Abstract
A species-specific latitudinal distribution of soybean rhizobia has been reported; Bradyrhizobium japonicum and B. elkanii dominate in nodules in northern and southern areas, respectively. The aim of this study was to elucidate whether temperature-dependent proliferation in soil or infection is more reliable for determining the latitudinal characteristic distribution of soybean-nodulating rhizobia under local climate conditions. Three study locations, Fukagawa (temperate continental climate), Matsue and Miyazaki (humid sub-tropical climate), were selected in Japan. Each soil sample was transported to the other study locations, and soybean cv. Orihime (non-Rj) was pot-cultivated using three soils at three study locations for two successive years. Species composition of Bradyrhizobium in the nodules was analyzed based on the partial 16S rRNA and 16S–23S rRNA ITS gene sequences. Two Bradyrhizobium japonicum (Bj11 and BjS10J) clusters and one B. elkanii (BeL7) cluster were phylogenetically sub-grouped into two (Bj11-1-2) and four clusters (BjS10J-1-4) based on the ITS sequence. In the Fukagawa soil, Bj11-1 dominated (80–87%) in all study locations. In the Matsue soil, the composition was similar in the Matsue and Miyazaki locations, in which BeL7 dominated (70–73%), while in the Fukagawa location, BeL7 decreased to 53% and Bj11-1 and BjS10J-3 increased. In the Miyazaki soil, BeL7 dominated at 77%, and BeL7 decreased to 13% and 33% in the Fukagawa and Matsue locations, respectively, while BjS10J-2 and BjS10J-4 increased. It was supposed that the B. japonicum strain preferably proliferated in the Fukagawa location, leading to its nodule dominancy, while in the Miyazaki location, temperature-dependent infection would lead to the nodule dominancy of B. elkanii, and both factors would be involved in the Matsue location.
1. Introduction
Soybean (Glycine max [L] Merr.) originated in north-eastern China and is presently cultivated around the globe under various soils and climatic conditions [1,2,3]. The high concentrations of protein and oil in soybean seeds indicate its significance in daily life. Soybean is an easy-to-cultivate crop belonging to the Leguminosae family that can grow in nitrogen-poor soils. Soybean-nodulating rhizobia can establish symbiosis with soybeans through effective nitrogen fixation.
Diverse soybean-nodulating rhizobia belong to the genera Bradyrhizobium, Sinorhizobium (Ensifer) and Mesorhizobium [4,5], among which Bradyrhizobium is recognized as a slow grower, while Sinorhizobium (Ensifer) is recognized as a fast grower and Mesorhizobium as a variable one [6]. B. japonicum and B. elakanii are the major soybean-nodulating rhizobia having a high nitrogen-fixing ability and have been used as inoculants for improving crop production. However, the inoculants could not dominate in nodules due to competition with indigenous rhizobia in the field [7]. Therefore, it is essential to evaluate the ecological behavior of the indigenous soybean-nodulating rhizobia in relation to the environmental conditions.
Saeki et al. [8] studied the geographical distribution of soybean-nodulating rhizobia in Japan using soil samples around the country as inoculants and showed the species-specific latitudinal distribution of B. japonicum and B. elkanii, in which the former dominated in nodules when the northern soils were used, while the latter did in southern soils. Shiro et al. [9] examined the genetic diversity of indigenous soybean-nodulating rhizobia in the USA using a similar method and found the same latitudinal distribution of B. japonicum and B. elkanii from north to south. Adhikari et al. [10] examined nodules from different locations in Nepal and found that B. japonicum dominated in temperate regions, while in subtropical locations, B. elkanii, B. yuanmingense and B. liaoningense dominated in acidic, moderately acidic and slightly alkaline soils, respectively. Li et al. [11] also reported the pH-dependent distribution of rhizobia in Chinese soils, in which B. japonicum and B. elkanii dominated in neutral soils, while B. yuanmingense, B. liaoningense and Sinorhizobium dominated in alkaline soils. These results suggest that temperature and soil pH determine the species-specific distribution of soybean-nodulating rhizobia in soils.
