Effect of Storage Conditions and Cooking Methods on Chlorophyll, Glucosinolate, and Sulforaphane Content in Broccoli Florets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatment
2.2. Chlorophyll Content Measurement
2.3. Determination of GSLs Content
2.4. Determination of Sulforaphane Content
2.5. Statistical Analysis
3. Results
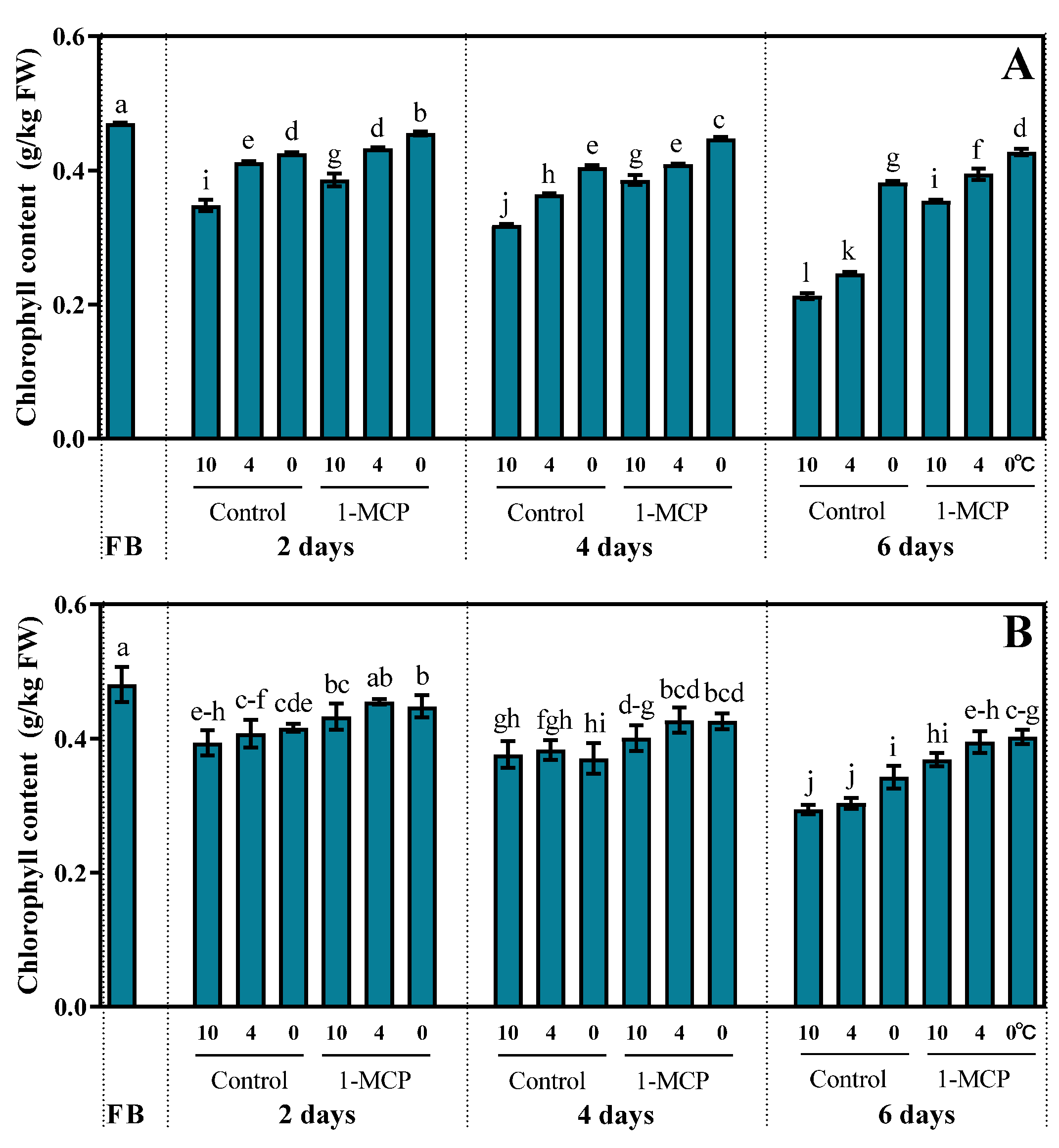
3.1. Effects of Storage Condition on Chlorophyll Content in Broccoli Florets
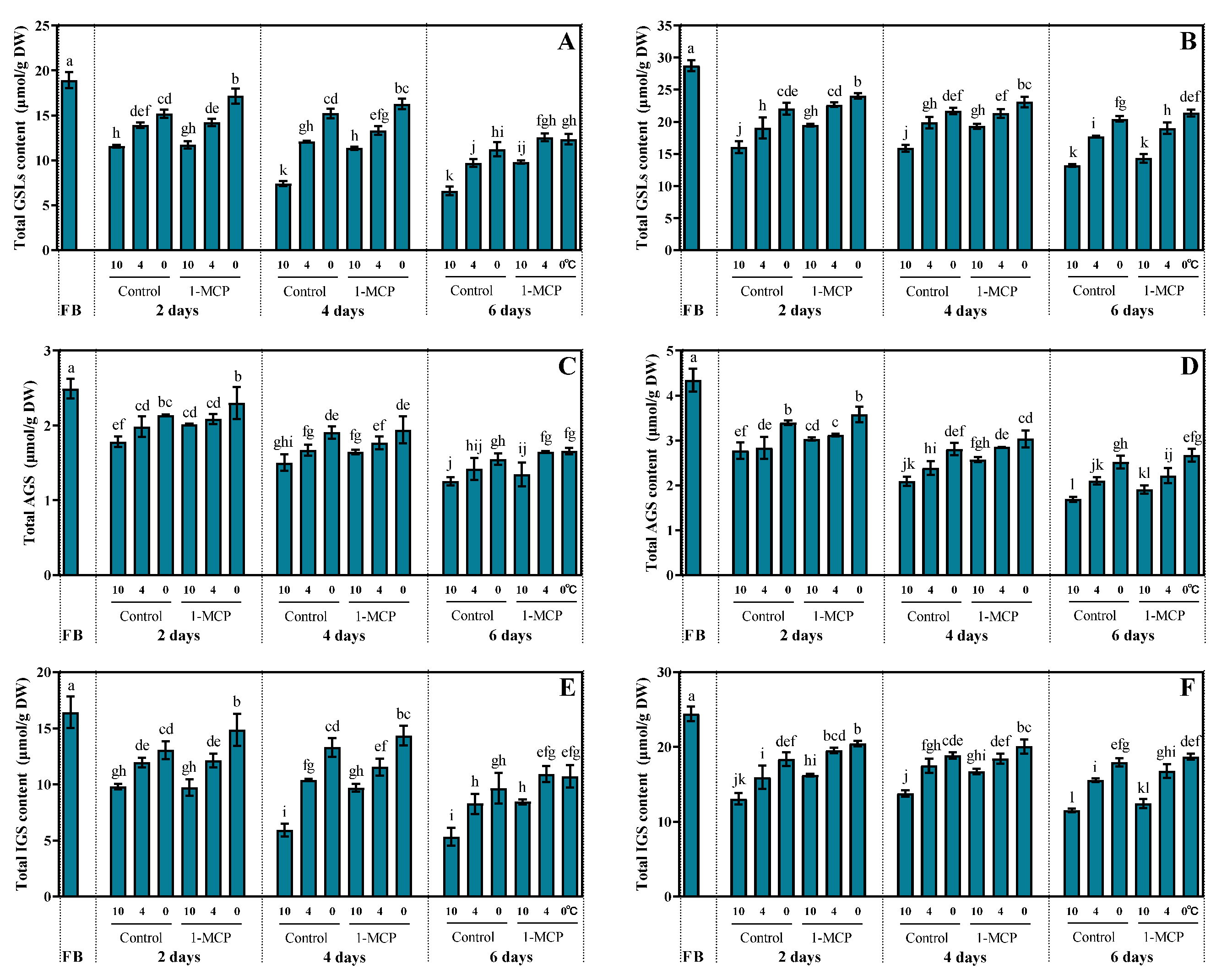
3.2. Effect of Storage Condition on GSL Content in Broccoli Florets
3.3. Effect of Storage Condition on Glucoraphanin and Sulforaphane Content in Broccoli Florets
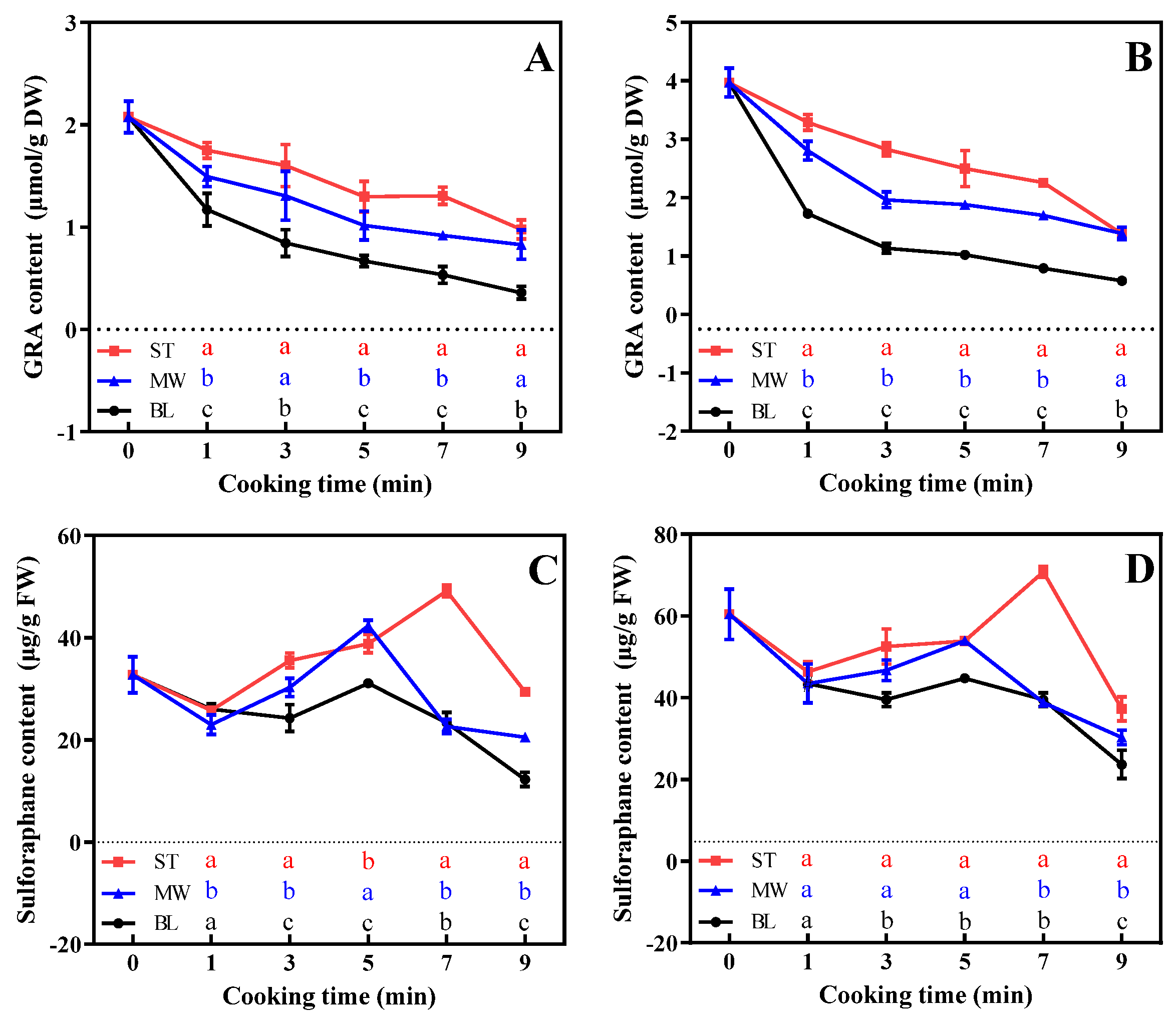
3.4. Effects of Cooking Method on GSL Content in Broccoli Florets
3.5. Effects of Cooking Method on Glucoraphanin and Sulforaphane Contents in Broccoli Florets
4. Discussion
4.1. Low Temperature with 1-MCP Delayed Yellowing and Nutrient Loss of Broccoli Florets
4.2. Steaming or Microwaving Reduced Nutrient Loss of Broccoli Florets
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, G.; Sun, B.; Yuan, J.; Wang, Q. Effects of different cooking methods on health-promoting compounds of broccoli. J. Zhejiang Univ. Sci. B 2009, 10, 580–588. [Google Scholar] [CrossRef] [Green Version]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef]
- Lage, M.Á.P.; Jiménez López, C.; Simal-Gandara, J. Glucosinolates: Molecular structure, breakdown, genetic, bioavailability, properties and healthy and adverse effects. Adv. Food Nutr. Res. 2019, 90, 305–350. [Google Scholar]
- Martínez-Ballesta, M.; Moreno, D.A.; Carvajal, M. The physiological importance of glucosinolates on plant response to abiotic atress in brassica. Int. J. Mol. Sci. 2013, 14, 11607–11625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matusheski, N.; Jeffery, E.H. Comparison of the bioactivity of two glucoraphanin hydrolysis products found in broccoli, sulforaphane and sulforaphane nitrile. J. Agric. Food Chem. 2001, 49, 5743–5749. [Google Scholar] [CrossRef]
