Effect of 5-Aminolevulinic Acid (5-ALA) on Leaf Chlorophyll Fast Fluorescence Characteristics and Mineral Element Content of Buxus megistophylla Grown along Urban Roadsides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Treatment
2.2. Determination of Leaf Morphology and Relative Chlorophyll Content
2.3. Rapid Fluorescence Determination of Chlorophyll in Leaves and Analysis of Fluorescence Parameters
2.4. Determination of Soluble Sugars and Free Proline in Leaves
2.5. Determination of the Activities of Antioxidant Enzymes in Leaves and Roots
2.6. Determination of Mineral Nutrient Elements in Leaves and Roots
2.7. Statistical Analysis
3. Results
3.1. Effect of 5-ALA Treatment on the Appearance of B. megistophylla on Both Sides of Urban Roads
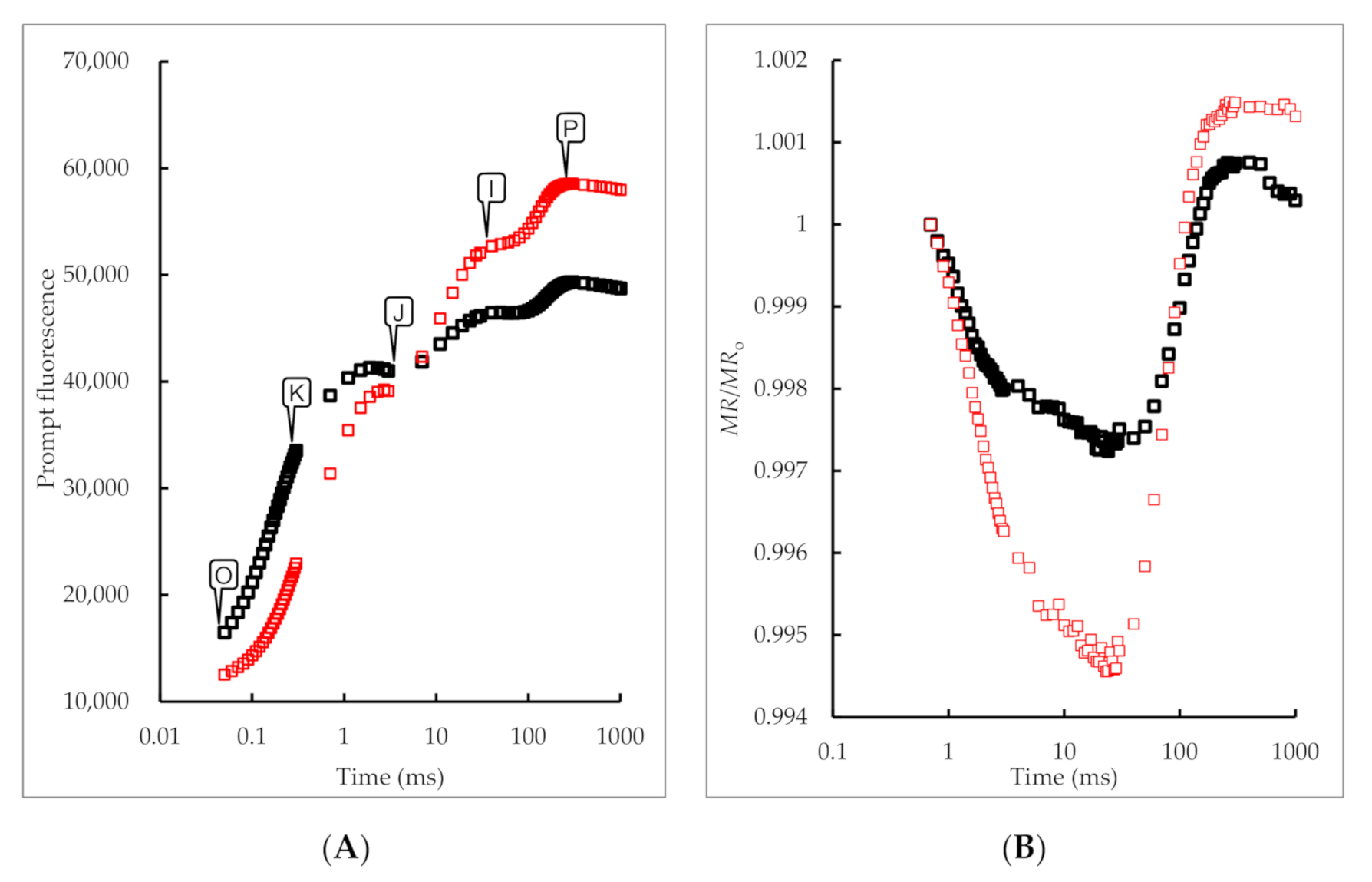
3.2. Effects of 5-ALA Treatment on Chlorophyll Fast Fluorescence and 820 nm Reflection Fluorescence Absorption Curve of B. megistophylla Leaves
3.3. Effect of 5-ALA on Chlorophyll Rapid Fluorescence Parameters in B. megistophylla Leaves
3.4. Effects of 5-ALA Treatment on the Content of Soluble Sugar and Free Proline in B. megistophylla Leaves
3.5. Effects of 5-ALA Treatment on the Activities of Antioxidant Enzymes in Leaves and Roots of B. megistophylla
3.6. Effect of 5-ALA Treatment on the Mineral Element Content of B. megistophylla
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, J.; Luo, W. Importance and design method of urban road greening. World Build. Mater. 2013, 34, 50–52. [Google Scholar]
- Gao, Y.D.; Liu, H.R. Physiological responses of road greening plants to traffic pollution. Heilongjiang Agric. Sci. 2016, 3, 93–97. [Google Scholar]
- Wu, Y.; Liao, W.; Dawuda, M.M.; Hu, L.; Yu, J. 5-Aminolevulinic acid (ALA) biosynthetic and metabolic pathways and its role in higher plants: A review. Plant Growth Regul. 2019, 87, 357–374. [Google Scholar] [CrossRef]
- Akram, N.A.; Ashraf, M. Regulation in plant stress tolerance by a potential plant growth regulator, 5-aminolevulinic acid. J. Plant Growth Regul. 2013, 32, 663–679. [Google Scholar] [CrossRef]
- Wang, L.J.; Jiang, W.B.; Zhang, Z.; Yao, Q.H.; Matsui, H.; Ohara, H. Biosynthesis and physiological activity of 5-aminolevulinic acid and its potential application in agriculture. Plant Physiol. Commun. 2003, 39, 185–192. [Google Scholar]
