Grapevine Red Blotch Disease Etiology and Its Impact on Grapevine Physiology and Berry and Wine Composition
Abstract
:1. Introduction
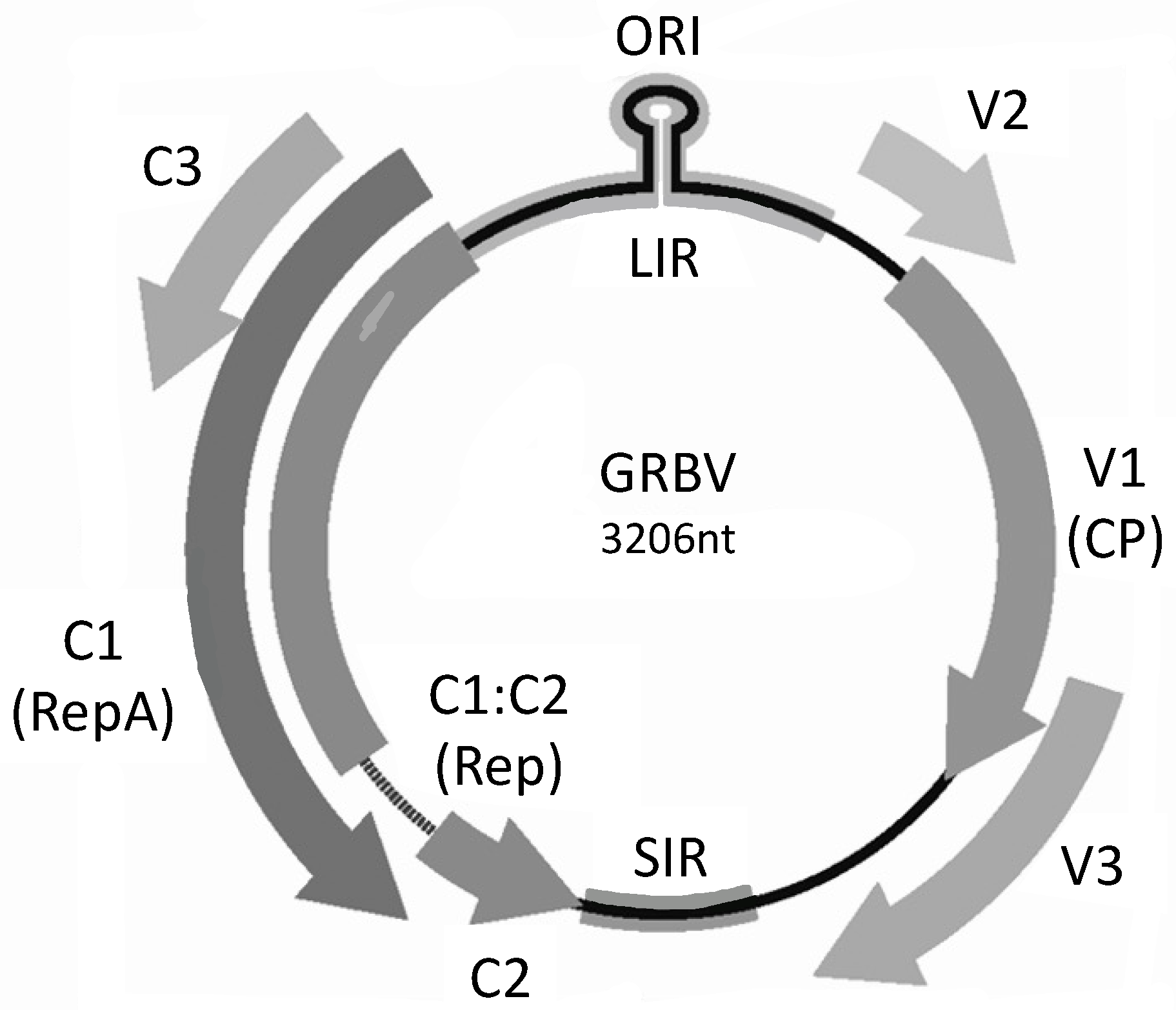
2. GRBV Genome and Taxonomy
3. Causative Role in GRBD: Symptoms, Diagnosis, and Transmission
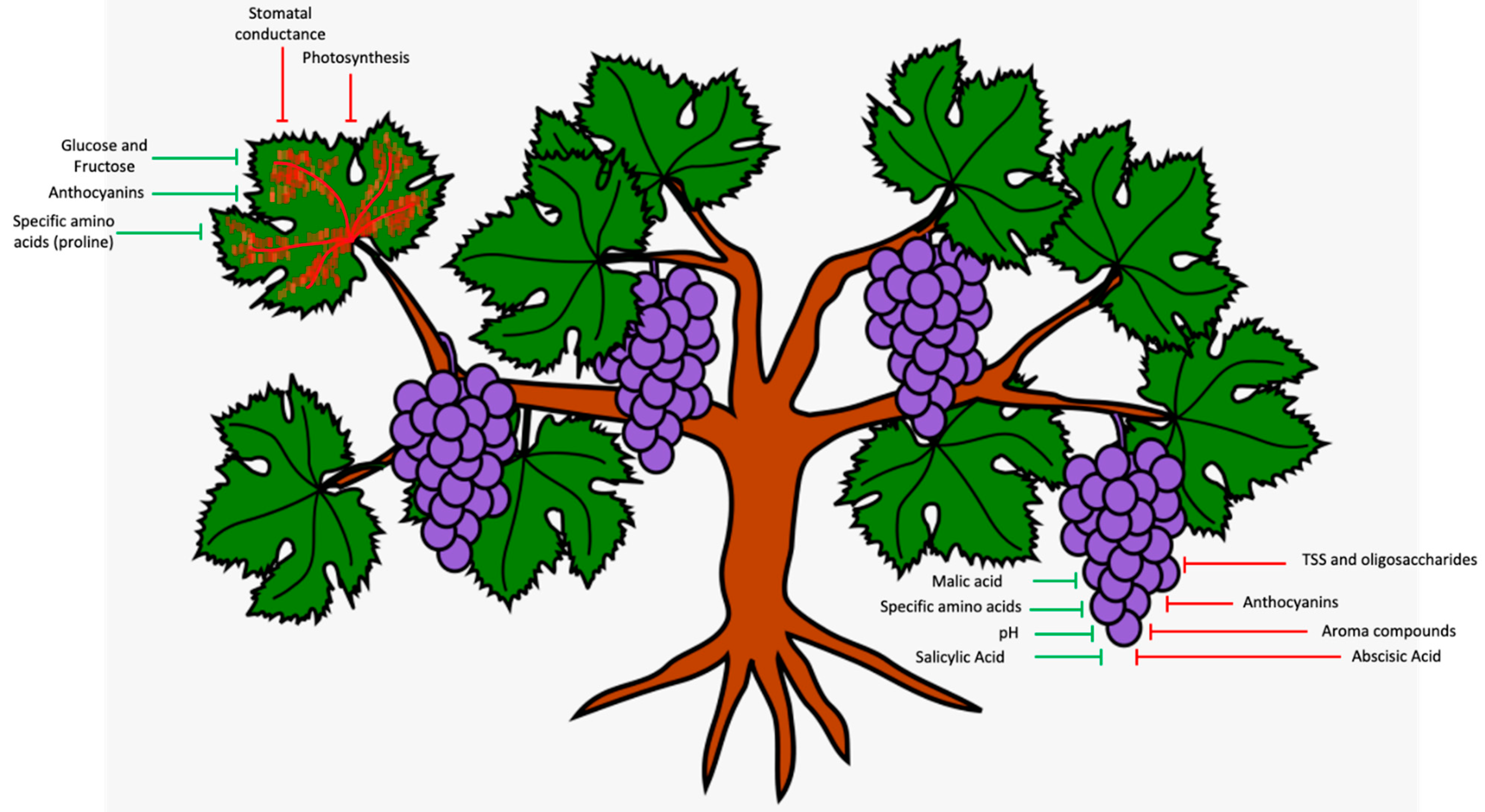
4. Impacts on Grapevine Physiology
5. Impairment to Grape Metabolism
6. Impact on Wine Composition
7. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fuchs, M. Grapevine viruses: A multitude of diverse species with simple but overall poorly adopted management solutions in the vineyard. J. Plant Pathol. 2020, 102, 643–653. [Google Scholar] [CrossRef]
- Dolja, V.V.; Meng, B.; Martelli, G.P. Evolutionary Aspects of Grapevine Virology. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Meng, B., Martelli, G.P., Golino, D.A., Fuchs, M., Eds.; Springer International Publishing: Bellin/Heidelberg, Germany, 2017; pp. 659–688. ISBN 978-3-319-57706-7. [Google Scholar]
- Martelli, G.P. DIRECTORY OF VIRUS AND VIRUS-LIKE DISEASES OF THE GRAPEVINE AND THEIR AGENTS. J. Plant Pathol. 2014, 96, 1–136. [Google Scholar]
- Ricketts, K.D.; Gómez, M.I.; Fuchs, M.F.; Martinson, T.E.; Smith, R.J.; Cooper, M.L.; Moyer, M.M.; Wise, A. Mitigating the economic impact of grapevine red blotch: Optimizing disease management strategies in U.S. vineyards. Am. J. Enol. Vitic. 2017, 68, 127–135. [Google Scholar] [CrossRef]
- Ricketts, K.D.; Gomez, M.I.; Atallah, S.S.; Fuchs, M.F.; Martinson, T.E.; Battany, M.C.; Bettiga, L.J.; Cooper, M.L.; Verdegaal, P.S.; Smith, R.J. Reducing the economic impact of grapevine leafroll disease in California: Identifying optimal disease management strategies. Am. J. Enol. Vitic. 2015, 66, 138–149. [Google Scholar] [CrossRef]
- Maree, H.J.; Almeida, R.P.P.; Bester, R.; Chooi, K.M.; Cohen, D.; Dolja, V.V.; Fuchs, M.F.; Golino, D.A.; Jooste, A.E.C.; Martelli, G.P.; et al. Grapevine leafroll-associated virus 3. Front. Microbiol. 2013, 4, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Vega, A.; Gutiérrez, R.A.; Peña-Neira, A.; Cramer, G.R.; Arce-Johnson, P. Compatible GLRaV-3 viral infections affect berry ripening decreasing sugar accumulation and anthocyanin biosynthesis in Vitis vinifera. Plant Mol. Biol. 2011, 77, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Gutha, L.R.; Casassa, L.F.; Harbertson, J.F.; Naidu, R.A. Modulation of flavonoid biosynthetic pathway genes and anthocyanins due to virus infection in grapevine (Vitis vinifera L.) leaves. BMC Plant Biol. 2010, 10, 187. [Google Scholar] [CrossRef] [Green Version]
- Vondras, A.M.; Lerno, L.; Massonnet, M.; Minio, A.; Rowhani, A.; Liang, D.; Garcia, J.; Quiroz, D.; Figueroa-Balderas, R.; Golino, D.A.; et al. Rootstock influences the effect of grapevine leafroll-associated viruses on berry development and metabolism via abscisic acid signalling. Mol. Plant Pathol. 2021, 1–22. [Google Scholar] [CrossRef]
