Multiple Amino Acids Inhibit Postharvest Senescence of Broccoli
Abstract
1. Introduction
2. Materials and Methods
2.1. Broccoli Treatment
2.2. Postharvest Quality Assessment
2.2.1. Green Life
2.2.2. Ethylene Production and Respiration Rate
2.2.3. Ion Leakage
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hildebrandt, T.M.; Nunes Nesi, A.; Araujo, W.L.; Braun, H.P. Amino acid catabolism in plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ding, Y.; Tang, X.; Wang, G.; Wu, S.; Li, X.; Huang, X.; Qu, T.; Chen, J.; Tang, X. Effect of L-arginine on maintaining storage quality of the white button mushroom (Agaricus bisporus). Food Bioprocess. Technol. 2019, 12, 563–574. [Google Scholar] [CrossRef]
- Wang, X.; Gu, S.; Chen, B.; Huang, J.; Xing, J. Effect of postharvest L-arginine or cholesterol treatment on the quality of green asparagus (Asparagus officinalis L.) spears during low temperature storage. Sci. Hortic. 2017, 225, 788–794. [Google Scholar] [CrossRef]
- Shu, P.; Min, D.; Ai, W.; Li, J.; Zhou, J.; Li, Z.; Zhang, X.; Shi, Z.; Sun, Y.; Jiang, Y.; et al. L-arginine treatment attenuates postharvest decay and maintains quality of strawberry fruit by promoting nitric oxide synthase pathway. Postharvest. Biol. Technol. 2020, 168, 111253. [Google Scholar] [CrossRef]
- Zheng, Y.; Sheng, J.; Zhao, R.; Zhang, J.; Lv, S.; Liu, L.; Shen, L. Preharvest L-arginine treatment induced postharvest disease resistance to Botrysis cinerea in tomato fruits. J. Agric. Food Chem. 2011, 59, 6543–6549. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Khan, A.S.; Malik, A.U. Postharvest L-cysteine application delayed pericarp browning, suppressed lipid peroxidation and maintained antioxidative activities of litchi fruit. Postharvest. Biol. Technol. 2016, 121, 135–142. [Google Scholar] [CrossRef]
- Li, T.; Wu, Q.; Zhou, Y.; Yun, Z.; Duan, X.; Jiang, Y. L-Cysteine hydrochloride delays senescence of harvested longan fruit in relation to modification of redox status. Postharvest. Biol. Technol. 2018, 143, 35–42. [Google Scholar] [CrossRef]