- Hotta, Y.; Tanaka, T.; Takaoka, H.; Takeuchi, Y.; Konnai, M. New physiological effects of 5-aminolevulinic acid in plants: The increase of photosynthesis, chlorophyll content, and plant growth. Biosci. Biotechnol. Biochem. 1997, 61, 2025–2028. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, E.; Kondo, K.; Parvez, M.M.; Takahashi, K.; Watanabe, K.; Tanaka, K. Role of 5-aminolevulinic acid (ALA) on active oxygen-scavenging system in NaCl-treated spinach (Spinacia oleracea). J. Plant Physiol. 2003, 160, 1085–1091. [Google Scholar] [CrossRef]
- Wang, L.J.; Jiang, W.B.; Huang, B.J. Promotion of 5-aminolevulinic acid on photosynthesis of melon (Cucumis melo) seedlings under low light and chilling stress conditions. Physiol. Plant 2004, 121, 258–264. [Google Scholar] [CrossRef]
- Ma, N.; Qi, L.; Gao, J.; Chao, K.C.; Hu, Q.F.; Jiang, H.G.; Wang, L.J. Effects of 5-ALA on growth and chlorophyll fluorescence of Ficus carica cutting seedlings under high temperature. J. Nanjing Agric. Univ. 2015, 38, 546–553. [Google Scholar]
- Liu, W.Q.; Kang, L.; Wang, L.J. Effect of ALA on photosynthesis of strawberry and its relationship with antioxidant enzymes. Acta Bot. Boreal. Occident. Sin. 2006, 26, 57–62. [Google Scholar]
- Aksakal, O.; Algur, O.F.; Icoglu, A.F.; Aysin, F. Exogenous 5-aminolevulinic acid alleviates the detrimental effects of UV-B stress on lettuce (Lactuca sativa L.) seedlings. Acta Physiol. Plant 2017, 39, 55. [Google Scholar] [CrossRef]
- Cai, C.Y.; He, S.S.; An, Y.Y.; Wang, L.J. Exogenous 5-aminolevulinic acid improves strawberry tolerance to osmotic stress and its possible mechanisms. Physiol. Plant 2020, 168, 948–962. [Google Scholar] [CrossRef]
- An, Y.Y.; Qi, L.; Wang, L.J. ALA pretreatment improves waterlogging tolerance of fig plants. PLoS ONE 2016, 11, e0147202. [Google Scholar] [CrossRef]
- Liu, L.Y.; El-Shemy, H.A.; Saneoka, H. Effects of 5-aminolevulinic acid on water uptake, ionic toxicity, and antioxidant capacity of Swiss chard (Beta vulgaris L.) under sodic-alkaline conditions. J. Plant Nutr. Soil Sci. 2017, 180, 535–543. [Google Scholar]
- Singh, R.; Kesavan, A.K.; Landi, M.; Kaur, S.; Thakur, S.; Zheng, B.S.; Bhardwaj, R.; Sharma, A. 5-Aminolevulinic acid regulates Krebs cycle, antioxidative system and gene expression in Brassica juncea L. to confer tolerance against lead toxicity. J. Biotechnol. 2020, 323, 283–292. [Google Scholar] [CrossRef]
- Taspinar, M.S.; Aydin, M.; Arslan, E.; Yaprak, M.; Agar, G. 5-Aminolevulinic acid improves DNA damage and DNA methylation changes in deltamethrin-exposed Phaseolus vulgaris seedlings. Plant Physiol. Biochem. 2017, 118, 267–273. [Google Scholar] [CrossRef]
