Abstract
The invasion of numerous countries by the armored scale Aulacaspis yasumatsui Takagi has caused widespread mortality of host Cycas species. Few studies have looked at reproductive biology responses of host plants to the herbivore. This study was conducted to determine the influence of direct Cycas seed integument infestation of A. yasumatsui on germination and seedling performance. An observational study in a Tinian ex situ Cycas micronesica K.D. Hill garden revealed that germination percentage was reduced two-thirds by heavy pre-harvest integument infestation, and more than half of the seedlings from infested seeds died in the nursery. Multi-year mortality of plants was six times greater for plants from habitats with infested seeds than for plants from minimally infested habitats. Stem height of nine-year-old plants from habitats with infested seeds was 64% of that of plants from habitats with un-infested seeds. A controlled study in a Philippine ex situ C. micronesica and Cycas edentata de Laub. garden corroborated these findings. Germination of infested seeds was about 30% of that for un-infested seeds. Seedling mortality in the nursery following experimental infestation of seed integuments with A. yasumatsui during seed maturation was about 50%, but was minimal for seedlings from un-infested seeds. These results indicate that limitations in regeneration and recruitment of host Cycas plants following A. yasumatsui herbivory may be partly due to the influence of direct seed infestations. Conservationists may use this new knowledge to improve in situ seed selection criteria and use prophylactic treatments to ensure lack of integument infestations for ex situ gardens.
1. Introduction
The armored scale A. yasumatsui has been invading new territories in recent years to threaten native and cultivated host cycad plants [1]. The invasion that has been most studied was the invasion of Guam in 2003 [2]. The high-density native C. micronesica population allowed the scale to rapidly spread throughout the island from the initial infestation site. The scale predator Rhyzobius lophanthae Blaisdell was released throughout the island beginning February 2005 [3]. The density of scale infestations exhibited substantial spatiotemporal variations among the areas of occupancy for several years as the non-native herbivore and predator interacted with the native host plant and other organisms [4].
This insular case study illuminated numerous developments about how the host plants responded to A. yasumatsui damage. For example, the C. micronesica seedlings and small juvenile plants were the first to be killed following the invasion [5,6]. Regeneration and recruitment of the host species were subsequently terminated by the invasion [7]. Direct infestation of the seed integuments during seed maturation decreased non-structural carbohydrates in seed gametophyte tissue [8]. Changes in sarcotesta tissue of the seed integument caused by A. yasumatsui infestations led to unprecedented herbivory of the integument by other non-native insect herbivores [9].
Persistent lack of regeneration may emerge as one of the most important C. micronesica conservation concerns in the future if mature tree mortality subsides. The youngest natural plants that remain alive on the island are an estimated 70 y in age [10]. In the event that C. micronesica regeneration is reestablished in the near future, this gap in plant size will likely exert a permanent influence on the genetic structure of the insular plant population. The severity of that change in genetic structure will increase with each passing year that regeneration remains nil.
Cycads are the most threatened plant group worldwide [11]. Invasive species pose international threats and cause substantial economic and biodiversity losses [12,13]. The continued study of the invasion of Guam by this cycad pest is of importance to more fully understanding the global threats to cycad conservation. Therefore, the ongoing scale-induced C. micronesica regeneration failure clearly deserves more dedicated research. In response to this heavily studied island invasion, off-site collections of Guam C. micronesica germplasm were established in several locations beginning 2006 [14,15]. Two of these off-site gardens were exploited for this study. The objectives were to determine how germination and seedling behaviors were influenced by direct scale infestations of seed integuments during source seed maturation. I hypothesized that direct infestation of seed surfaces during seed maturation would directly damage germination, seedling survival, and seedling growth.
2. Materials and Methods
2.1. Tinian Observational Study
Cycas micronesica seeds were harvested throughout Guam from 219 female trees beginning January 2006 and segregated among sites defined as 15 areas of occupancy. A total of 5867 seeds were harvested, for a mean of 27 seeds per tree. Although the armored scale was present in every area of occupancy at the time of seed harvests, the density of infestations was highly heterogeneous among the individual trees and locations. One of the recorded metrics at the time of harvest was the extent of direct scale infestation of seed integument tissue.