- Cai, C.; Miao, H.; Qian, H.; Yao, L.; Wang, B.; Wang, Q. Effects of industrial pre-freezing processing and freezing handling on glucosinolates and antioxidant attributes in broccoli florets. Food Chem. 2016, 210, 451–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valgimigli, L.; Iori, R. Antioxidant and pro-oxidant capacities of ITCs. Environ. Mol. Mutagen. 2010, 50, 222–237. [Google Scholar] [CrossRef]
- Lippmann, D.; Lehmann, C.; Florian, S.; Barknowitz, G.; Haack, M.; Mewis, I.; Wiesner, M.; Schreiner, M.; Glatt, H.; Brigelius-Flohé, R.; et al. Glucosinolates from pak choi and broccoli induce enzymes and inhibit inflammation and colon cancer differently. Food Funct. 2014, 5, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Lee, H.E.; Lee, J.Y. A pharmacological inhibitor of NLRP3 inflammasome prevents non-alcoholic fatty liver disease in a mouse model induced by high fat diet. Sci. Rep. UK 2016, 6, 24399. [Google Scholar] [CrossRef]
- Traka, M.; Mithen, R. Glucosinolates, isothiocyanates and human health. Phytochem. Rev. 2009, 8, 269–282. [Google Scholar] [CrossRef]
- Brennan, P.S.; Shewfelt, R.L. Effect of cooling delay at harvest on broccoli quality during postharvest storage. J. Food Qual. 2010, 12, 13–22. [Google Scholar] [CrossRef]
- Ilahy, R.; Tlili, I.; Zoltán, P.; Montefusco, A.; Siddiqui, M.W.; Homa, F.; Hdider, C.; R’Him, T.; Lajos, H.; Lenucci, M.S. Pre-and post-harvest factors affecting glucosinolate content in broccoli. Front. Nutr. 2020, 7, 00147. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.; Wagstaff, C. Enhancement of glucosinolate & isothiocyanate profiles in Brassicaceae crops: Addressing challenges in breeding for cultivation, storage, and consumer related traits. J. Agric. Food Chem. 2017, 65, 9379–9403. [Google Scholar] [PubMed]
- Casajús, V.; Demkura, P.; Civello, P.; Lobato, M.G.; Martínez, G. Harvesting at different time-points of day affects glucosinolate metabolism during postharvest storage of broccoli. Food Res. Int. 2020, 136, 109529. [Google Scholar] [CrossRef] [PubMed]