- Xu, L.; Islam, F.; Zhang, W.F.; Ghani, M.A.; Ali, B. 5-Aminolevulinic acid alleviates herbicide-induced physiological and ultrastructural changes in Brassica napus. J. Integr. Agric. 2018, 17, 579–592. [Google Scholar] [CrossRef]
- Elansary, H.O.; El-Ansary, D.O.; Al-Mana, F.A. 5-Aminolevulinic acid and soil fertility enhance the resistance of rosemary to Alternaria dauci and Rhizoctonia solani and modulate plant biochemistry. Plants 2019, 8, 585. [Google Scholar] [CrossRef]
- Chen, H.; Xu, L.; Li, X.; Wang, D.Y.; An, Y.Y.; Wang, L.J. Effect of 5-aminolevulinic acid on cold tolerance of Rhododendron simsii and Cinnamomum camphora leaves. Plant Physiol. J. 2017, 53, 2103–2113. [Google Scholar]
- Wang, D.Y.; Li, X.; Xu, L.; An, Y.Y.; Wang, L.J. Effect of 5-aminolevulinic acid on leaf heat tolerance in Ligustrum japonicum and Spiraea japonica. Bot. Res. 2018, 7, 350–365. [Google Scholar]
- Qiu, M.H.; Li, D.Z. Study on chemotaxonomy of Buxaceae. Chin. J. Appl. Environ. Biol. 2002, 8, 387–391. [Google Scholar]
- Shen, Y.M.; Ma, G.S. Occurrence regularity and ecological control technology of diseases of hedgerow commonly used in greening in five cities of southern Jiangsu. J. Landsc. Res. 2019, 11, 141–144. [Google Scholar]
- Tian, B. Effects of drought stress on protective enzymes and osmotic substances of Buxus megistophylla Levl. Shandong For. Sci. Technol. 2019, 4, 64–67. [Google Scholar]
- Hou, L.Q. Effects of sustained low temperature stress on physiological characteristics of Buxus megistophylla Levl. Shandong For. Sci. Technol. 2019, 4, 68–71. [Google Scholar]
- Li, J.X.; He, J.; Sun, Y.M.; Zhao, A.; Tain, Q. Physiological and ecological responses of ten garden plant functional traits to air pollution. Ecol. Environ. Sci. 2020, 29, 1205–1214. [Google Scholar]
- Li, H.S. Principles and Techniques of Plant Physiological and Biochemical Experiments; Higher Education Press: Beijing, China, 2020. [Google Scholar]
- Zhang, D.Z.; Wang, P.H.; Zhao, H.X. Determination of free proline content in wheat leaves. Plant Physiol. Commun. 1990, 4, 62–65. [Google Scholar]
- Tan, W.; Liu, J.; Dai, T.; Jing, Q.; Cao, W.; Jiang, D. Alterations in photosynthesis and antioxidant enzyme activity in winter wheat subjected to post-anthesis water-logging. Photosynthetica 2008, 46, 21–27. [Google Scholar] [CrossRef]
- Zhang, W.F.; Zhang, F.; Raziuddin, R.; Gong, H.J.; Yang, Z.M.; Lu, L.; Ye, Q.F.; Zhou, W.J. Effects of 5-aminolevulinic acid on oilseed rape seeding growth under herbicide toxicity stress. J. Plant Growth Regul. 2008, 27, 159–169. [Google Scholar] [CrossRef]