An initial seed storage period occurred on Guam until sarcotesta tissue had been manually removed from all seeds. A subsequent storage period occurred on Tinian to enable embryo maturation prior to sowing. The seeds were sown on beds of perlite in December 2006. Each germinated seed was removed from the germination beds when the radicle reached 4+ cm in length and planted individually in 2.6-L containers with medium comprised of approximately equal parts of field soil, perlite, and peat. The seedlings were grown under shadecloth with 50% light exclusion until the field site was planted.
The nursery exhibited a mean of 22% germination. However, this varied greatly among individual source trees and areas of occupancy. The number of seeds sown and the number of seeds that germinated were recorded for each female source tree. The number of germinated seedlings that survived in the nursery phase was recorded for each female source tree. The data were sorted into two groups defined by scale infestations at the time of seed harvests. Six areas of occupancy were identified that exhibited copious direct scale infestations of seed integuments at the time of harvest, and five areas of occupancy were identified that exhibited seeds relatively free of seed integument infestations.
The container-grown plants included in this study were transplanted from the nursery in June–August 2008. The field site was a native forest supported by karst outcrop soils (loamy-skeletal, carbonatic, isohyperthermic Lithic Haplustolls). The number of Guam areas of occupancy included in the field planting was reduced to 12 as a result of 100% mortality of seedlings from some areas of occupancy during the nursery phase. Short-term field survival data were quantified in year four, and long-term survival data were quantified in year nine. Stem height of living plants was recorded in year nine. The data were sorted into two groups defined by scale infestations at the time of seed harvest. Two of the heavily infested areas of occupancy that were represented in the nursery phase data were excluded from the field data due to 100% mortality of the seedlings in the nursery. Therefore, four areas of occupancy were identified that exhibited copious direct scale infestations of seed integuments at the time of harvest, and five areas of occupancy were identified that exhibited seeds relatively free of seed integument infestations.
These observational data were not appropriate for statistical comparisons for numerous reasons. Therefore, the mean ± standard error was calculated for all nursery and field response variables for each of the two groups defined by extent of scale infestation.
2.2. Philippine Experimental Study
The experimental site was in Barangay Sapang Bato, Angeles City, Philippines in an open field with a loam soil (coarse loamy, isohyperthermic, Typic Untipsamment). Megastrobili from Yap-sourced C. micronesica and Philippine-sourced C. edentata trees were hand-pollinated in May–June 2018. When the megastrobili were 10–11 weeks old, individual megasporophylls were easily observed as they were re-orienting from the initial immature vertical orientation (Figure 1a). Experimental treatments were begun at this stage because developing seeds can be distinguished from unpollinated persistent ovules. Nothing is known about source–sink relations of individual Cycas sporophylls on mega- or microstrobili. Therefore, the number of developing seeds per sporophyll was standardized. For C. micronesica, sporophylls with two developing seeds were selected for the treatments. For C. edentata, sporophylls with four developing seeds were selected for the treatments.

Figure 1.
Cycas micronesica megastrobilus appearance for experimental manipulation of Aulacaspis yasumatsui infestations. (a) Ten weeks in age with sporophylls marked for experimental inclusion. Yellow arrow shows sporophyll with two developing seeds and two unpollinated ovules. Blue arrow shows sporophyll with two developing seeds and four unpollinated ovules; (b) Twelve months in age with individual sporophylls enclosed in polyester fabric to facilitate scale infestation treatments.
The urban areas of central Luzon are infested with A. yasumatsui, but parasitoid biological control maintains the population such that infested plants are not threatened. In order to obtain scale-infested Cycas organ surfaces, the surfaces require protection from parasitoid access. This was achieved by use of the methods employed by Marler and Cascasan [16]. The apical portion of each selected sporophyll was enclosed in polyester fabric with 130 holes per centimeter (Figure 1b). To create scale-infested replications, Cycas leaflets with mature A. yasumatsui infestations were placed into each of the enclosed sporophylls for 30 min to allow crawlers to move to the sporophyll and seed surfaces. Removal of the infested leaflets after a short window of time reduced the possibility of a parasitoid adult emerging from one or more of the A. yasumatsui individuals on the infested leaflets. This procedure was repeated four consecutive days. Twenty sporophylls per species received this treatment. A total of 15 sporophylls were successfully infested for C. edentata, and a total of 12 sporophylls were successfully infested for C. micronesica. An adjacent paired sporophyll with no scale infestation was also enclosed in the polyester fabric to ensure incident light and microenvironment were homogeneous for the infested and un-infested treatments.