To examine the possible reasons for the temperature-dependent distribution of soybean-nodulating rhizobia, competitive inoculation experiments at different temperatures have been conducted. Kluson et al. [12] reported that B. japonicum USDA 6 and B. diazoefficiens USDA 110 dominated in nodules at lower temperatures, while B. elkanii USDA 76 and B. elkanii USDA 94 did at higher temperature. Suzuki et al. [13] examined the nodule occupancy as well as relative population of B. japonicum and B. elkanii strains in the rhizosphere of soybean cultivated using sterilized vermiculite. Under competitive conditions, B. japonicum strains dominated in nodules at lower temperature even though the relative populations of both strains were similar in the rhizosphere, while at higher temperature, B. elkanii strains dominated in nodules due to their larger relative population in the rhizosphere. Shiro et al. [14] examined the gene expression of nodC and the nodule occupancy of Bradyrhizobia at different temperatures. In the inoculation experiment with mixes of three strains, the nodule occupancy of B. elkanii USDA 31 increased at higher temperatures, whereas that of B. japonicum USDA 123 increased at lower temperatures, corresponding to their temperature-dependent nodC gene expressions. These results support the temperature-dependent distribution of soybean-nodulating rhizobia in the field and suggest that the temperature influenced their preference for infection and/or proliferation in soils. Since the species-specific distribution of rhizobia in field soils is evaluated by their distribution in nodules, it is uncertain which factor, namely, temperature-dependent infection or proliferation in soil, contributes to the temperature-dependent distribution of rhizobia in nodules.
Considering the two above-mentioned factors, we selected three study locations with different climatic conditions in Japan, and each soil sample of the sites was used for soybean cultivation at all the locations for two successive years to examine the changes in the distribution of rhizobia in the nodules after the transfer of the soil samples to the different climatic conditions and to follow the changes in the second year in the new environments. If the predominance of some rhizobia in the soil determines the nodule occupancy, changing climatic conditions would not affect the nodule occupancy; on the other hand, if temperature-dependent infection determines the nodule occupancy, it would be changed in different climatic conditions. The aim of this study is to elucidate the possible reasons for the latitudinal characteristic distribution of soybean-nodulating rhizobia in local climate conditions.
2. Materials and Methods
2.1. Study Locations
To examine the temperature-dependent nodule occupancy of soybean rhizobia, three study locations, Fukagawa (fu), Matsue (ma) and Miyazaki (mi), were selected in Japan. According to Koppen’s climatic classification, Fukagawa belongs to the Dfb (temperate continental climate) region, and Matsue and Miyazaki belong to the Cfa (humid sub-tropical climate) region. Soil samples were collected from the experimental fields of Takushoku University of Hokkaido College, Shimane University and Miyazaki University and used for soybean cultivation at all study locations. There had been no history of legumes cultivation in all soils. Basic information on the site and climatic parameters are presented in Table 1. The soil properties were reported previously (Table S1, [15]).

Table 1.
Geographical and climatic characteristics of the study locations in Japan.
2.2. Soybean Cultivation
The soil samples with a total weight of about 25 Kg were collected from several sites of the experimental field and mixed together for each study location. Each soil sample was divided into three parts (ca. 7.5 Kg) and used for soybean cultivation at each study location. Each soil sample was put in three plastic pots (20 cm in diameter and 25 cm in height), which were placed on a plastic sheet or a wooden duck board in the open field. Seeds of soybean cv. Orihime (non-Rj) from the same lot were used at all study locations. Three healthy seedlings per pot remained at seven days after germination, then they were cultivated for ca. 2–3 months depending on the conditions of the study locations in each year until harvest without fertilization and then the fresh weight of the whole plant and number of nodules were measured. After harvesting of the soybean plants in 2016, in the case of the Matsue location, the pots with the soil were kept in the open filed until the next cultivation season. For the Miyazaki and Fukagawa locations, the triplicate soil samples were mixed and kept in a paper bag in a warehouse under the same temperature conditions as outdoors until the next cultivation in 2017. The different procedures were due to space issues at the study locations.
2.3. Nodule Sampling and Isolation of Rhizobia
After harvesting from flowering to the early fruiting period, the roots were washed carefully with tap water and the whole plant fresh weight was measured after removal of surface water with tissue towel, and the number of nodules in each plant was counted, then the nodules were preserved at low temperature in a vial containing desiccating silica gel until isolation of rhizobia.