- Al Ubeed, H.M.S.; Wills, R.B.H.; Bowyer, M.C.; Golding, J.B. Inhibition of postharvest senescence of green leafy vegetables by exogenous D-cysteine and L-cysteine as precursors of hydrogen sulphide. J. Hortic. Sci. Biotechnol. 2019, 94, 620–626. [Google Scholar] [CrossRef]
- Babalar, M.; Pirzad, F.; Sarcheshmeh, M.A.A.; Talaei, A.; Lessani, H. Arginine treatment attenuates chilling injury of pomegranate fruit during cold storage by enhancing antioxidant system activity. Postharvest. Biol. Technol. 2018, 137, 31–37. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, L.; Li, F.; Meng, D.; Sheng, J. Amelioration of chilling stress by arginine in tomato fruit: Changes in endogenous arginine catabolism. Postharvest. Biol. Technol. 2013, 76, 106–111. [Google Scholar] [CrossRef]
- Banin Sogvar, O.; Razavi, F.; Rabiei, V.; Gohari, G. Postharvest application of L-cysteine to prevent enzymatic browning of “Stanley” plum fruit during cold storage. J. Food Process. Preserv. 2020, 14788. [Google Scholar] [CrossRef]
- Wills, R.B.H.; Li, Y. Use of arginine to inhibit browning on fresh cut apple and lettuce. Postharvest. Biol. Technol. 2016, 113, 66–68. [Google Scholar] [CrossRef]
- Pace, B.; Capotorto, I.; Ventura, M.; Cefola, M. Evaluation of L-cysteine as anti-browning agent in fresh-cut lettuce processing. J. Food Proces. Preserv. 2015, 39, 985–993. [Google Scholar] [CrossRef]
- Cerit, İ.; Pfaff, A.; Ercal, N.; Demirkol, O. Postharvest application of thiol compounds affects surface browning and antioxidant activity of fresh-cut potatoes. J. Food Biochem. 2020, 44, 13378. [Google Scholar] [CrossRef]
- Zhou, X.; Xiao, Y.; Meng, X.; Liu, B. Full inhibition of Whangkeumbae pear polyphenol oxidase enzymatic browning reaction by L-cysteine. Food Chem. 2018, 266, 1–8. [Google Scholar] [CrossRef]
- Arpita, S.; Subroto, D.; Pinaki, B.; Bidyut, B. Inhibition of polyphenol oxidase in banana, apple and mushroom by using different antibrowning agents under different conditions. Int. J. Chem. Sci. 2010, 8, S550–S558. [Google Scholar]