All seeds were harvested in February 2020. Sarcotesta tissue was immediately removed and cleaned seeds were stored in ambient conditions until August 2020 when they were sown in a bed of washed river sand and maintained under 50% shadecloth. Germinated seeds were removed from the bed and planted into individual 2.6-L containers with a medium comprised of washed river sand. The number of seeds that germinated was recorded for each replication. The container-grown plants were maintained under shadecloth with daily irrigation. Plant nutrition was maintained with monthly drench of soluble fertilizer solution at 200 mL per container. The stock solution was comprised of water-soluble fertilizer (Scotts Miracle-Gro, Marysville, OH, U.S.A.; 24% nitrogen, 3.5% phosphorus, 13.2% potassium, 0.02% boron, 0.07% copper, 0.15% iron, 0.05% manganese, 0.0005% molybdenum, 0.06% zinc) at 1 g·L−1 and calcium nitrate at 0.5 g·L−1. Final measurements were conducted on 2 November 2021. The number of seedlings that died in the nursery was recorded. Stem height and leaf number were the growth response variables that were measured.
Each sporophyll was considered one replication. For each response variable, the mean of the four (C. edentata) or two (C. micronesica) seeds or seedlings for each sporophyll was used as the value for each replication. The data for each species were analyzed separately. Percentage data were square root transformed prior to analysis. A paired t-test was performed for germination percentage, mortality percentage, stem height, and leaf number to determine significance of scale-infested versus un-infested seeds.
3. Results
