 b | 0.35 ± 0.01 b | 0.77 ± 0.00 a |
| T3 | 0.40 ± 0.02 c | 0.21 ± 0.01 c | 0.66 ± 0.01 a | 1.31 ± 0.07 a | 0.33 ± 0.01 b | 0.42 ± 0.01 a | 0.25 ± 0.01 c | 0.78 ± 0.01 a |
| T4 | 0.12 ± 0.01 d | 0.05 ± 0.00 d | 0.31 ± 0.01 c | 0.31 ± 0.02 c | 0.71 ± 0.01 a | 0.22 ± 0.01 d | 0.07 ± 0.00 d | 0.64 ± 0.02 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, J.; Song, S.; Yu, J.; Liu, H. Effect of Daily Light Integral on Cucumber Plug Seedlings in Artificial Light Plant Factory. Horticulturae 2021, 7, 139. https://doi.org/10.3390/horticulturae7060139
Cui J, Song S, Yu J, Liu H. Effect of Daily Light Integral on Cucumber Plug Seedlings in Artificial Light Plant Factory. Horticulturae. 2021; 7(6):139. https://doi.org/10.3390/horticulturae7060139
Chicago/Turabian StyleCui, Jiawei, Shiwei Song, Jizhu Yu, and Houcheng Liu. 2021. "Effect of Daily Light Integral on Cucumber Plug Seedlings in Artificial Light Plant Factory" Horticulturae 7, no. 6: 139. https://doi.org/10.3390/horticulturae7060139
APA StyleCui, J., Song, S., Yu, J., & Liu, H. (2021). Effect of Daily Light Integral on Cucumber Plug Seedlings in Artificial Light Plant Factory. Horticulturae, 7(6), 139. https://doi.org/10.3390/horticulturae7060139







