Influence of the Position of Mango Fruit on the Tree (Mangifera indica L. CV. ‘Zibda’) on Chilling Sensitivity and Antioxidant Enzyme Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Materials and Experimental Setup
2.2. Ci-Index and Electrolyte Leakage (El%)
2.3. Antioxidant Enzyme Activity Assays
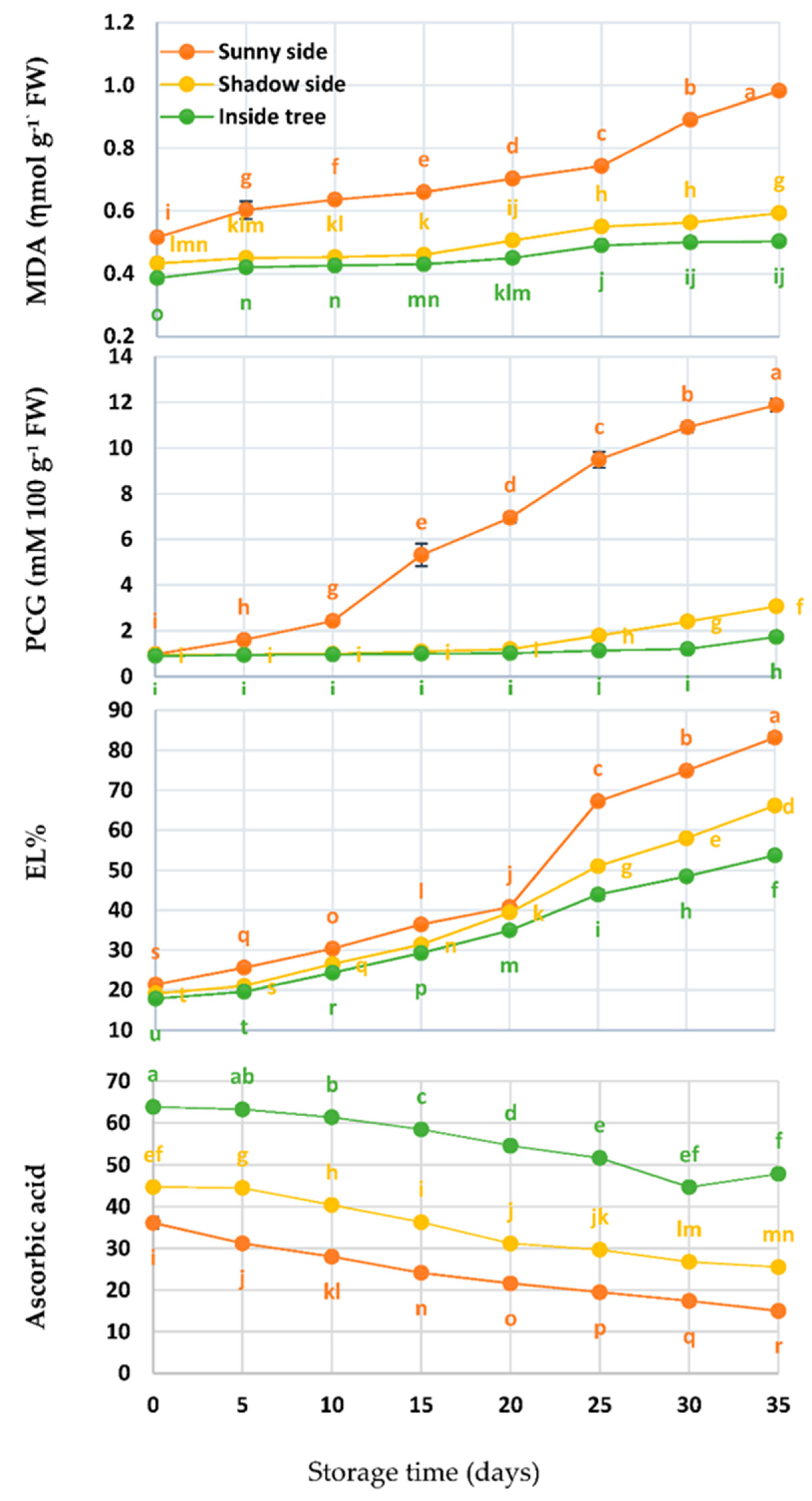
2.4. MDA and PCG Contents
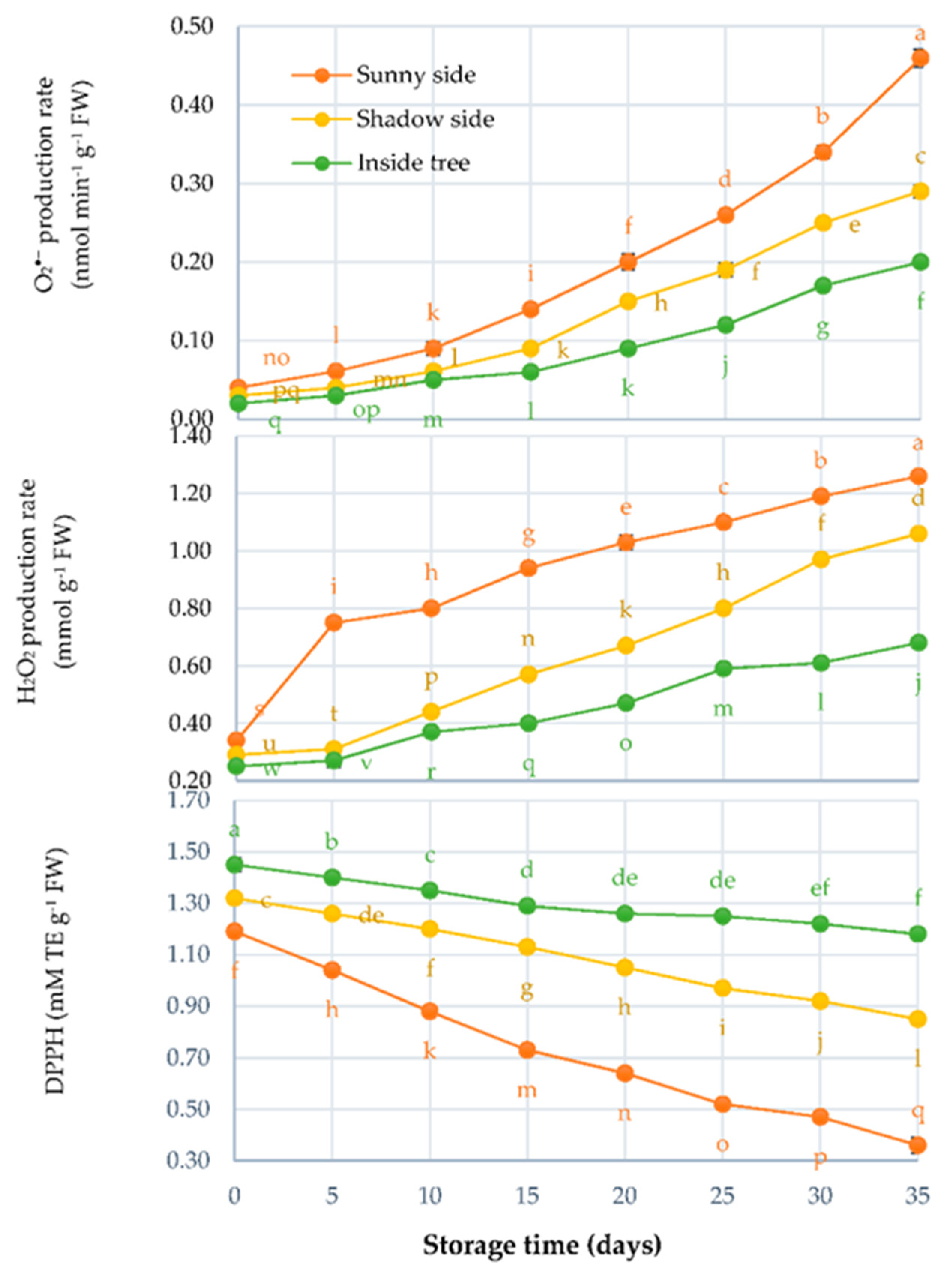