In the absence of biological control, A. yasumatsui effectively covers the entire integument tissue surfaces of young developing Cycas seeds (Figure 2a,b). The chronic infestations throughout maturation bring about substantial changes in appearance of individual seeds and the entire megastrobilus at the time of seed harvest (Figure 2c,d).

Figure 2.
Cycas micronesica seeds with or without direct Aulacaspis yasumatsui infestations. (a,b) Six months in age; (c,d) 18 months in age.
3.1. Tinian Observational Study
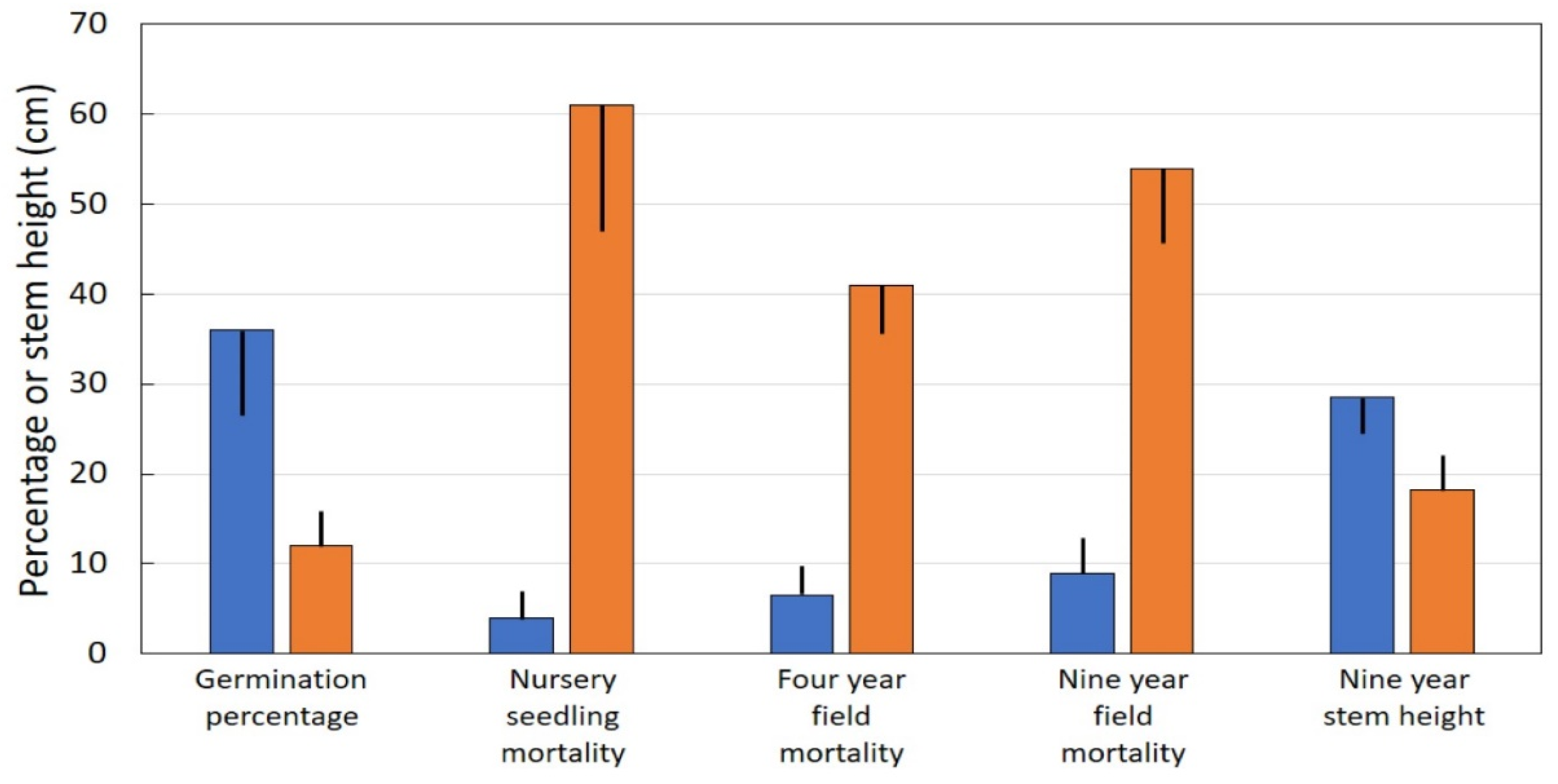
Locations throughout Guam with direct seed infestations at the time of seed harvest exhibited germination percentage that was one-third of that for locations with minimal seed infestations (Figure 3). Very few seedlings from locations with minimal seed infestations died in the nursery, but more than half of the seedlings from locations with direct seed infestations died while still under the luxurious conditions of the container nursery. Similar trends occurred after transplanting to field conditions. Mortality of seedlings from locations with direct seed infestations was more than six-fold greater than that for seedlings from locations with minimal infestations of seeds. This trend was evident by four years, and continued through the end of the study at nine years. Nine-year-old stem height of seedlings from locations with minimal scale infestations was 57% greater than that for seedlings from locations with direct seed infestations.
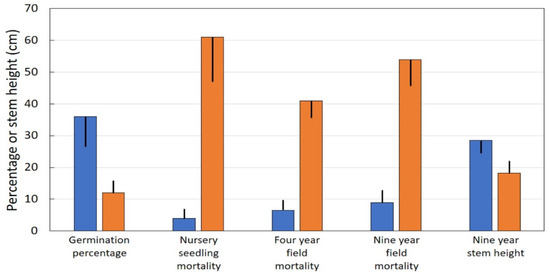
Figure 3.
Performance of Cycas micronesica seeds with or without substantial Aulacaspis yasumatsui infestations. Blue bars = minimal scale infestation, orange bars = considerable scale infestation. Mean plus or minus SE.