For isolation of rhizobia, ten nodules were randomly selected from one plant for each replication and kept in sterilized distilled water overnight. When the number of nodules was less than 10, two plants were used. After surface sterilization with 95% ethanol for 30 s followed by 3% sodium hypochlorite solution for 30 s, and rinsing in sterilized distilled water at least seven times, each nodule was crushed in an Eppendorf tube with 1 mL of sterilized distilled water, then a drop of suspension was streaked onto yeast mannitol agar (YMA) medium [16] and incubated at 25 °C for 5–12 days. Two randomly selected colonies per nodule were purified, and a total of 540 isolates (3 replications, 3 soils from 3 study locations, 10 nodules per plant and 2 isolates per nodule) were further analyzed molecularly.
2.4. Phylogenetic Analysis of the Rhizobia Based on Genes of 16S rRNA and 16S–23S rRNA Internal Transcribed Spacer (ITS) Region
A small amount of the colony was directly subjected as the template for the PCR amplification of the partial 16S rRNA gene using the universal primers fD1 and rP2 [17]. The components of the PCR mixtures and the PCR running conditions are summarized in Tables S2 and S3, respectively. PCR products were purified and subjected to PCR cycle sequencing, according to the procedures described previously [10]. Taxonomic position of the isolates was determined based on the database (https://www.ncbi.nlm.nih.gov/) using a BLAST [18] search. Multiple sequence alignments were constructed using ClustalW 2.1 [19]. Alignments were manually edited and phylogenetic trees with the related reference strains were constructed using ClustalW 2.1 with the neighbor-joining method and the tree was visualized by MEGA 7 [20].
Among the isolates with the same phylogeny in the 16S rRNA gene in each Soil–Location–Year combination, two representatives were randomly selected for analysis of the ITS region. PCR amplification of the ITS region was conducted using the universal ITS primers 1512F and 23R [21]. The procedures were the same as described above.
2.5. Nucleotide Sequence Accession Numbers
The sequence data generated in this study were deposited in the DDBJ Nucleotide Submission System under the accession numbers LC582850 to LC582907 for the 16S rRNA gene, and LC579845 to LC579902 for the 16S–23S rRNA ITS region.
2.6. Statistical Analysis
Statistical analysis of the soybean cultivation data was carried out using the MSTAT-C 6.1.4 [22] software package. The data were subjected to Duncan’s multiple range test after one-way ANOVA.
3. Results
3.1. Fresh Plant Weight and Number of Nodules of Soybean
At each study location, the fresh plant weight and the nodule numbers were not significantly different among soils in both years with a few exceptions (Figure 1). When all data in each study location were analyzed, the fresh plant weight showed the tendency of increasing from northern (FU) to southern (MI) sites, whereas the nodule number showed the opposite tendency of significantly decreasing from northern to southern sites (Figure 2).
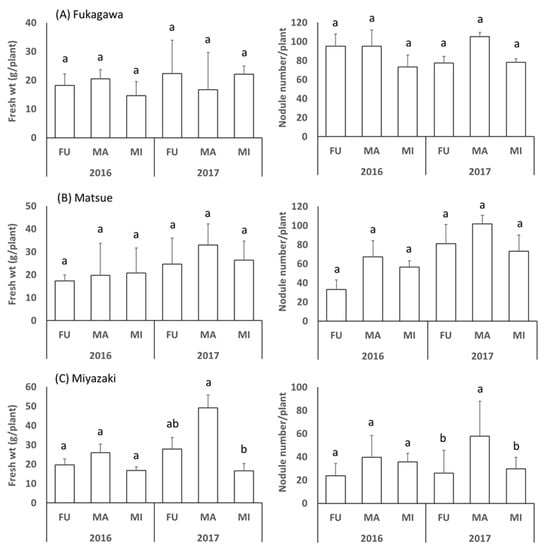
Figure 1.
Fresh weight and number of nodules of soybean cultivated at Fukagawa (A), Matsue (B) and Miyazaki (C) locations using the soil samples (FU, MA and MI) collected from the corresponding study locations. The bars represent standard deviation (n = 3) and different letters indicate significant differences at p < 0.05 by Duncan’s test.
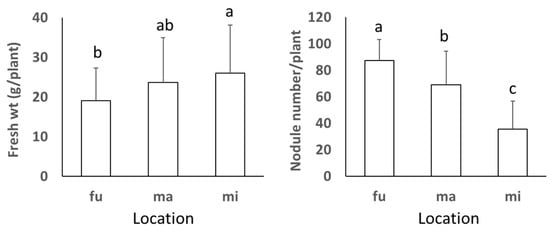
Figure 2.