- Kumar Patel, M.; Maurer, D.; Feygenberg, O.; Ovadia, A.; Elad, Y.; Oren-Shamir, M.; Alkan, N. Phenylalanine: A promising inducer of fruit resistance to postharvest pathogens. Foods 2020, 9, 646. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Cai, Y.; Sun, C.; Huang, Y.; Yu, T. Exogenous L-glutamate treatment could induce resistance against Penicillium expansum in pear fruit by activating defense-related proteins and amino acids metabolism. Postharvest. Biol. Technol. 2019, 150, 148–157. [Google Scholar] [CrossRef]
- Yang, J.; Sun, C.; Fu, D.; Yu, T. Test for L-glutamate inhibition of growth of Alternaria alternata by inducing resistance in tomato fruit. Food Chem. 2017, 230, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Khan, A.S.; Malik, A.U.; Shaheen, T.; Shahid, M. Pre-storage methionine treatment inhibits postharvest enzymatic browning of cold stored ‘Gola’ litchi fruit. Postharv. Biol. Technol. 2018, 140, 100–106. [Google Scholar] [CrossRef]
- Ali, H.M.; El-Gizawy, A.M.; El-Bassiouny, R.E.I.; Saleh, M.A. The role of various amino acids in enzymatic browning process in potato tubers and identifying the browning products. Food Chem. 2016, 192, 879–885. [Google Scholar] [CrossRef]
- Sohail, M.; Wills, R.B.H.; Bowyer, M.C.; Pristijono, P. Beneficial impact of exogenous arginine, cysteine and methionine on postharvest senescence of broccoli. Food Chem. 2021, 338, 128055. [Google Scholar] [CrossRef] [PubMed]
- Wills, R.B.H.; Warton, M.A.; Ku, V.V.V. Ethylene levels associated with fruit and vegetables during marketing. Aust. J. Exp. Agric. 2000, 40, 465–470. [Google Scholar] [CrossRef]
- Al Ubeed, H.M.S.; Wills, R.B.H.; Bowyer, M.C.; Vuong, Q.V.; Golding, J.B. Interaction of exogenous hydrogen sulphide and ethylene on senescence of green leafy vegetables. Postharvest. Biol. Technol. 2017, 133, 81–87. [Google Scholar] [CrossRef]
- Sohail, M.; Wills, R.B.H.; Bowyer, M.C.; Pristijono, P. Impact of exogenous arginine, cysteine and methionine on the postharvest senescence of six green leafy vegetables. J. Hort. Postharvest. Res. 2021, 4, 1–10. [Google Scholar] [CrossRef]