- Naidu, R.; Rowhani, A.; Fuchs, M.; Golino, D.; Martelli, G.P. Grapevine Leafroll: A complex viral disease affecting a high-value fruit crop. Plant Dis. 2014, 98, 1172–1185. [Google Scholar] [CrossRef] [Green Version]
- Oliver, J.E.; Fuchs, M. Tolerance and resistance to viruses and their vectors in vitis sp.: A virologist’s perspective of the literature. Am. J. Enol. Vitic. 2011, 62, 438–451. [Google Scholar] [CrossRef] [Green Version]
- Krenz, B.; Thompson, J.R.; Fuchs, M.; Perry, K.L. Complete Genome Sequence of a New Circular DNA Virus from Grapevine. J. Virol. 2012, 86, 7715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Rwahnih, M.; Dave, A.; Anderson, M.M.; Rowhani, A.; Uyemoto, J.K.; Sudarshana, M.R. Association of a DNA Virus with Grapevines Affected by Red Blotch Disease in California. Phytopathology 2013, 103, 1069–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seguin, J.; Rajeswaran, R.; Malpica-López, N.; Martin, R.R.; Kasschau, K.; Dolja, V.V.; Otten, P.; Farinelli, L.; Pooggin, M.M. De novo reconstruction of consensus master genomes of plant RNA and DNA viruses from siRNAs. PLoS ONE 2014, 9, e88513. [Google Scholar]
- Krenz, B.; Thompson, J.R.; Mclane, H.L.; Fuchs, M.; Perry, K.L. Grapevine red blotch-associated virus Is Widespread in the United States. Phytopathology 2014, 104, 1232–1240. [Google Scholar] [CrossRef] [Green Version]
- Sudarshana, M.R.; Perry, K.L.; Fuchs, M.F. Grapevine Red Blotch-Associated Virus, an Emerging Threat to the Grapevine Industry Mysore. Phytopathology 2015, 105, 1026–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yepes, L.M.; Cieniewicz, E.; Krenz, B.; McLane, H.; Thompson, J.R.; Perry, K.L.; Fuchs, M. Causative Role of Grapevine Red Blotch Virus in Red Blotch Disease. Phytopathology 2018, 108, 902–909. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.; Igori, D.; Zhao, F.; Moon, J.; Cho, I.-S.; Choi, G.-S. First report of Grapevine red blotch-associated virus on grapevine in Korea. Plant Dis. 2016, 100, 1957. [Google Scholar] [CrossRef]
- Gasperin-Bulbarela, J.; Licea-Navarro, A.F.; Pino-Villar, C.; Hernandez-Martínez, R.; Carrillo-Tripp, J. First report of grapevine red blotch virus in Mexico. Plant Dis. 2019, 103, 381. [Google Scholar] [CrossRef]
- Luna, F.; Debat, H.; Gomez-Talquenca, S.; Moyano, S.; Zavallo, D.; Asurmendi, S. First report of grapevine red blotch virus infecting grapevine in Argentina. J. Plant Pathol. 2019, 101, 1239. [Google Scholar] [CrossRef] [Green Version]
- Marwal, A.; Kumar, R.; Paul Khurana, S.M.; Gaur, R.K. Complete nucleotide sequence of a new geminivirus isolated from Vitis vinifera in India: A symptomless host of Grapevine red blotch virus. VirusDisease 2019, 30, 106–111. [Google Scholar] [CrossRef]