Fresh weight and number of nodules of soybean cultivated at Fukagawa (fu), Matsue (ma) and Miyazaki (mi) locations. The bars represent standard deviation (n = 18) and different letters indicate significant differences at p < 0.05 by Duncan’s test.
3.2. Phylogenetical Characterizations of the Rhizobia
Based on the 16S rRNA gene analysis, the isolated rhizobia were most closely related to one of the three groups, Bradyrhizobium japonicum Bj11 (KY000638), Bradyrhizobium japonicum S10J (MF664374) and Bradyrhizobium elkanii L7 (KY412842). Similarities (%) of the sequences between the isolates and the corresponding type strains were 98–100%, 96–100% and 97–100% for B. japonicum Bj11, B. japonicum S10J and B. elkanii L7, respectively. The phylogenetic tree of the ITS region of the selected isolates indicated that the rhizobial strains were further grouped into sub-groups (Figure 3). The most similar sequences in the database are listed in Table 2.
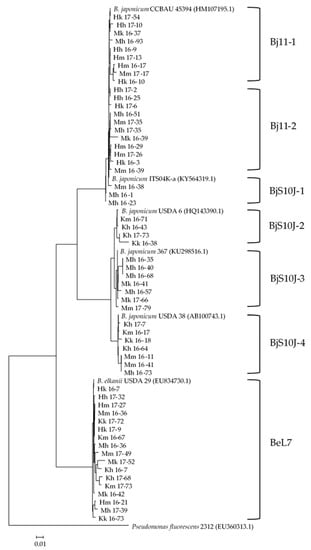
Figure 3.
Phylogenetic tree of the 16S–23S rRNA ITS gene regions of the soybean rhizobial strains isolated in this study with reference strains. The isolates were designated by the soil [Fukagawa (H), Matsue (M) and Miyazaki (K)], the study location (h, m and k), year of the cultivation and the strain number. The scale bar indicates the number of substitutions per site.

Table 2.
Group of soybean rhizobial strains isolated in this study based on phylogeny of 16S–23S rRNA genes’ ITS region.
B. japonicum Bj11 was grouped into Bj11-1 and Bj11-2 based on the ITS sequence, and the two groups were characterized by their physiological properties, that is, it took more than one week for Bj11-1 to form visible colonies on the YMA agar plate, compared to 5–6 days for Bj11-2. B. japonicum S10J was grouped from BjS10J-1 to BjS10J-4 based on the ITS sequence. The ITS sequences of BjS10J-1 had more similarity to those of B. japonicum Bj11 than the other groups of B. japonicum S10J. BjS10J-2 and BjS10J-3 were characterized by their origin, that is, BjS10J-2 and BjS10J-3 were isolated from soybeans cultivated in Miyazaki and Matsue soils, respectively. B. elkanii L7 was isolated from soybeans cultivated in all soils and study locations, and its ITS sequences were not distinguished among them.
3.3. Relative Composition of the Strains in Relation to Soil and Climate in 2016 and 2017
In the Fukagawa soil, the soybean rhizobia consisted of Bj11-1, Bj11-2 and BeL7 in all study locations (Table 3 and Figure 4). Bj11-1 dominated (80–87%) in all study locations in 2016. In 2017, Bj11-1 was maintained in the Fukagawa soil at 80%; however, the compositions decreased in the Matsue and Miyazaki locations at 53% and 60%, respectively, along with the increase in BeL7 to 40% and 30%, respectively. Bj11-2 was present minorly at 7–17% in all study locations and in both years.

Table 3.
Relative composition (%) of rhizobial strains isolated in this study based on phylogeny of the 16S–23S rRNA ITS gene regions.
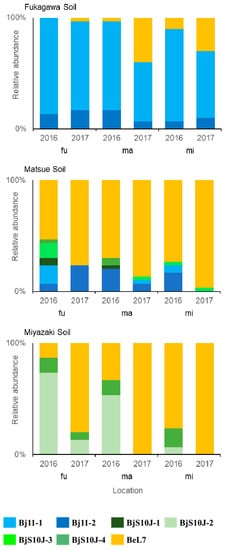
Figure 4.
Relative abundance of the soybean rhizobial strains.