3.2. Philippine Experimental Study
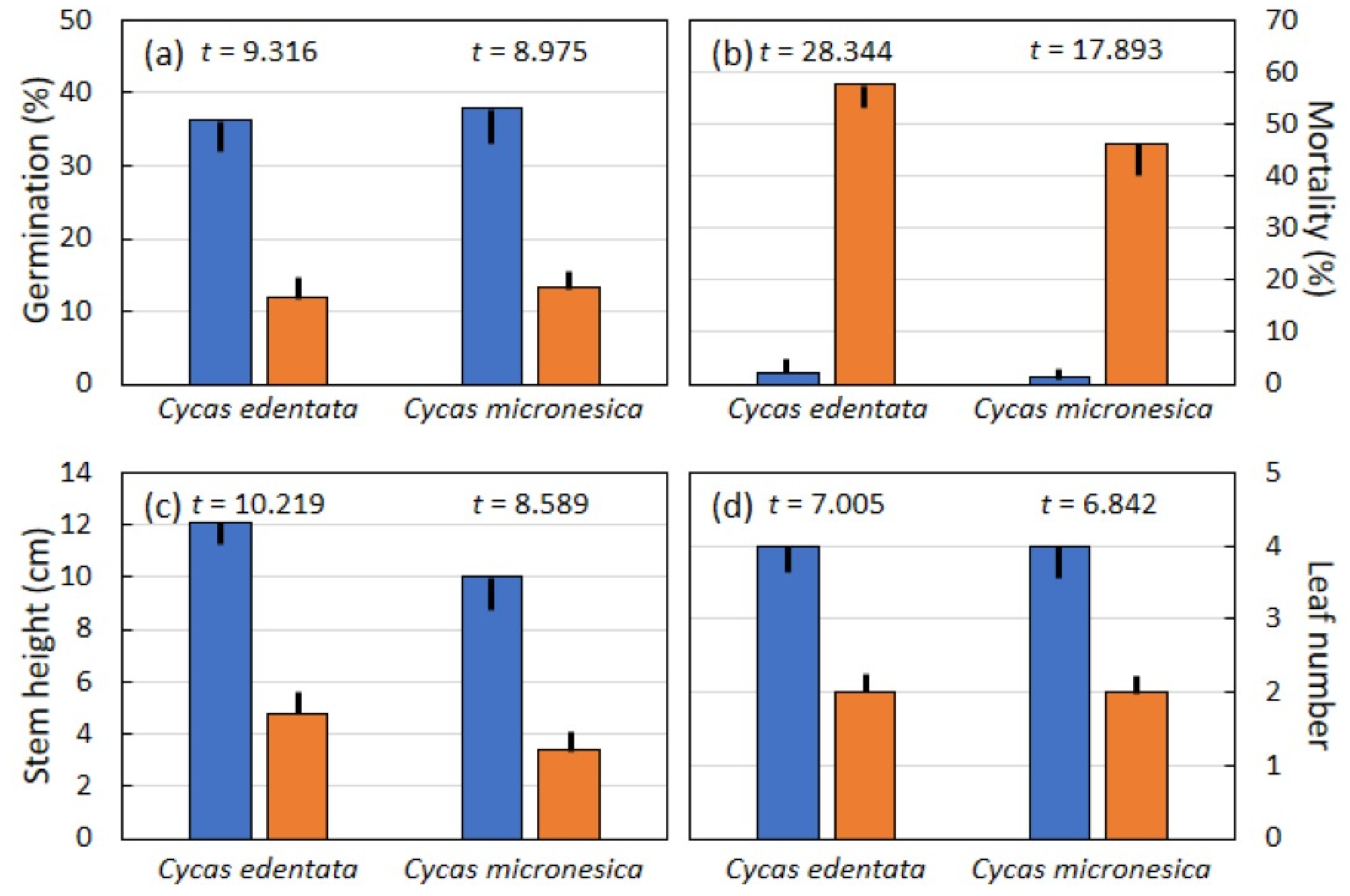
Experimental infestation of A. yasumatsui on seed integument tissue caused germination percentage of C. edentata and C. micronesica seeds to decline to about 30% of germination for un-infested seeds (Figure 4a). Post-germination mortality of nursery-grown seedlings exceeded 50% for C. edentata infested seeds and was almost 50% for plants grown from C. micronesica infested seeds, while seedling mortality was minimal for plants grown from un-infested seeds (Figure 4b). Stem height of the 15-month-old seedlings grown from scale-infested seeds was 40% of that for un-infested seeds for C. edentata and 35% of that for un-infested seeds for C. micronesica (Figure 4c). Leaf number for each 15-month-old seedling was reduced in half by experimentally infesting developing Cycas seeds with A. yasumatsui (Figure 4d).
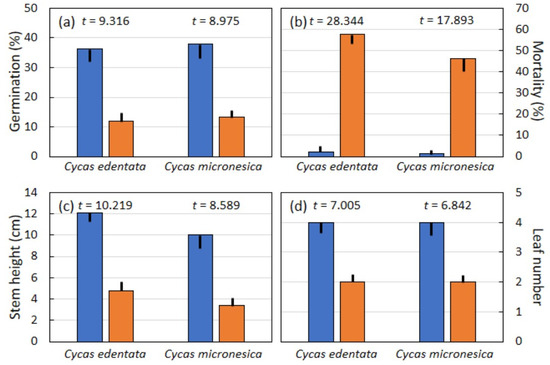
Figure 4.