- Rybarczyk, A.; Hagen, S.F.; Borge, G.I.A.; Bengtsson, G.B.; Hansen, M.K.; Wold, A.-B. Glucosinolates in broccoli (Brassica oleracea L. var. italica) as affected by postharvest temperature and radiation treatments. Postharvest Biol. Technol. 2016, 116, 16–25. [Google Scholar] [CrossRef]
- Hansen, M.; Møller, P.; Sørensen, H.; Trejo, M.C.D. Glucosinolates in broccoli stored under controlled atmosphere. J. Am. Soc. Hortic. Sci. 1995, 120, 1069–1074. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.S.; Rosa, E.A. Effect of post-harvest treatments on the level of glucosinolates in broccoli. J. Sci. Food Agric. 1999, 79, 1028–1032. [Google Scholar] [CrossRef]
- Ku, V.; Wills, R. Effect of 1-methylcyclopropene on the storage life of broccoli. Postharvest Biol. Technol. 1999, 17, 127–132. [Google Scholar] [CrossRef]
- Fan, X.T.; Mattheis, J.P. Yellowing of broccoli in storage is reduced by 1-methylcyclopropene. HortScience 2000, 35, 885–887. [Google Scholar] [CrossRef] [Green Version]
- Yuan, G.; Sun, B.; Yuan, J.; Wang, Q. Effect of 1-methylcyclopropene on shelf life, visual quality, antioxidant enzymes and health-promoting compounds in broccoli florets. Food Chem. 2010, 118, 774–781. [Google Scholar] [CrossRef]
- Fernández-León, M.F.; Fernández-León, A.M.; Lozano, M.; Ayuso, M.C.; González-Gómez, D. Different postharvest strategies to preserve broccoli quality during storage and shelf life: Controlled atmosphere and 1-MCP. Food Chem. 2013, 138, 564–573. [Google Scholar] [CrossRef]
- Bongoni, R.; Verkerk, R.; Steenbekkers, B.; Dekker, M.; Stieger, M. Evaluation of different cooking conditions on broccoli (Brassica oleracea var. italica) to improve the nutritional value and consumer acceptance. Plant Food Hum. Nutr. 2014, 69, 228–234. [Google Scholar] [CrossRef]
- Chiu, Y.-C.; Matak, K.; Ku, K.-M. Methyl jasmonate treatment of broccoli enhanced glucosinolate concentration, which was retained after boiling, steaming, or microwaving. Foods 2020, 9, 758. [Google Scholar] [CrossRef]