In the Matsue soil, Bj11, BjS10J and BeL7 were isolated in all study locations (Table 3 and Figure 4). The composition and behavior were similar between the Matsue and Miyazaki locations, with Bj11 decreasing from 20% and 24% in 2016 to 10% and 0% in 2017, respectively, while BeL7 increased from 70% and 73% in 2016 to 87% and 97% in 2017, respectively. BjS10J was present minorly at 3–10% in both years. In the Fukagawa location, Bj11 was present at 24% in 2016 and was maintained at 23% in 2017, although the major group shifted from Bj11-1 to Bj11-2. The dominant group BeL7 increased from 53% in 2016 to 77% in 2017 as with the other study locations, while BjS10J, which was 23% in 2016, disappeared in 2017.
In the Miyazaki soil, the rhizobia consisted of BjS10J-2, BjS10J-4 and BeL7 (Table 3 and Figure 4). In the Miyazaki location, BeL7 was dominant at 77% in 2016 and completely eliminated BjS10J in 2017. In the Fukagawa and Matsue locations, BjS10J-2, which was dominant at 73% and 53% in 2016, decreased to 13% and 0% in 2017, respectively, while BeL7 increased from 13% and 33% in 2016 to 80% and 100% in 2017, respectively. BjS10J-4 also decreased from 13% in 2016 to 0–7% in 2017, respectively.
When the Fukagawa soil was moved to the Matsue and Miyazaki locations, the dominant rhizobia changed from Bj11 to BeL7 in the second year. For the Matsue and Miyazaki soils, BeL7 decreased in the Fukagawa and Matsue locations in the first year and recovered to the original level in the second year.
4. Discussion
Although the fresh weight and number of nodules were not significantly different among soils at all study locations (Figure 1), the fresh weight increased from northern to southern study locations, while the number of nodules showed the opposite tendency depending on the study location (Figure 2). The cultivation temperature might be involved in the change in the parameters (Table 1), and a similar tendency of the temperature-dependent growth of soybean has been reported [12,23,24,25].
In the case of the number of nodules, previous reports showed opposite temperature-dependent tendencies from ours [23,24,25]. Reduction in nodules at higher temperature might be due to strain-specific properties. Shiro et al. [14] reported that the nodule numbers were different by about 10 times depending on the strains, and the temperature-dependent expression of the nodC gene was also strain-specific but it was not related to the nodule numbers of the corresponding strains. Hungria and Vargas [26] showed an example of adverse effects of high temperature on the soil population of bradyrhizobia and the nodule number of soybean. Strain-dependent tolerance against high temperature in soil was also reported [13]. Since the bradyrhizobial community structure was changed at the different study locations, the microbial transition might be a reason for the reduction in the nodules at the southern study location.
The phylogenetic analysis of the 16S rRNA genes of the Bradyrhizobium spp. isolates showed three clusters, Bj11, BjS10J and BeL, which mostly corresponded to the three clusters in the phylogeny of the ITS sequences. As the pHs of the soil samples were slightly acidic (Table S1, [15]), the dominant presence of B. japonicum and B. elkanii in the nodules is reasonable [11]. The three clusters were phylogenetically comparable with the results of Saeki et al. [27] and Willems et al. [28] (data not shown). Willems et al. [28] showed that each cluster had more than 95.5% similarity in ITS sequences, whereas the similarity within each cluster in this study ranged 95–97%, and those within the subclusters (Bj11-1-2 and BjS10J-1-4) were 98–99% (data not shown). These results suggest that the Bradyrhizobium spp. isolates in this study were phylogenetically positioned in the same groups as previously reported, and they were further grouped by physiological property (Bj11-1 and 2), phylogeny of 16S rRNA genes (BjS10J-1) and origin of the soil (BjS10J-1, 2 and 3).
The topology of the phylogenetic trees of 16S rDNA and the ITS region was almost the same except for BjS10J-1. The variable position of a subcluster of B. japonicum has also been reported in the reports of Saeki et al. [8] and Adhikari et al. [10]. The ITS nucleotide sequence similarity of the BjS10J-1 strains was more than 98% with those of the Bj11 strains, while it was 88 to 90% with those of the other BjS10J strains having the same 16S rRNA gene sequences. Horizontal gene transfer among them would be one of the possible reasons for the discrepancy in their topologies.
Each cluster of BjS10J was characterized by its origin; on the other hand, BeL7 originated from all soils having undistinguishable gene sequences, and Bj11 was isolated only from Fukagawa and Matsue soils. These results suggest that the range of distribution of the strains differed among the groups. As the wide range of distributions was generally reported in previous studies [10,29], the limited range of distribution of the BjS10J strains suggests that their presence might depend on soil characteristics.