2.5. O2− and H2O2 Production Rates
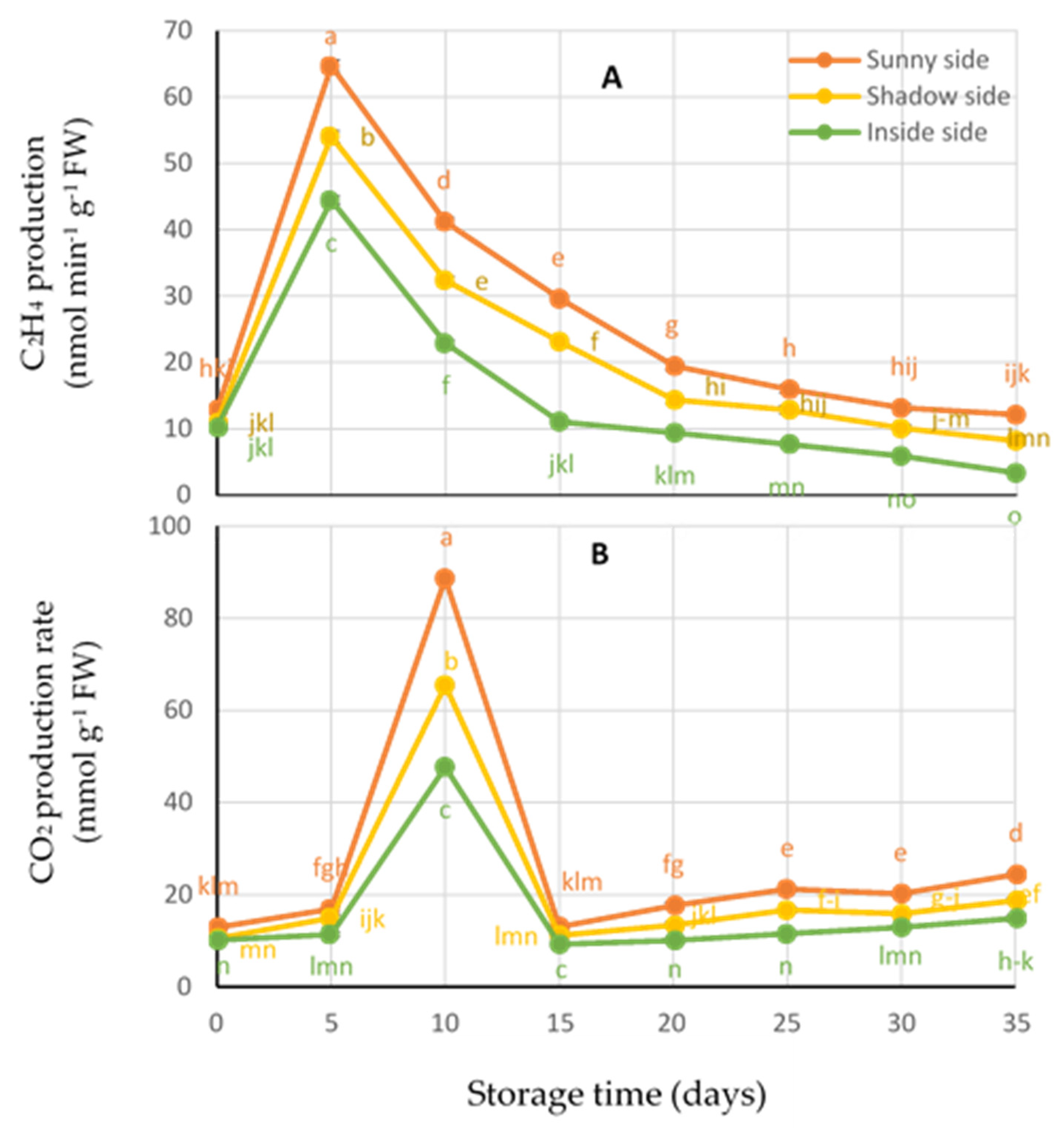
2.6. Ethylene and Respiration
2.7. Statistical Analysis
3. Results
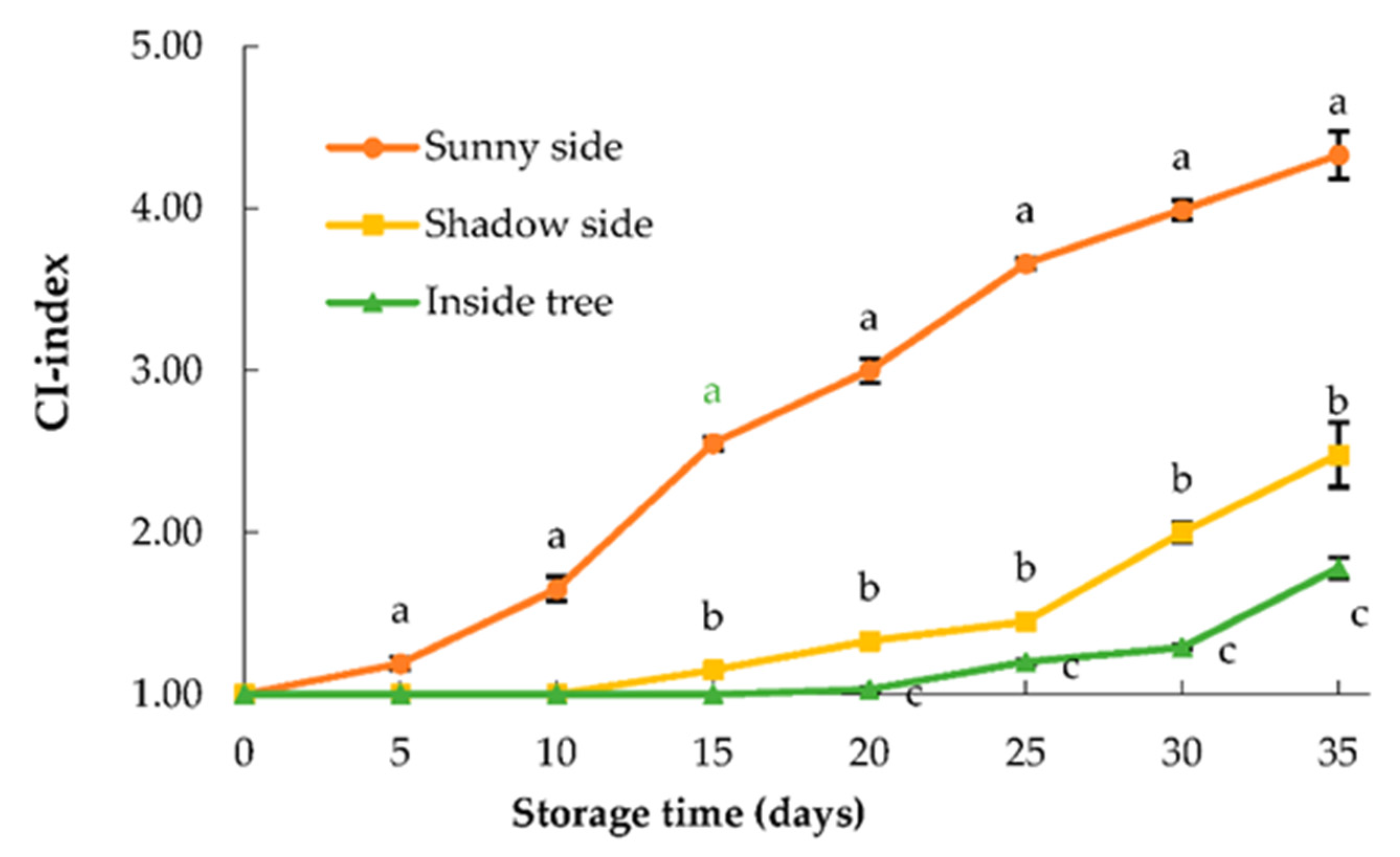
3.1. Chilling Injury Symptoms
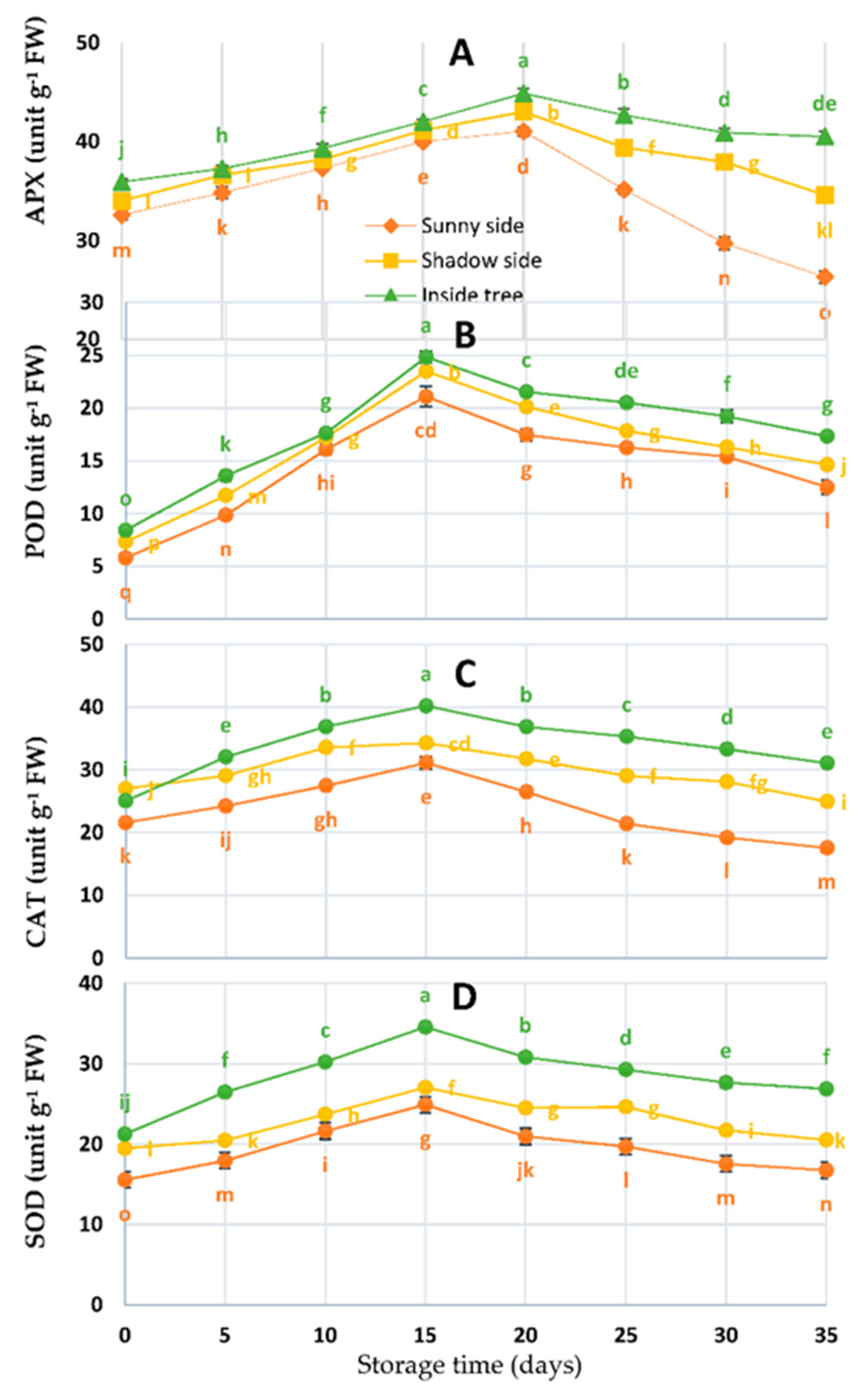
3.2. Antioxidant Enzyme Activities
3.3. MDA, PCG, EL%, and Ascorbic Acid
3.4. O2−; H2O2 Production Rates, and DPPH*
3.5. Ethelene and Respiration Production