- Change, B.; Maehly, A.C. Assay of catalases and peroxidase. Methods Enzym. 1995, 2, 764–775. [Google Scholar]
- Wang, J.B. The role of urban road greening. New Silk Road Horiz. 2011, 8, 88–90. [Google Scholar]
- Yang, X.X.; Feng, L.H.; Wei, P. Automobile exhaust pollution and its harm. Front. Sci. 2012, 6, 10–22. [Google Scholar]
- Ling, Q.Y. Physiological response and resistance of landscape plants to automobile exhaust. Green Technol. 2015, 6, 206–207. [Google Scholar]
- Chen, J.N.; Yuan, X.W.; Xiang, X.Z.; Yuan, X.H.; Li, H. Evaluation on the resistance of fourteen ground cover plants to automobile exhaust. Resour. Environ. Arid Areas 2018, 32, 176–181. [Google Scholar]
- Li, L.B.; Kuang, T.Y. An Overview of Primary Light Energy Conversion in Photosynthesis, Principle and Regulation of Primary Energy Transformation Process of Photosynthesis; Kuang, T.Y., Ed.; Jiangsu Science and Technology Press: Nanjing, China, 2003; pp. 3–21. [Google Scholar]
- Li, M.A.; Ma, L.; Hao, Q.; An, Y.Y.; Wang, L.J. Effect of 5-aminolevulinic acid on leaf photosynthetic characteristics, yield and quality of potato. China Veg. 2020, 11, 43–52. [Google Scholar]
- Ou, C.; Yao, X.M.; Yao, X.J.; Ji, J.; Wang, W.G.; Guo, J. Effects of exogenous 5-aminolevulinic acid and PEG on photosynthetic and antioxidant characteristics of Gardenia jasminoides seedlings. Agric. Res. Arid Areas 2016, 34, 235–242. [Google Scholar]
- Naeem, M.S.; Jin, Z.L.; Wan, G.L.; Liu, D.; Liu, H.B.; Yoneyama, K.; Zhou, W.J. 5-Aminolevulinic acid improves photosynthetic gas exchange capacity and ion uptake under salinity stress in oilseed rape (Brassica napus L.). Plant Soil 2010, 332, 405–415. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Feng, X.X.; Gao, J.J.; An, Y.Y.; Tian, F.; Li, J.; Zhang, Z.P.; Wang, L.J. Effects of ALA solution on leaf physiological characteristics and fruit quality of apple under rhizosphere irrigation. Jiangsu J. Agric. Sci. 2015, 1, 158–165. [Google Scholar]
- Anwar, A.; Yan, Y.; Liu, Y.; Li, Y.; Yu, X. 5-Aminolevulinic acid improves nutrient uptake and endogenous hormone accumulation, enhancing low-temperature stress tolerance in cucumbers. Int. J. Mol. Sci. 2018, 19, 3379. [Google Scholar] [CrossRef]
- Wei, Z.Y.; Zhang, Z.P.; Lee, M.R.; Sun, Y.P.; Wang, L.J. Effect of 5-aminolevulinic acid on leaf senescence and nitrogen metabolism of pakchoi under different nitrates. J. Plant Nutr. 2012, 35, 49–63. [Google Scholar] [CrossRef]