It has been well known that the species-specific distribution of soybean-nodulating rhizobia in the field is temperature-dependent [8,9,10] and that the temperature effect is mainly due to their infection preference [14] and/or proliferation in soil [12,13]. However, it is uncertain which factor, temperature-dependent infection or proliferation in soil, contributes to the temperature-dependent distribution of the rhizobia in nodules.
In the case of the Fukagawa soil, B. japonicum was dominant in the nodules in the high-latitude Fukagawa location, and the dominancy was maintained for two years (Table 3 and Figure 4). This tendency is the same as the temperature-dependent nodule dominancy of B. japonicum as previously reported [8,9,10]. When the Fukagawa soil was moved to the warmer Matsue and Miyazaki locations, the nodule composition of B. japonicum and B. elkanii was not changed, suggesting an originally lower population of B. elkanii in the Fukagawa soil. If B. elkanii was present in the Fukagawa soil to a certain extent and low temperature prevented their infection, thus resulting in their lower nodule dominancy, the nodule dominancy of B. elkanii would increase when the temperature increased in the sub-tropical study locations. In the second year, however, the nodule dominancy of B. elkanii increased in the Matsue and Miyazaki locations, suggesting an increase in the soil population of B. elkanii in the warmer environment. Regarding B. japonicum Bj11 in the Fukagawa soil, the composition of Bj11-2 was maintained in both years in the Matsue and Miyazaki locations, while that of Bj11-1 decreased in the second year, suggesting a higher sensitivity of Bj11-1 to high temperature.
In the case of the Matsue soil, the dominancy of B. elaknii was observed in the Matsue and Miyazaki locations (Table 3 and Figure 4). Similar temperature-dependent nodule occupancy of B. elaknii has been reported [8,9,10]. The dominancy of B. elkanii increased in both study locations in the second year. When this soil was moved to the cooler Fukagawa location, the dominancy of B. japonicum increased in the first year, suggesting that B. japonicum was originally present in the Matsue soil and its nodule dominancy increased due to the lower temperature. The composition of B. elkanii increased in the second year, supposing a decrease in the population of B. japonicum and/or an increase in that of B. elkanii. Although the average minimum and maximun temperatures during the cultivation period were similar between both years, those during one month after sowing, when frequent nodulation would be expected, seemed to be a little higher in the second year (Table 1). It was supposed that the higher temperature in the second year might cause the increase in the relative dominancy of B. elaknii. Among the B. japonicum strains in the first year, Bj11-2 was dominant in the Matsue and Miyazaki locations, while Bj11-1 and BjS10J were dominant in the Fukagawa location. Along with the decrease in Bj11-1 and BjS10J in the second year, the relative dominancy of Bj11-2 increased. The transition of the B. japonicum strains might be due the difference in sensitivity to high temperature among them.
In the Miyazaki soil, BeL7 was dominant and BjS10J-4 was minor in the Miyazaki location in 2016, while in the Fukagawa and Matsue locations, BjS10J-2 appeared dominant (Table 3 and Figure 4), suggesting that B. japonicum was originally present in the Miyazaki soil and its nodulation increased due to the lower temperature in the cooler environments. In the second year, however, BeL7 recovered to 80–100%. A slightly higher temperature during one month after sowing in the second year might be the reason (Table 1), but the temperature in the Fukagawa location in 2017 was lower than that in the Matsue location in 2016; therefore, only the change in temperature could not explain the increase in BjS10J-2 and BeL7 in the Matsue (2016) and Fukagawa (2017) locations, respectively. The difference in rainfall that changed at all study locations each year might be another possible reason (Table 1). As the nodule occupancy of BeL7 increased in the Matsue and Miyazaki soils in 2017, but not in the Fukagawa soil in the Fukagawa location, BeL7 in the different soils might have different properties for a competitive relationship with the coexisting Bj strains.
5. Conclusions
Various conditions of soil storage until the next year in the study location might be differentiated in environmental conditions even in the same temperature conditions as outdoors. Potentially other environmental factors and their correlation with temperature might also affect the microorganisms. In addition, rainfall might affect the soil conditions between outdoor and indoor storage during the winter. Although the effects could not be verified, the shift of the nodule composition in the second year showed the same tendency in all soils and study locations, suggesting that the effects of the difference in the soil storage conditions did not seem to be serious on the composition of rhizobia. Fluctuating rainfall over two successive years in the study locations (Table 1) also suggests that the difference in rainfall would not significantly affect the nodule composition.