- Poojari, S.; Lowery, D.T.; Rott, M.; Schmidt, A.M.; Úrbez-Torres, J.R. Incidence, distribution and genetic diversity of Grapevine red blotch virus in British Columbia. Can. J. Plant Pathol. 2017, 39, 201–211. [Google Scholar] [CrossRef]
- Al Rwahnih, M.; Rowhani, A.; Golino, D.A.; Islas, C.M.; Preece, J.E.; Sudarshana, M.R. Detection and genetic diversity of Grapevine red blotch-associated virus isolates in table grape accessions in the National Clonal Germplasm Repository in California. Can. J. Plant Pathol. 2015, 37, 130–135. [Google Scholar] [CrossRef]
- Reynard, J.S.; Brodard, J.; Dubuis, N.; Zufferey, V.; Schumpp, O.; Schaerer, S.; Gugerli, P. Grapevine red blotch virus: Absence in Swiss vineyards and analysis of potential detrimental effect on viticultural performance. Plant Dis. 2018, 102, 651–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Rwahnih, M.; Dave, A.; Anderson, M.M.; Uyemoto, J.K.; Sudarshana, M.R. Association of a circular DNA virus in grapevines affected by red blotch disease in California. Congress of the International Council for the Study of Virus and Virus-Like Diseases of the Grapevine (ICVG), Davis, CA, USA, 8–11 October 2012. [Google Scholar]
- Poojari, S.; Alabi, O.J.; Fofanov, V.Y.; Naidu, R.A. A Leafhopper-Transmissible DNA Virus with Novel Evolutionary Lineage in the Family Geminiviridae Implicated in Grapevine Redleaf Disease by Next-Generation Sequencing. PLoS ONE 2013, 8, e0147510. [Google Scholar]
- National Clean Plant Network Fact Sheet: Grapevine Red Blotch Disease. Available online: http://ucanr.edu/sites/NCPNGrapes/files/161782.pdf (accessed on 2 December 2021).
- Al Rwahnih, M.; Rowhani, A.; Golino, D. First Report of Grapevine red blotch-associated virus in Archival Grapevine Material From Sonoma County, California. Dis. Notes 2015, 99, 895. [Google Scholar] [CrossRef]
- Perry, K.L.; McLane, H.; Hyder, M.Z.; Dangl, G.S.; Thompson, J.R.; Fuchs, M.F. Grapevine red blotch-associated virus is Present in Free-Living Vitis spp. Proximal to Cultivated Grapevines. Phytopathology 2016, 106, 663–670. [Google Scholar] [CrossRef] [Green Version]
- Bahder, B.W.; Zalom, F.G.; Sudarshana, M.R. An evaluation of the flora adjacent to wine grape vineyards for the presence of alternative host plants of grapevine red blotch-associated virus. Plant Dis. 2016, 100, 1571–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brannen, P.M.; Deom, C.M.; Alabi, O.J.; Naidu, R.A. Prevalence of viruses in commercial wine grape vineyards in Georgia. Plant Health Prog. 2018, 19, 342–346. [Google Scholar] [CrossRef]
- Schoelz, J.E.; Adhab, M.; Qiu, W.; Petersen, S.; Volenberg, D. First Report of Grapevine Red Blotch Virus in Hybrid Grapes in Missouri. Plant Dis. 2018, 103, 379. [Google Scholar] [CrossRef]