- Gomes, M.H.; Rosa, E. Free amino acid composition in primary and secondary inflorescences of 11 broccoli (Brassica oleracea var italica) cultivars and its variation between growing seasons. J. Sci. Food Agric. 2000, 81, 295–299. [Google Scholar] [CrossRef]
- Wills, R.B.H. Low ethylene technology in non-optimal storage temperatures. In Advances in Postharvest Fruit and Vegetable Technology; Wills, R.B.H., Golding, J.B., Eds.; CRC Press: Boca Raton, FL, USA, 2015; Chapter 8; pp. 167–190. [Google Scholar] [CrossRef]
- Kumar, V.; Hatan, E.; Bar, E.; Davidovich-Rikanati, R.; Doron-Faigenboim, A.; Spitzer-Rimon, B.; Elad, Y.; Alkan, N.; Lewinsohn, E.; Oren-Shamir, M. Phenylalanine increases chrysanthemum flower immunity against Botrytis cinerea attack. Plant J. 2020, 104, 226–240. [Google Scholar] [CrossRef] [PubMed]
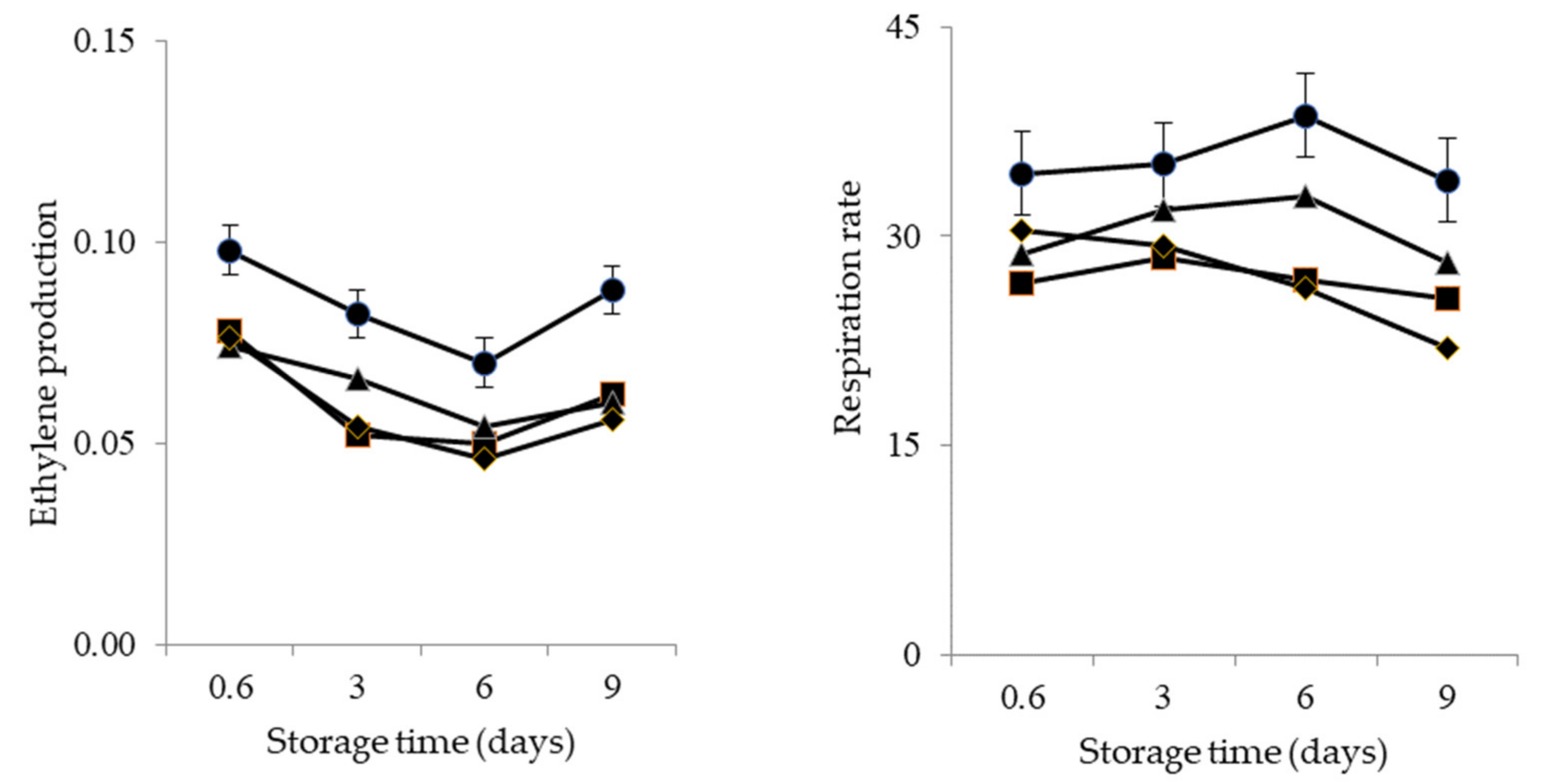
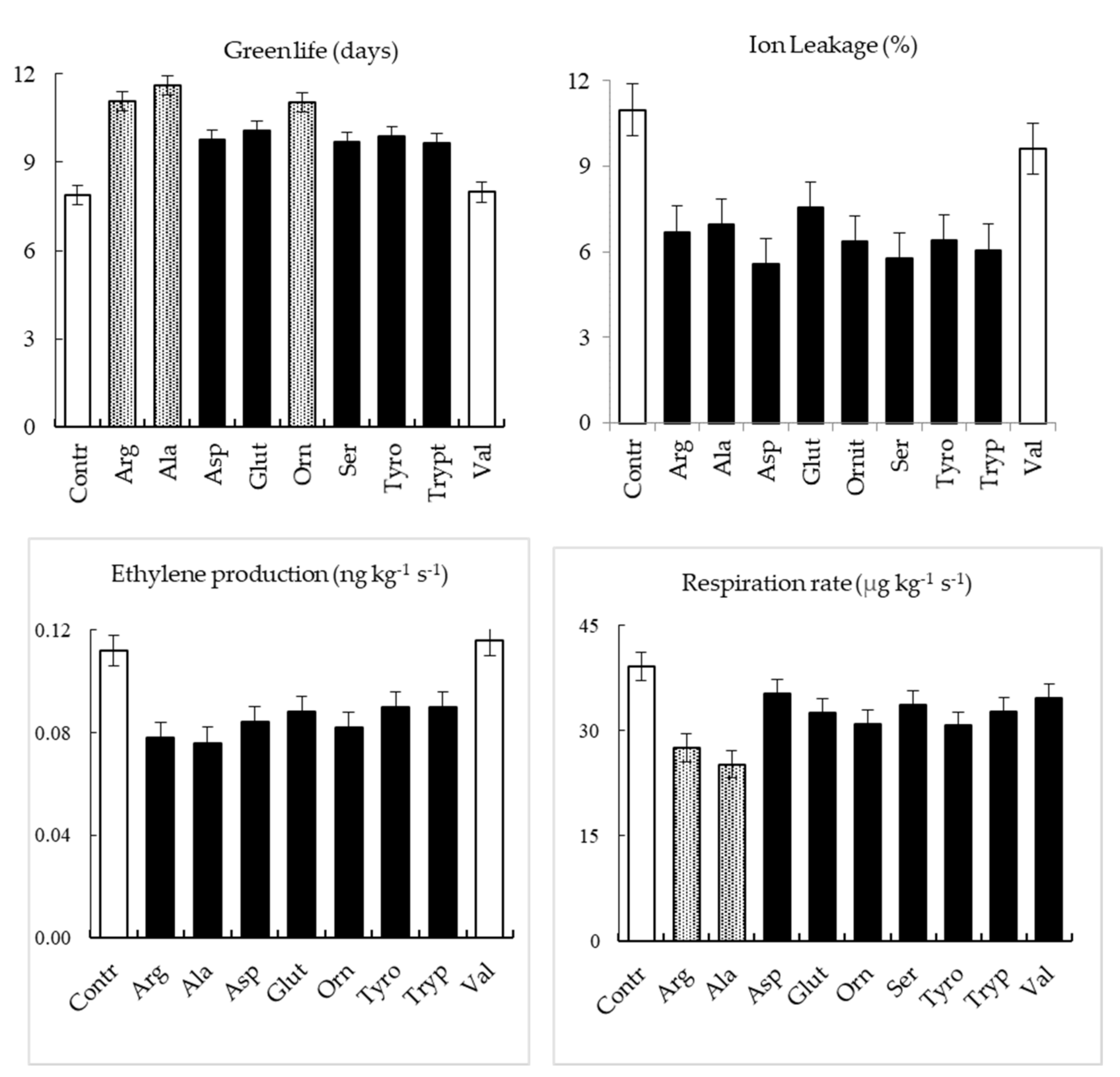
| Treatment | Green Life (Days) | Ethylene Production (ng kg−1 s−1) | Respiration Rate (µg kg−1 s−1) |
|---|---|---|---|
| Control | 9.2 a | 0.082 a | 35.3 a |
| Arginine | 11.1 b | 0.060 b | 26.8 c |
| Glycine | 11.0 b | 0.064 b | 30.4 b |
| Phenylalanine | 12.1 c | 0.058 b | 27.0 c |
| LSD (p = 0.05) | 0.5 | 0.015 | 3.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohail, M.; Wills, R.B.H.; Bowyer, M.C.; Pristijono, P. Multiple Amino Acids Inhibit Postharvest Senescence of Broccoli. Horticulturae 2021, 7, 71. https://doi.org/10.3390/horticulturae7040071
Sohail M, Wills RBH, Bowyer MC, Pristijono P. Multiple Amino Acids Inhibit Postharvest Senescence of Broccoli. Horticulturae. 2021; 7(4):71. https://doi.org/10.3390/horticulturae7040071
Chicago/Turabian StyleSohail, Muhammad, Ron Baden Howe Wills, Michael C. Bowyer, and Penta Pristijono. 2021. "Multiple Amino Acids Inhibit Postharvest Senescence of Broccoli" Horticulturae 7, no. 4: 71. https://doi.org/10.3390/horticulturae7040071
APA StyleSohail, M., Wills, R. B. H., Bowyer, M. C., & Pristijono, P. (2021). Multiple Amino Acids Inhibit Postharvest Senescence of Broccoli. Horticulturae, 7(4), 71. https://doi.org/10.3390/horticulturae7040071