By the novel methodology used in this study, we could assume that B. japonicum (Bj11-1) dominantly proliferated in the Fukagawa soil and led to its dominant nodule composition and that both B. japonicum (BjS10J-2) and B. elkanii (BeL7) existed in the Miyazaki soil and the dominant nodule composition of B. elkanii (BeL7) was due to the temperature-dependent infection.
Supplementary Materials
The following are available online at https://www.mdpi.com/2311-7524/7/2/22/s1, Table S1: Soil property of the study sites [15], Table S2: PCR ingredients for amplification of 16S rRNA and 16S-23S rRNA ITS region, Table S3: PCR running conditions.
Author Contributions
M.H.R.H. and K.I. conceptualized the study and designed the experiments; M.H.R.H. performed the experiments; F.A., M.O., Y.S., A.Y. performed the field experiment; S.H., A.S. helped to conduct the experiment and data analysis; M.H.R.H. wrote the article, with a substantial contribution from K.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hymowitz, T.; Harlan, J.R. Introduction of soybean to north America by Samuel Bowen in 1765. Econ. Bot. 1983, 37, 371–379. [Google Scholar] [CrossRef]
- Dupare, B.U.; Billore, S.D.; Joshi, O.P.; Husain, S.M. Origin, domestication, introduction, and success of soybean in India. Asian Agrihist 2008, 12, 179–195. [Google Scholar]
- Khojely, D.M.; Ibrahim, S.E.; Sapey, E.; Han, T. History, current status, and prospects of soybean production and research in sub-Saharan Africa. Crop J. 2018, 6, 226–235. [Google Scholar] [CrossRef]
- Vinuesa, P.; Rojas-Jimenez, K.; Contreras-Moreira, B.; Mahna, S.K.; Prasad, B.N.; Moe, H.; Selvaraju, S.B.; Thierfelder, H.; Werner, D. Multilocus sequence analysis for assessment of the biogeography and evolutionary genetics of four Bradyrhizobium species that nodulate soybeans on the asiatic continent. Appl. Environ. Microbiol. 2008, 74, 6987–6996. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Z.; XU, X.D.; Wang, E.T.; Gao, J.I.; Martinez-Romero, E.; Chen, W.X. Phylogenetic and genetic relationships of Mesorhizobium tianshanense and related rhizobia. Int. J. Syst. Bacteriol. 1997, 47, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, E.; Wang, S.; Li, Y.; Chen, X.; Li, Y. Characteristics of Rhizobium tianshanense sp. nov., a moderately and slowly growing root nodule bacterium isolated from an arid saline environment in Xinjiang, People’s Republic of China. Int. J. Syst. Bacteriol. 1995, 45, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Streeter, J.G. Failure of inoculant rhizobia to overcome the dominance of indigenous strains for nodule formation. Can J. Microbiol. 1994, 40, 513–522. [Google Scholar] [CrossRef]
- Saeki, Y.; Aimi, N.; Tsukamoto, S.; Yamakawa, T.; Nagatomo, Y.; Akao, S. Diversity and geographical distribution of indigenous soybean-nodulating bradyrhizobia in Japan. Soil Sci. Plant Nutr. 2006, 52, 418–426. [Google Scholar] [CrossRef]
- Shiro, S.; Matsuura, S.; Saiki, R.; Sigua, G.C.; Yamamoto, A.; Umehara, Y.; Hayashi, M.; Saeki, Y. Genetic diversity and geographical distribution of indigenous soybean-nodulating bradyrhizobia in the United States. Appl. Environ. Microbiol. 2013, 79, 3610–3618. [Google Scholar]
- Adhikari, D.; Kaneto, M.; Itoh, K.; Suyama, K.; Pokharel, B.B.; Gaihre, Y.K. Genetic diversity of soybean-nodulating rhizobia in Nepal in relation to climate and soil properties. Plant Soil 2012, 357, 131–145. [Google Scholar] [CrossRef]