- Xiao, H.; Shabanian, M.; Moore, C.; Li, C.; Meng, B. Survey for major viruses in commercial Vitis vinifera wine grapes in Ontario. Virol. J. 2018, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Soltani, N.; Hu, R.; Hensley, D.D.; Lockwood, D.L.; Perry, K.L.; Hajimorad, M.R. A Survery for Nine Major Viruses of Grapevines in Tennessee Vineyards. Plant Health Prog. 2020, 21, 157–161. [Google Scholar] [CrossRef]
- Fall, M.L.; Xu, D.; Lemoyne, P.; Ben Moussa, I.E.; Beaulieu, C.; Carisse, O. A diverse virome of leafroll-infected grapevine unveiled by dsRNA sequencing. Viruses 2020, 12, 1142. [Google Scholar] [CrossRef] [PubMed]
- Poojari, S.; Moreau, D.L.; Kahl, D.; Ritchie, M.; Ali, S.; Úrbez-Torres, J.R. Disease incidence and genetic variability of economically important grapevine viruses in Nova Scotia. Can. J. Plant Pathol. 2020, 42, 584–594. [Google Scholar] [CrossRef]
- Lee, J.; Rennaker, C.D.; Thompson, B.D.; Karasev, A.V. Influence of Grapevine red blotch virus (GRBV) on Idaho ‘Syrah’ grape composition. Sci. Hortic. 2021, 282, 110055. [Google Scholar] [CrossRef]
- Varsani, A.; Roumagnac, P.; Fuchs, M.; Navas-Castillo, J.; Moriones, E.; Idris, A.; Briddon, R.W.; Rivera-Bustamante, R.; Murilo Zerbini, F.; Martin, D.P. Capulavirus and Grablovirus: Two new genera in the family Geminiviridae. Arch. Virol. 2017, 162, 1819–1831. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Asencio, J.; Liou, H.; Perry, K.L.; Thompson, J.R. Evidence for the splicing of grablovirus transcripts reveals a putative novel open reading frame. J. Gen. Virol. 2019, 100, 709–720. [Google Scholar] [CrossRef]
- Perry, K.L.; McLane, H.; Thompson, J.R.; Fuchs, M. A novel grablovirus from non-cultivated grapevine (Vitis sp.) in North America. Arch. Virol. 2018, 163, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Al Rwahnih, M.; Alabi, O.J.; Westrick, N.M.; Golino, D. Prunus geminivirus A: A novel grablovirus infecting prunus spp. Plant Dis. 2018, 102, 1246–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, E.A.; Heckel, T.; Groenendijk, J.; Davies, J.W.; Boulton, M.I. Splicing features in maize streak virus virion- and complementary- sense gene expression. Plant J. 1997, 12, 1285–1297. [Google Scholar] [CrossRef]
- Bernardo, P.; Golden, M.; Akram, M.; Naimuddin; Nadarajan, N.; Fernandez, E.; Granier, M.; Rebelo, A.G.; Peterschmitt, M.; Martin, D.P.; et al. Identification and characterisation of a highly divergent geminivirus: Evolutionary and taxonomic implications. Virus Res. 2013, 177, 35–45. [Google Scholar] [CrossRef] [PubMed]
- International Committee on Taxonomy of Viruses ICTV. Available online: https://talk.ictvonline.org/ (accessed on 26 July 2021).