- Gliszczyńska-Świglo, A.; Ciska, E.; Pawlak-Lemańska, K.; Chmielewski, J.; Borkowski, T.; Tyrakowska, B. Changes in the content of health-promoting compounds and antioxidant activity of broccoli after domestic processing. Food Addit. Contam. 2006, 23, 1088–1098. [Google Scholar] [CrossRef]
- Palermo, M.; Pellegrini, N.; Fogliano, V. The effect of cooking on the phytochemical content of vegetables. J. Sci. Food Agric. 2014, 94, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Inskeep, W.P.; Bloom, P.R. Extinction coefficients of chlorophyll a and b in N,N-dimethylformamide and 80% acetone. Plant Physiol. 1985, 77, 483–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.-B.; Li, X.; Kim, S.-J.; Kim, H.-H.; Lee, J.; Kim, H.; Sang, U.P. MYB transcription factors regulate glucosinolate biosynthesis in different organs of Chinese cabbage (Brassica rapa ssp. pekinensis). Molecules 2013, 18, 8682–8695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schonhof, I.; Krumbein, A.; Brückner, B. Genotypic effects on glucosinolates and sensory properties of broccoli and cauliflower. Mol. Nutr. Food Res. 2010, 2010. 48, 25–33. [Google Scholar] [CrossRef]
- Huang, K.; Lin, J.C.; Wu, Q.Y.; Yan, J.Y.; Liu, M.Y.; Zhang, S.; Xiao, W.J. Changes in sulforaphane and selenocysteine methyltransferase transcript levels in broccoli treated with sodium selenite. Plant Mol. Biol. Rep. 2015, 34, 1–8. [Google Scholar] [CrossRef]
- Rawson, A.; Patras, A.; Kumar, T.B.; Noci, F.; Koutchma, T.; Brunton, N.P. Effect of thermal and non-thermal processing technologies on the bioactive content of exotic fruits and their products. Food Res. Int. 2011, 44, 1875–1887. [Google Scholar] [CrossRef]
- Xu, F.; Chen, X.; Yang, Z.; Jin, P.; Wang, K.; Shang, H.; Wang, X.; Zheng, Y. Maintaining quality and bioactive compounds of broccoli by combined treatment with 1-methylcyclopropene and 6-benzylaminopurine. J. Sci. Food Agric. 2013, 93, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Bhatia, S.; Kaur, P. Influence of packaging and storage conditions on biochemical quality and enzymatic activity in relation to shelf life enhancement of fresh basil leaf. J. Food Sci. Technol. 