- Li, Q.Q.; Wang, E.T.; Zhang, Y.Z.; Zhang, Y.M.; Tian, C.F.; Sui, X.H.; Chen, W.F.; Chen, W.X. Diversity and biogeography of rhizobia isolated from root nodules of Glycine max grown in Hebei province, China. Microb. Ecol. 2011, 61, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Kluson, R.A.; Kenworthy, W.J.; Weber, D.F. Soil temperature effects on competitiveness and growth of Rhizobium japonicum and on Rhizobium-induced chlorosis of soybeans. Plant Soil 1986, 95, 201–207. [Google Scholar] [CrossRef]
- Suzuki, Y.; Adhikari, D.; Itoh, K.; Suyama, K. Effects of temperature on competition and relative dominance of Bradyrhizobium japonicum and Bradyrhizobium elkanii in the process of soybean nodulation. Plant Soil 2014, 374, 915–924. [Google Scholar] [CrossRef]
- Shiro, S.; Kuranaga, C.; Yamamoto, A.; Sameshima-Saito, R.; Saeki, Y. Temperature-dependent expression of NodC and community structure of soybean-nodulating bradyrhizobia. Microbes Environ. 2016, 31, 27–32. [Google Scholar] [CrossRef]
- Puri, R.R.; Adachi, F.; Omichi, M.; Saeki, Y.; Yamamoto, A.; Hayashi, S.; Itoh, K. Culture-dependent analysis of endophytic bacterial community of sweet potato (Ipomoea batatas) in different soils and climates. J. Adv. Microbiol. 2018, 13, 1–12. [Google Scholar]
- Vincent, J.M. A manual for practical study of root nodule bacteria. In IBP Handbook No. 15; Blackwell Scientific Publishers: Oxford, UK, 1970; p. 164. [Google Scholar]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar]
- Hiraishi, A.; Inagaki, K.; Tanimoto, Y.; Iwasaki, M.; Kishimoto, N.; Tanaka, I. Phylogenetic characterization of a new thermoacidophilic bacterium isolated from hot springs in Japan. J. Gen. Appl. Microbiol. 1997, 43, 295–304. [Google Scholar] [CrossRef][Green Version]
- Freed, R. MSTAT: A software program for plant breeder. In Principles of Plant Genetics and Breeding, 2nd ed.; Acquaah, G., Ed.; Blackwell Publishing: Malden, MA, USA, 2007; Volume 1, pp. 426–431. [Google Scholar]
- Stoyanova, J. Growth, nodulation and nitrogen fixation in soybean as affected by air humidity and root temperature. Biol. Plant 1996, 38, 537–544. [Google Scholar] [CrossRef]
- Zhang, F.; Smith, D.L. Effects of low root zone temperatures on the early stages of symbiosis establishment between soybean [Glycine max (L.) Merr.] and Bradyrhizobium japonicum. J. Exp. Bot. 1994, 45, 1467–1473. [Google Scholar]
- Montañez, A.; Danso, S.K.A.; Hardarson, G. The effect of temperature on nodulation and nitrogen fixation by five Bradyrhizobium japonicum strains. Appl. Soil Ecol. 1995, 2, 165–174. [Google Scholar] [CrossRef]
- Hungria, M.; Vargas, M.A.T. Environmental factors affecting N2 fixation in grain legumes in the tropics, with an emphasis on Brazil. F. Crop Res. 2000, 65, 151–164. [Google Scholar] [CrossRef]
- Saeki, Y.; Aimi, N.; Hashimoto, M.; Tsukamoto, S.; Kaneko, A.; Yoshida, N.; Nagatomo, Y.; Akao, S. Grouping of bradyrhizobium usda strains by sequence analysis of 168 rRDNA and 168-238 rDNA internal transcribed spacer region. Soil Sci. Plant Nutr. 2004, 50, 517–525. [Google Scholar]
- Willems, A.; Munive, A.; de Lajudie, P.; Gillis, M. In most Bradyrhizobium groups sequence comparison of 16S-23S rDNA internal transcribed spacer regions corroborates DNA-DNA hybridizations. Syst. Appl. Microbiol. 2003, 26, 203–210. [Google Scholar] [CrossRef]
- Risal, C.P.; Yokoyama, T.; Ohkama-Ohtsu, N.; Djedidi, S.; Sekimoto, H. Genetic diversity of native soybean bradyrhizobia from different topographical regions along the southern slopes of the Himalayan Mountains in Nepal. Syst. Appl. Microbiol. 2010, 33, 416–425. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).