The influence of Aulacaspis yasumatsui infestation on Cycas edentata (n = 15) and Cycas micronesica (n = 12) seed integument tissues on germination and seedling responses. (a) Germination percentage; (b) seedling mortality; (c) stem height; (d) leaf number per seedling. Mean plus or minus standard error. Blue bars = un-infested seeds, orange bars = infested seeds. Significance was p < 0.001 for every t-test.
4. Discussion
The combination of observational and experimental approaches has confirmed the hypothesis that pre-harvest infestations of Cycas seed integuments by A. yasumatsui damaged germination, seedling survival, and seedling growth for up to nine years. This influence of A. yasumatsui herbivory was independent of how this armored scale influenced whole-plant responses. For example, plants with experimentally infested seeds contained non-threatening infestations of A. yasumatsui on stems and leaves that were similar among all source trees.
What mechanism could A. yasumatsui employ to exert such a devastating outcome for regeneration and recruitment of the host species? We have shown that non-structural carbohydrates decline in gametophyte storage tissues as a result of direct seed integument infestation [8]. Although a limited supply of gametophyte non-structural resources may explain initial seedling mortality in a nursery setting, this seed trait is an unlikely candidate for explaining long-term sustained mortality and multi-year suppression of plant growth. A second explanation for these plant behaviors following A. yasumatsui herbivory may be changes to the epigenome as a result of the stress during seed growth and maturation [17]. Studies designed to understand the sustained epigenetic memory imparted by pre-harvest scale infestation of seeds are warranted.
Ongoing C. micronesica conservation projects on Guam are using seed harvests to grow and outplant seedlings as part of the conservation agenda. Current knowledge indicates this conservation action is ill-conceived because effective biological control of A. yasumatsui on small plants remains absent from Guam. Therefore, a commitment to 70 years of prophylactic insecticide applications may be required to ensure the seedlings reach sufficient size to avoid ubiquitous scale-induced mortality (see [10]). For restoration and conservation programs that rely on seeding methods, knowledge is needed to select the seeds with the greatest potential of vigor and success. Indeed, the maternal effect is well-known whereby seedling performance is directly influenced by the source plant’s historical environment in addition to the contemporary environment of the seedling [18,19,20]. The results herein indicate that collection of C. micronesica seeds that exhibit no history of direct A. yasumatsui is a requirement for success in a conservation nursery. For populations that are within managed in situ conservation plots and within ex situ gardens, some form of prophylactic treatment may be employed to ensure the lack of A. yasumatsui herbivory on seed surfaces. This could be achieved with exclusion screen material as exemplified in the methods herein or with chemical pesticides.
Conservation efforts in off-site germplasm gardens augment in situ conservation efforts and are crucial for mitigating the global biodiversity crisis [21,22]. This study is an example of how some conservation questions may be answered in ex situ settings where threats are mitigated more efficiently than in disturbed in situ settings where threats are ubiquitous.
In summary, herbivory of C. micronesica and C. edentata seed surfaces by A. yasumatsui influenced regeneration and recruitment traits in a manner that was independent of whole-plant herbivory. The percentage of seeds that germinated was reduced and early seedling mortality was increased by direct seed herbivory. Growth of seedlings for up to nine years in an ex situ setting was also reduced by direct A. yasumatsui infestations prior to seed harvests. This cycad pest continues to threaten cycad horticulture and conservation efforts, and these new findings may be used to improve conservation decisions.
Funding
This research was funded by SAIC grant numbers 850-40801043 and 302-40827265 and United States Forest Service grant numbers 13-DG-11052021-210 and 17-DG-11052021-217.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon request.
Acknowledgments
I thank Nirmala Dongol and Gil Cruz for nursery and field assistance.