2018, 55, 3199–3211. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Zhu, Z.; Sun, D.-W. Effects of pretreatments on quality attributes of long-term deep frozen storage of vegetables: A review. Crit. Rev. Food Sci. 2018, 59, 743–757. [Google Scholar] [CrossRef]
- Björkman, M.; Klingen, I.; Birch, A.N.E.; Bones, A.M.; Bruce, T.J.A.; Johansen, T.J.; Meadow, R.; Mølmann, J.; Seljåsen, R.; Smart, L.E.; et al. Phytochemicals of Brassicaceae in plant protection and human health-influences of climate, environment and agronomic practice. Phytochemistry 2011, 72, 538–556. [Google Scholar] [CrossRef]
- Guo, L.; Yang, R.; Wang, Z.; Guo, Q.; Gu, Z. Glucoraphanin, sulforaphane and myrosinase activity in germinating broccoli sprouts as affected by growth temperature and plant organs. J. Funct. Foods 2014, 9, 70–77. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Schreiner, M. Isothiocyanates, nitriles, and epithionitriles from glucosinolates are affected by genotype and developmental stage in Brassica oleracea varieties. Front. Plant Sci. 2017, 8, 01095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrino, R.; Wheeler, J.; Sams, C.E.; Luckett, C.R. Storage time and temperature on the sensory properties broccoli. Foods 2019, 8, 162. [Google Scholar] [CrossRef] [Green Version]
- Bones, A.M.; Rossiter, J.T. The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry 2006, 67, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Zinoviadou, K.; Galanakis, C.M. Glucosinolates and respective derivatives (isothiocyanates) from plants. Food Bioact. 2017, 3–22. [Google Scholar]
- Li, R.; Zhu, Y. The primary active components, antioxidant properties, and differential metabolite profiles of radish sprouts (Raphanus sativus L.) upon domestic storage: Analysis of nutritional quality. J. Sci. Food Agric. 2018, 98, 5853–5860. [Google Scholar] [CrossRef]