- Yao, S.M.; Wang, W.J.; Chen, G.X. Effects of 5-aminolevulinic acid on the uptake and distribution of 32P in rice plants. Acta Phytonutri Fertil 2006, 12, 70–75. [Google Scholar]
- Montanaro, G.; Dichio, B.; Lang, A.; Mininni, A.; Xiloyannis, C. Fruit calcium accumulation coupled and uncoupled from its transpiration in kiwifruit. J. Plant Physiol. 2015, 181, 67–74. [Google Scholar] [CrossRef]
- An, Y.; Xiong, L.J.; Hu, S.; Wang, L.J. PP2A and microtubules function in 5-aminolevulinic acid-mediated H2O2 signaling in Arabidopsis guard cells. Physiol. Plant 2020, 168, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.W.; He, S.S.; An, Y.Y.; Cao, R.X.; Sun, Y.P.; Tang, Q.; Wang, L.J. Hydrogen peroxide as a mediator of 5-aminolevulinic acid-induced Na+ retention in roots for improving salt tolerance of strawberries. Physiol. Plant 2019, 167, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, B. Study on Heavy Metal Distribution and Pollution Characteristics of Crops in Highway Area—Taking Xisan and Xiyan Expressways as Examples. Master’s Thesis, Chang’an University, Xi’an, China, 2012. [Google Scholar]
- Ali, B.; Wang, B.; Ali, S.; Ghani, M.A.; Hayat, M.T.; Yang, C.; Xu, L.; Zhou, W.J. 5-Aminolevulinic acid ameliorates the growth, photosynthetic gas exchange capacity, and ultrastructural changes under cadmium stress in Brassica napus L. J. Plant Growth Regul. 2013, 32, 604–614. [Google Scholar] [CrossRef]
- Xu, L.; Li, J.J.; Najee, U.; Li, X.; Pan, J.M.; Huang, Q.; Zhou, W.J.; Liang, Z.S. Synergistic effects of EDDS and ALA on phytoextraction of cadmium as revealed by biochemical and ultrastructural changes in sunflower (Helianthus annuus L.) tissues. J. Hazard. Mater. 2020, 407, 124764. [Google Scholar] [CrossRef]
- Zhang, X.H.; Guo, Y.L.; Lin, A.J.; Huang, Y.Z. Effects of arbuscular mycorrhizal fungi colonization on toxicity of soil contaminated by heavy metals to Vicia faba. Chin. J. Environ. Eng. 2008, 2, 274–278. [Google Scholar]
- Wang, H.X.; Han, L.L.; Li, Y.; Zhang, L.L.; Li, X.; Yuan, Z.L. Study on mitigation of cadmium stress in wheat seedlings by inoculation with arbuscular mycorrhizal fungi. J. Henan Agric. Univ. 2021, 55, 29–34. [Google Scholar]
| Treatment | Leaf Length (cm) | Leaf Width (cm) | Leaf Thickness (cm) | SPAD |
|---|---|---|---|---|
| Control | 3.25 ± 0.08 B z | 2.38 ± 0.09 B | 0.30 ± 0.002 b | 41.70 ± 0.23 B |
| 5-ALA | 4.84 ± 0.03 A | 3.34 ± 0.06 A | 0.35 ± 0.002 a | 67.16 ± 0.25 A |
| Fluorescence Parameters | Treatment | Fluorescence Parameters | Treatment | ||