- Hewitt, W.B.; Goheen, A.C.; Raski, D.J.; Gooding, G.V. Studies on Virus Diseases of the Grapevine in California. Vitis 1962, 3, 57–83. [Google Scholar]
- Girardello, R.C.; Rich, V.; Smith, R.J.; Brenneman, C.; Heymann, H.; Oberholster, A. The impact of grapevine red blotch disease on Vitis vinifera L. Chardonnay grape and wine composition and sensory attributes over three seasons. J. Sci. Food Agric. 2019, 100, 1436–1447. [Google Scholar] [CrossRef] [PubMed]
- Buchs, N.; Braga-Lagache, S.; Uldry, A.C.; Brodard, J.; Debonneville, C.; Reynard, J.S.; Heller, M. Absolute quantification of grapevine red blotch virus in grapevine leaf and petiole tissues by proteomics. Front. Plant Sci. 2018, 9, 1735. [Google Scholar] [CrossRef] [PubMed]
- Cieniewicz, E.; Flasco, M.; Brunelli, M.; Onwumelu, A.; Wise, A.; Fuchs, M.F. Differential spread of grapevine red blotch virus in California and New York vineyards. Phytobiomes J. 2019, 3, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Cieniewicz, E.J.; Pethybridge, S.J.; Gorny, A.; Madden, L.V.; McLane, H.; Perry, K.L.; Fuchs, M. Spatiotemporal spread of grapevine red blotch-associated virus in a California vineyard. Virus Res. 2017, 241, 156–162. [Google Scholar] [CrossRef]
- Dalton, D.T.; Hilton, R.J.; Kaiser, C.; Daane, K.M.; Sudarshana, M.R.; Vo, J.; Zalom, F.G.; Buser, J.Z.; Walton, V.M. Spatial associations of vines infected with grapevine red blotch virus in oregon vineyards. Plant Dis. 2019, 103, 1507–1514. [Google Scholar] [CrossRef]
- Bahder, B.W.; Zalom, F.G.; Jayanth, M.; Sudarshana, M.R. Phylogeny of Geminivirus Coat Protein Sequences and Digital PCR Aid in Identifying Spissistilus festinus as a Vector of Grapevine red blotch-associated virus. Phytopathology 2016, 106, 1223–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cieniewicz, E.J.; Pethybridge, S.J.; Loeb, G.; Perry, K.; Fuchs, M. Insights Into the Ecology of Grapevine red blotch virus in a Diseased Vineyard. Phytopathology 2017, 108, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Flasco, M.; Hoyle, V.; Cieniewicz, E.; Roy, B.; McLane, H.; Perry, K.L.; Loeb, G.M.; Nault, B.; Cilia, M.; Fuchs, M. Grapevine red blotch virus is transmitted by the three-cornered alfalfa hopper in a circulative, nonpropagative mode with unique attributes. Phytopathology 2021, 1–50. [Google Scholar] [CrossRef]
- Kahl, D.; Úrbez-Torres, J.R.; Kits, J.; Hart, M.; Nyirfa, A.; Lowery, D.T. Identification of candidate insect vectors of Grapevine red blotch virus by means of an artificial feeding diet. Can. J. Plant Pathol. 2021, 43, 1–9. [Google Scholar] [CrossRef]
- Bowen, P.; Bogdanoff, C.; Poojari, S.; Usher, K.; Lowery, T.; Úrbez-Torres, J.R. Effects of grapevine red blotch disease on cabernet franc vine physiology, bud hardiness, and fruit and wine quality. Am. J. Enol. Vitic. 2020, 71, 308–318. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Plank, C.M.; Brillante, L.; Cooper, M.L.; Smith, R.J.; Al-Rwahnih, M.; Yu, R.; Oberholster, A.; Girardello, R.; Kurtural, S.K. Grapevine Red Blotch Virus May Reduce Carbon Translocation Leading to Impaired Grape Berry Ripening. J. Agric. Food Chem. 2019, 67, 2437–2448. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.D.; KC, A.N. Water Deficits Do Not Improve Fruit Quality in Grapevine Red Blotch Virus-Infected Grapevines (Vitis vinifera L.). Front. Plant Sci. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Reynard, J.; Gugerli, P. Effects of Grapevine red blotch-associated virus on vine physiology and fruit composition of field grown grapevine cv. Gamay. In Proceedings of the 18th Congress of the International Council for the Study of Virus and Virus-like Diseases of the Grapevine (ICVG), Ankara, Turquie, 7–11 September 2015; pp. 234–235. [Google Scholar]
- Wallis, C.M.; Sudarshana, M.R. Effects of Grapevine red blotch-associated virus (GRBaV) infection on foliar metabolism of grapevines. Can. J. Plant Pathol. 2016, 38, 358–366. [Google Scholar] [CrossRef]