- Oliviero, T.; Verkerk, R.; Dekker, M. Effect of water content and temperature on glucosinolate degradation kinetics in broccoli (Brassica oleracea var. italica). Food Chem. 2012, 132, 2037–2045. [Google Scholar] [CrossRef]
- Fahey, J.W.; Wehage, S.L.; Holtzclaw, W.D.; Kensler, T.W.; Egner, P.A.; Shapiro, T.A.; Talalay, P. Protection of humans by plant glucosinolates: Efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev. Res. 2012, 5, 603–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Shen, Y.; Wu, X.; Zhu, Y.; Mupunga, J.; Bao, W.; Huang, J.; Mao, J.; Liu, S.; You, Y. Hydrolysis before stir-frying increases the isothiocyanate content of broccoli. J. Agric. Food Chem. 2018, 66, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wang, M.; Rosen, R.T.; Ho, C.-T. Thermal degradation of sulforaphane in aqueous solution. J. Agric. Food Chem. 1999, 47, 3121–3123. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Mao, S.; Yuan, Y.; Zhang, N.; Wu, Q.; Liang, M.; Wang, S.; Huang, K.; Wu, Q. Effect of Storage Conditions and Cooking Methods on Chlorophyll, Glucosinolate, and Sulforaphane Content in Broccoli Florets. Horticulturae 2021, 7, 519. https://doi.org/10.3390/horticulturae7120519
Wang J, Mao S, Yuan Y, Zhang N, Wu Q, Liang M, Wang S, Huang K, Wu Q. Effect of Storage Conditions and Cooking Methods on Chlorophyll, Glucosinolate, and Sulforaphane Content in Broccoli Florets. Horticulturae. 2021; 7(12):519. https://doi.org/10.3390/horticulturae7120519
Chicago/Turabian StyleWang, Junwei, Shuxiang Mao, Yiming Yuan, Na Zhang, Qi Wu, Mantian Liang, Shengze Wang, Ke Huang, and Qiuyun Wu. 2021. "Effect of Storage Conditions and Cooking Methods on Chlorophyll, Glucosinolate, and Sulforaphane Content in Broccoli Florets" Horticulturae 7, no. 12: 519. https://doi.org/10.3390/horticulturae7120519
APA StyleWang, J., Mao, S., Yuan, Y., Zhang, N., Wu, Q., Liang, M., Wang, S., Huang, K., & Wu, Q. (2021). Effect of Storage Conditions and Cooking Methods on Chlorophyll, Glucosinolate, and Sulforaphane Content in Broccoli Florets. Horticulturae, 7(12), 519. https://doi.org/10.3390/horticulturae7120519