Conflicts of Interest
The author declares no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Marler, T.E.; Lindström, A.J.; Watson, G.W. Aulacaspis yasumatsui delivers a blow to international cycad horticulture. Horticulturae 2021, 7, 147. [Google Scholar] [CrossRef]
- Marler, T.E. Cycad aulacaspis scale invades the Mariana Islands. Mem. N. Y. Bot. Gard. 2012, 106, 20–35. [Google Scholar]
- Moore, A.; Marler, T.; Miller, R.H.; Muniappan, R. Biological control of cycad aulacaspis scale on Guam. Cycad Newsl. 2005, 28, 6–8. [Google Scholar]
- Marler, T.E. Temporal variations in leaf miner, butterfly, and stem borer infestations of Cycas micronesica in relation to Aulacaspis yasumatsui incidence. HortScience 2013, 48, 1334–1338. [Google Scholar] [CrossRef]
- Marler, T.E.; Lawrence, J.H. Demography of Cycas micronesica on Guam following introduction of the armoured scale Aulacaspis yasumatsui. J. Trop. Ecol. 2012, 28, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Marler, T.E.; Krishnapillai, M.V. Longitude, forest fragmentation, and plant size influence Cycas micronesica mortality following island insect invasions. Diversity 2020, 12, 194. [Google Scholar] [CrossRef]
- Marler, T.E.; Terry, L.I. Arthropod invasion disrupts Cycas micronesica seedling recruitment. Communic. Integr. Biol. 2011, 4, 778–780. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E.; Cruz, G.N. Source and sink relations mediate depletion of intrinsic cycad seed carbohydrates by Aulacaspis yasumatsui infestation. HortScience 2019, 54, 1712–1717. [Google Scholar] [CrossRef] [Green Version]
- Deloso, B.E.; Terry, L.I.; Yudin, L.S.; Marler, T.E. Biotic threats to Cycas micronesica continue to expand to complicate conservation decisions. Insects 2020, 11, 888. [Google Scholar] [CrossRef]
- Marler, T.E.; Griffith, M.P.; Krishnapillai, M.V. Height increment of Cycas micronesica informs conservation decisions. Plant Sig. Behav. 2020, 15, e1830237. [Google Scholar] [CrossRef]
- Fragniere, Y.; Bétrisey, S.; Cardinaux, L.; Stoffel, M.; Kozlowski, G. Fighting their last stand? A global analysis of the distribution and conservation status of gymnosperms. J. Biogeogr. 2015, 42, 809–820. [Google Scholar] [CrossRef]
- Hulme, P.E. Unwelcome exchange: International trade as a direct and indirect driver of biological invasions worldwide. One Earth 2021, 4, 666–679. [Google Scholar] [CrossRef]
- Diagne, C.; Leroy, B.; Vaissière, A.C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.M.; Bradshaw, C.J.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef]
- International Union for Conservation of Nature Cycad Specialist Group. Report and Recommendations on Cycad Aulacaspis Scale, Aulacaspis yasumatsui Takagi (Hemiptera: Diaspididae); IUCN: Gland, Switzerland, 2006.
- Anonymous. Conserving our nation’s only native cycad species. Currents 2014, Fall issue, 28–31. [Google Scholar]
- Marler, T.E.; Cascasan, A.N. Carbohydrate depletion during lethal infestation of Aulacaspis yasumatsui on Cycas revoluta. Int. J. Plant Sci. 2018, 179, 497–504. [Google Scholar] [CrossRef]
- Narsai, R.; Secco, D.; Schultz, M.D.; Ecker, J.R.; Lister, R.; Whelan, J. Dynamic and rapid changes in the transcriptome and epigenome during germination and in developing rice (Oryza sativa) coleoptiles under anoxia and re-oxygenation. Plant J. 2017, 89, 805–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donohue, K. Completing the cycle: Maternal effects as the missing link in plant life histories. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1059–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, J.; Michelangeli, J.A.C.; Gezan, S.A.; Lee, H.; Vallejos, C.E. Maternal effects on seed and seedling phenotypes in reciprocal F1 hybrids of the common bean (Phaseolus vulgaris L.). Front. Plant Sci. 2017, 8, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geshnizjani, N.; Khorami, S.S.; Willems, L.A.J.; Snoek, B.L.; Hilhorst, H.W.M.; Ligterink, W. The interaction between genotype and maternal nutritional environments affects tomato seed and seedling quality. J. Exp. Bot. 2019, 70, 2905–2918. [Google Scholar] [CrossRef]
- Westwood, M.; Cavender, N.; Meyer, A.; Smith, P. Botanic garden solutions to the plant extinction crisis. Plants People Planet 2020, 3, 22–32. [Google Scholar] [CrossRef]
- Kovács, Z.; Csergő, A.M.; Csontos, P.; Höhn, M. Ex situ conservation in botanical gardens—challenges and scientific potential preserving plant biodiversity. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12334. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).