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L.; et al. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, G.; Moshelion, M.; David-Schwartz, R.; Halperin, O.; Wallach, R.; Attia, Z.; Belausov, E.; Granot, D. Hexokinase mediates stomatal closure. Plant J. 2013, 75, 977–988. [Google Scholar] [CrossRef]
- Swiech, R.; Browning, S.; Molsen, D.; Stenger, D.C.; Holbrook, G.P. Photosynthetic responses of sugar beet and Nicotiana benthamiana Domin. infected with beet curly top virus. Physiol. Mol. Plant Pathol. 2001, 58, 43–52. [Google Scholar] [CrossRef]
- Fabro, G.; Kovács, I.; Pavet, V.; Szabados, L.; Alvarez, M.E. Proline accumulation and AtP5CS2 gene activation are induced by plant-pathogen incompatible interactions in Arabidopsis. Mol. Plant-Microbe Interact. 2004, 17, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Ulate, B.; Hopfer, H.; Figueroa-Balderas, R.; Ye, Z.; Rivero, R.M.; Albacete, A.; Pérez-Alfocea, F.; Koyama, R.; Anderson, M.M.; Smith, R.J.; et al. Red blotch disease alters grape berry development and metabolism by interfering with the transcriptional and hormonal regulation of ripening. J. Exp. Bot. 2017, 68, 1225–1238. [Google Scholar] [CrossRef] [Green Version]
- Girardello, R.C.; Cooper, M.L.; Smith, R.J.; Lerno, L.A.; Bruce, R.C.; Eridon, S.; Oberholster, A. Impact of Grapevine Red Blotch Disease on Grape Composition of Vitis vinifera Cabernet Sauvignon, Merlot, and Chardonnay. J. Agric. Food Chem. 2019, 67, 5496–5511. [Google Scholar] [CrossRef]
- Calvi, B.L. Effects of Red-leaf Disease on Cabernet Sauvignon at the Oakville Experimental Vineyard and Mitigation by Harvest Delay and Crop Adjustment; University of California: Davis, CA, USA, 2011. [Google Scholar]
- Girardello, R.C.; Cooper, M.L.; Lerno, L.A.; Brenneman, C.; Eridon, S.; Sokolowsky, M.; Heymann, H.; Oberholster, A. Impact of Grapevine Red Blotch Disease on Cabernet Sauvignon and Merlot Wine Composition and Sensory Attributes. Molecules 2020, 25, 3299. [Google Scholar] [CrossRef]
- Riesmeier, J.W.; Willmitzer, L.; Frommer, W.B. Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J. 1992, 11, 4705–4713. [Google Scholar] [CrossRef] [PubMed]
- Amtmann, A.; Troufflard, S.; Armengaud, P. The effect of potassium nutrition on pest and disease resistance in plants. Physiol. Plant. 2008, 133, 682–691. [Google Scholar] [CrossRef]
- Perrenoud, S. Potassium and plant health. Potash Rev. 1990, 85, 1–5. [Google Scholar]
- Zhou, L.; He, H.; Liu, R.; Han, Q.; Shou, H.; Liu, B. Overexpression of GmAKT2 potassium channel enhances resistance to soybean mosaic virus. BMC Plant Biol. 2014, 14, 154. [Google Scholar] [CrossRef] [Green Version]
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural practice and environmental impacts on the flavonoid composition of grapes and wine: A review of recent research. Am. J. Enol. Vitic. 2006, 57, 257–268. [Google Scholar]
- Sherman, E.; Greenwood, D.R.; Villas-Boâs, S.G.; Heymann, H.; Harbertson, J.F. Impact of grape maturity and ethanol concentration on sensory properties of Washington State merlot wines. Am. J. Enol. Vitic. 2017, 68, 344–356. [Google Scholar] [CrossRef]
- Casassa, L.F.; Beaver, C.W.; Mireles, M.; Larsen, R.C.; Hopfer, H.; Heymann, H.; Harbertson, J.F. Influence of fruit maturity, maceration length, and ethanol amount on chemical and sensory properties of Merlot wines. Am. J. Enol. Vitic. 2013, 64, 437–449. [Google Scholar] [CrossRef]
- Bergqvist, J.; Dokoozlian, N.; Ebisuda, N. Sunlight exposure and temperature effects on berry growth and composition of Cabernet Sauvignon and Grenache in the central San Joaquin Valley of California. Am. J. Enol. Vitic. 2001, 52, 1–7. [Google Scholar]