|---|---|---|---|---|---|
| Control | 5-ALA | Control | 5-ALA | ||
| Fo | (1.63 ± 0.12) × 104 A z | (1.23 ± 0.03) × 104 B | ETo/CS | (2.79 ± 0.19) × 103 B | (4.51 ± 0.19) × 103 A |
| Fm | (4.91 ± 0.22) × 104 B | (5.71 ± 0.17) × 104 A | DIo/CS | (5.83 ± 0.83) × 103 A | (2.65 ± 0.01) × 103 B |
| Fv | (3.28 ± 0.22) × 104 B | (4.49 ± 0.15) × 104 A | ABS/RC | 4.43 ± 0.40 A | 2.18 ± 0.07 B |
| Wk | 0.70 ± 0.04 A | 0.43 ± 0.01 B | TRo/RC | 2.80 ± 0.14 A | 1.70 ± 0.04 B |
| Ψo | 0.28 ± 0.03 B | 0.47 ± 0.02 A | ETo/RC | 0.73 ± 0.04 a | 0.79 ± 0.02 a |
| Mo | 2.06 ± 0.16 A | 0.91 ± 0.05 B | DIo/RC | 1.64 ± 0.27 A | 0.47 ± 0.02 B |
| φPo | 0.66 ± 0.03 B | 0.78 ± 0.00 A | RC/CS | (3.82 ± 0.20) × 103 B | (5.70 ± 0.18) × 103 A |
| φEo | 0.19 ± 0.02 B | 0.37 ± 0.01A | VPSI | (8.72 ± 3.22) × 10−4 B | (16.23 ± 0.18) × 10−4 A |
| φRo | 0.07 ± 0.01 B | 0.11 ± 0.00 A | VPSII-PSI | (2.12 ± 1.47) × 10−5 B | (6.72 ± 1.38) × 10−5 A |
| φDo | 0.34 ± 0.03 A | 0.22 ± 0.00 B | PIABS | 0.33 ± 0.09 B | 1.62 ± 0.19 A |
| ABS/CS | (1.63 ± 0.12) × 104 A | (1.23 ± 0.03) × 104 B | PItotal | 0.17 ± 0.04 B | 0.66 ± 0.07 A |
| TRo/CS | (1.04 ± 0.05) × 104 A | (0.96 ± 0.03) ×104 B | |||
| Treatment | Soluble Sugar (mg g−1FW) | Free Proline (mg g−1FW) |
|---|---|---|
| Control | 0.58 ± 0.07 b z | 0.12 ± 0.05 b |
| 5-ALA | 1.29 ± 0.07 a | 0.30 ± 0.07 a |
| Treatments | SOD (U g−1FW) | POD (U g−1FW min−1) | CAT (U g−1FW min−1) | |||
|---|---|---|---|---|---|---|
| Leaves | Roots | Leaves | Roots | Leaves | Roots | |
| Control | 36.05 ± 0.51 b z | 34.35 ± 0.70 b | 29.73 ± 5.45 B | 11.09 ± 1.95 B | 15.97 ± 0.72 B | 39.85 ± 4.99 B |
| 5-ALA | 38.11 ± 0.07 a | 36.95 ± 0.39 a | 85.69 ± 2.74 A | 37.44 ± 0.68 A | 62.42 ± 1.58 A | 63.76 ± 14.69 A |
| Element | Leaves | Roots | Ratio of Leaf to Root | |||
|---|---|---|---|---|---|---|
| Control | 5-ALA | Control | 5-ALA | Control | 5-ALA | |
| N (mg/g) | 7.01 ± 0.06 B z | 13.71 ± 0.82 A | 11.29 ± 0.17 B | 14.23 ± 0.21 A | 0.62 ± 0.01 B | 0.97 ± 0.07 A |
| P (mg/g) | 3.20 ± 0.78 b | 8.39 ± 1.44 a | 0.32 ± 0.05 b | 1.55 ± 0.21 a | 11.50 ± 4.83 a | 5.50 ± 0.82 a |
| K (mg/g) | 10.45 ± 1.40 a | 15.55 ± 3.23 a | 4.67 ± 0.62 a | 6.29 ± 0.62 a | 2.26 ± 0.33 a | 2.56 ± 0.67 a |
| Ca (mg/g) | 27.40 ± 2.67 b | 48.38 ± 5.18 a | 17.73 ± 0.77 b | 24.37 ± 0.81 a | 1.55 ± 0.14 a | 2.00 ± 0.29 a |
| Mg (mg/g) | 0.76 ± 0.13 b | 1.80 ± 0.25 a | 2.40 ± 0.20 a | 2.79 ± 0.34 a | 0.31 ± 0.03 b | 0.69 ± 0.18 a |
| Fe (mg/g) | 0.11 ± 0.01 B | 0.29 ± 0.01 A | 4.39 ± 0.34 B | 9.34 ± 0.47 A | 0.03 ± 0.00 a | 0.03 ± 0.00 a |
| Cu (mg/kg) | 26.50 ± 0.50 b | 32.30 ± 1.52 a | 231.91 ± 71.74 b | 463.19 ± 14.49 a | 0.13 ± 0.03 a | 0.07 ± 0.00 a |
| Zn (mg/kg) | 28.21 ± 6.54 a | 29.98 ± 6.08 a | 66.78 ± 2.27 a | 66.73 ± 1.22 a | 0.43 ± 0.11 a | 0.45 ± 0.09 a |
| Mn (mg/kg) | 24.08 ± 5.23 a | 23.52 ± 4.39 a | 194.60 ± 37.62 a | 90.67 ± 4.12 b | 0.14 ± 0.01 a | 0.26 ± 0.06 a |
| B (mg/kg) | 28.07 ± 4.08 b | 74.95 ± 10.61 a | 121.24 ± 10.28 b | 257.60 ± 18.56 a | 0.23 ± 0.02 a | 0.30 ± 0.07 a |
| Na (mg/g) | 0.97 ± 0.00 a | 1.04 ± 0.06 a | 1.25 ± 0.04 b | 1.45 ± 0.06 a | 0.78 ± 0.03 a | 0.72 ± 0.05 a |
| Al (mg/g) | 0.05 ± 0.00 a | 0.08 ± 0.01 a | 5.11 ± 0.67 a | 2.60 ± 0.27 b | 0.01 ± 0.00 a | 0.03 ± 0.00 a |
| Cd (mg/kg) | 0.43 ± 0.04 a | 0.28 ± 0.01 b | 0.35 ± 0.10 b | 0.76 ± 0.06 a | 1.48 ± 0.51 a | 0.37 ± 0.04 b |
| Hg (mg/kg) | 69.45 ± 4.37 a | 53.35 ± 1.46 b | 213.78 ± 13.29 b | 292.75 ± 20.48 a | 0.32 ± 0.00 a | 0.18 ± 0.01 b |
| Cr (mg/g) | 0.36 ± 0.05 a | 0.16 ± 0.01 b | 1.29 ± 0.24 b | 3.36 ± 0.39 a | 0.30 ± 0.08 a | 0.05 ± 0.01 b |
| Pb (mg/kg) | 1.32 ± 0.10 a | 0.90 ± 0.04 b | 6.92 ± 1.15 b | 19.11 ± 1.89 a | 0.20 ± 0.04 a | 0.05 ± 0.01 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Zhang, J.; Zhang, H.; Xu, Y.; An, Y.; Wang, L. Effect of 5-Aminolevulinic Acid (5-ALA) on Leaf Chlorophyll Fast Fluorescence Characteristics and Mineral Element Content of Buxus megistophylla Grown along Urban Roadsides. Horticulturae 2021, 7, 95. https://doi.org/10.3390/horticulturae7050095
Yang H, Zhang J, Zhang H, Xu Y, An Y, Wang L. Effect of 5-Aminolevulinic Acid (5-ALA) on Leaf Chlorophyll Fast Fluorescence Characteristics and Mineral Element Content of Buxus megistophylla Grown along Urban Roadsides. Horticulturae. 2021; 7(5):95. https://doi.org/10.3390/horticulturae7050095
Chicago/Turabian StyleYang, Hao, Jianting Zhang, Haiwen Zhang, Yi Xu, Yuyan An, and Liangju Wang. 2021. "Effect of 5-Aminolevulinic Acid (5-ALA) on Leaf Chlorophyll Fast Fluorescence Characteristics and Mineral Element Content of Buxus megistophylla Grown along Urban Roadsides" Horticulturae 7, no. 5: 95. https://doi.org/10.3390/horticulturae7050095
APA StyleYang, H., Zhang, J., Zhang, H., Xu, Y., An, Y., & Wang, L. (2021). Effect of 5-Aminolevulinic Acid (5-ALA) on Leaf Chlorophyll Fast Fluorescence Characteristics and Mineral Element Content of Buxus megistophylla Grown along Urban Roadsides. Horticulturae, 7(5), 95. https://doi.org/10.3390/horticulturae7050095