- Alabi, O.J.; Casassa, L.F.; Gutha, L.R.; Larsen, R.C.; Henick-Kling, T.; Harbertson, J.F.; Naidu, R.A. Impacts of Grapevine Leafroll Disease on Fruit Yield and Grape and Wine Chemistry in a Wine Grape (Vitis vinifera L.) Cultivar. PLoS ONE 2016, 11, e0149666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treutter, D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol. 2005, 7, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Rumbaugh, A.C.; Girardello, R.C.; Cooper, M.L.; Plank, C.M.; Kurtural, S.K.; Oberholster, A. Impact of Rootstock and Season on Red Blotch Disease Expression in Cabernet Sauvignon (V. vinifera). Plants 2021, 10, 1583. [Google Scholar] [CrossRef]
- Gambetta, G.A.; Matthews, M.A.; Shaghasi, T.H.; McElrone, A.J.; Castellarin, S.D. Sugar and abscisic acid signaling orthologs are activated at the onset of ripening in grape. Planta 2010, 232, 219–234. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, S.; Loveys, B.; Ford, C.; Davies, C. The relationship between the expression of abscisic acid biosynthesis genes, accumulation of abscisic acid and the promotion of Vitis vinifera L. berry ripening by abscisic acid. Aust. J. Grape Wine Res. 2009, 15, 195–204. [Google Scholar] [CrossRef]
- Kalua, C.M.; Boss, P.K. Evolution of volatile compounds during the development of cabernet sauvignon grapes (vitis vinifera L.). J. Agric. Food Chem. 2009, 57, 3818–3830. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; John Wiley & Sons, Ltd: West Sussex, UK, 2016; ISBN 9781118627808. [Google Scholar]
- Kassara, S.; Kennedy, J.A. Relationship between red wine grade and phenolics. 2. Tannin composition and size. J. Agric. Food Chem. 2011, 59, 8409–8412. [Google Scholar] [CrossRef] [PubMed]
- Buck, K.W. Geminiviruses (Geminiviridae). In Encyclopedia of Virology; Granoff, A., Webster, R.G., Eds.; Academic Press: Cambridge, MA, USA, 1999; pp. 597–606. [Google Scholar]
- Nunan, K.J.; Sims, I.M.; Bacic, A.; Robinson, S.P.; Fincher, G.B. Changes in cell wall composition during ripening of grape berries. Plant Physiol. 1998, 118, 783–792. [Google Scholar] [CrossRef] [Green Version]
- Otulak-Kozieł, K.; Kozieł, E.; Lockhart, B.E.L. Plant cell wall dynamics in compatible and incompatible potato response to infection caused by Potato virus Y (PVYNTN). Int. J. Mol. Sci. 2018, 19, 862. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Location | Country | Cultivar | Reference |
|---|---|---|---|
| California | USA | Cabernet franc | Al Rwahnih et al. 2012, 2013 [13,25] |
| California | USA | Zinfandel | Al Rwahnih et al. 2012, 2013 [13,25] |
| New York | USA | Cabernet franc | Krenz et al. 2012 [12] |
| Washington | USA | Merlot | Poojari et al. 2013 [26] |
| Washington | USA | Cabernet franc | Poojari et al. 2013 [26] |
| Texas | USA | Unknown | National Clean Plant Network 2013 [27] |
| Pennsylvania | USA | Merlot | Krenz et al. 2014 [15] |
| Pennsylvania | USA | Cabernet franc | Krenz et al. 2014 [15] |
| New York | USA | Pinot noir | Krenz et al. 2014 [15] |
| California | USA | Chardonnay | Krenz et al. 2014 [15] |
| California | USA | Pinot noir | Krenz et al. 2014 [15] |
| California | USA | Cabernet Sauvignon | Krenz et al. 2014 [15] |
| California | USA | Malbec | Krenz et al. 2014 [15] |
| California | USA | Petit Verdot | Krenz et al. 2014 [15] |
| California | USA | Cabernet franc | Krenz et al. 2014 [15] |
| California | USA | Riesling | Krenz et al. 2014 [15] |
| California | USA | Zinfandel | Krenz et al. 2014 [15] |
| Maryland | USA | Merlot | Krenz et al. 2014 [15] |
| Maryland | USA | Cabernet franc | Krenz et al. 2014 [15] |
| Virginia | USA | Unknown | Krenz et al. 2014 [15] |
| New Jersey | USA | Cabernet franc | Krenz et al. 2014 [15] |
| Oregon | USA | Pinot noir | Krenz et al. 2014 [15]; Seguin et al. 2014 [14] |
| California (herbarium) | USA | Early Burgundy | Al Rwahnih et al. 2015 [28] |
| California (National Clonal Germplasm Repository) | USA | Table grapes | Al Rwahnih et al. 2015 [23] |
| Arkansas | USA | Unknown | Sudarshana et al. 2015 [16] |
| Unknown | USA | Chambourcin (interspecific hybrid) | Sudarshana et al. 2015 [16] |
| California | USA | Free-living Vitis spp. | Perry et al. 2016 [29] |
| California | USA | Free-living Vitis spp. | Bahder et al. 2016 [30] |
| Suwon and Gyeongsan | South Korea | Unknown | Lim et al. 2016 [18] |
| Ontario | Canada | Cabernet franc | Poojari et al. 2017 [22] |
| Ontario | Canada | Chardonnay | Poojari et al. 2017 [22] |
| Ontario | Canada | Riesling | Poojari et al. 2017 [22] |
| Ontario | Canada | Cabernet franc | Poojari et al. 2017 [22] |
| Ontario | Canada | Syrah | Poojari et al. 2017 [22] |
| British Columbia | Canada | Muscat | Poojari et al. 2017 [22] |
| British Columbia | Canada | Cabernet franc | Poojari et al. 2017 [22] |
| British Columbia | Canada | Chardonnay | Poojari et al. 2017 [22] |
| British Columbia | Canada | Zinfandel | Poojari et al. 2017 [22] |
| British Columbia | Canada | Grenache | Poojari et al. 2017 [22] |
| British Columbia | Canada | Petit Verdot | Poojari et al. 2017 [22] |
| Nyon (Agroscope grapevine virus collection) * | Switzerland | Gamay | Reynard et al. 2018 [24] |
| Georgia | USA | Cynthiana (Norton, interspecific hybrid) | Brannen et al. 2018 [31] |
| Georgia | USA | Cabernet franc | Brannen et al. 2018 [31] |
| Missouri | USA | Crimson Cabernet | Schoelz et al. 2018 [32] |
| Ontario | Canada | Cabernet Franc | Xiao et al. 2018 [33] |
| Ontario | Canada | Cabernet Sauvignon | Xiao et al. 2018 [33] |
| Ontario | Canada | Pinot noir | Xiao et al. 2018 [33] |
| Ontario | Canada | Merlot | Xiao et al. 2018 [33] |
| Ontario | Canada | Syrah | Xiao et al. 2018 [33] |
| Ontario | Canada | Pinot Gris | Xiao et al. 2018 [33] |
| Ontario | Canada | Sauvignon Blanc | Xiao et al. 2018 [33] |
| Ontario | Canada | Chardonnay | Xiao et al. 2018 [33] |
| Ontario | Canada | Riesling | Xiao et al. 2018 [33] |
| Ontario | Canada | Gewürz traminer | Xiao et al. 2018 [33] |
| San Juan and Mendoza | Argentina | Flame Seedless | Luna et al. 2019 [20] |
| Baja California and Ensenada | Mexico | Pinot noir | Gasperin-Bulbarela et al. 2019 [19] |
| Baja California and Ensenada | Mexico | Merlot | Gasperin-Bulbarela et al. 2019 [19] |
| Baja California and Ensenada | Mexico | Nebbiolo | Gasperin-Bulbarela et al. 2019 [19] |
| Punjab | India | Unknown | Marwal et al. 2019 [21] |
| Tennessee | USA | Several cultivars | Soltani et al. 2020 [34] |
| Quebec | Canada | Pinot noir | Fall et al. 2020 [35] |
| Nova Scotia | Canada | Chardonnay | Poojari et al. 2020 [36] |
| Nova Scotia | Canada | Pinot noir | Poojari et al. 2020 [36] |
| Nova Scotia | Canada | New York Muscat (Interspecific hybrid) | Poojari et al. 2020 [36] |
| Nova Scotia | Canada | Marechal Foch (Interspecific hybrid) | Poojari et al. 2020 [36] |
| Idaho | USA | Syrah | Lee et al. 2021 [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rumbaugh, A.C.; Sudarshana, M.R.; Oberholster, A. Grapevine Red Blotch Disease Etiology and Its Impact on Grapevine Physiology and Berry and Wine Composition. Horticulturae 2021, 7, 552. https://doi.org/10.3390/horticulturae7120552
Rumbaugh AC, Sudarshana MR, Oberholster A. Grapevine Red Blotch Disease Etiology and Its Impact on Grapevine Physiology and Berry and Wine Composition. Horticulturae. 2021; 7(12):552. https://doi.org/10.3390/horticulturae7120552
Chicago/Turabian StyleRumbaugh, Arran C., Mysore R. Sudarshana, and Anita Oberholster. 2021. "Grapevine Red Blotch Disease Etiology and Its Impact on Grapevine Physiology and Berry and Wine Composition" Horticulturae 7, no. 12: 552. https://doi.org/10.3390/horticulturae7120552
APA StyleRumbaugh, A. C., Sudarshana, M. R., & Oberholster, A. (2021). Grapevine Red Blotch Disease Etiology and Its Impact on Grapevine Physiology and Berry and Wine Composition. Horticulturae, 7(12), 552. https://doi.org/10.3390/horticulturae7120552