3.6. Data Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yasunaga, E.; Fukida, S.; Nagle, M.; Spreer, W. Effect of storage conditions on the postharvest quality changes of fresh mango fruits for export during transportation. Environ. Control Biol. 2018, 56, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Sivakumar, D.; Jiang, Y.; Yahia, E.M. Maintaining mango (Mangifera indica L.) fruit quality during the export chain. Food Res. Int. 2011, 44, 1254–1263. [Google Scholar] [CrossRef]
- FROSTAT. 2019. Available online: http://www.fao.org/faostat/ (accessed on 21 July 2021).
- Barman, K.; Asery, R. Salicylic acid pre-treatment alleviates chilling injury, preserves bioactive compounds and enhances shelf life of mango fruit during cold storage. J. Sci. Ind. Res. 2014, 73, 713–718. [Google Scholar]
- Moretti, C.L.; Mattos, L.M.; Calbo, A.G.; Sargent, S.A. Climate changes and potential impacts on postharvest quality of fruit and vegetable crops: A review. Food Res. Int. 2010, 43, 1824–1832. [Google Scholar] [CrossRef]
- Henson, R. The Rough Guide to Climate Change, 2nd ed.; Penguim Books: London, UK, 2008; p. 384. [Google Scholar]
- Kays, S.J. Postharvest Physiology of Perishable Plant Products; AVI: Athens, Greece, 1997; 532p. [Google Scholar]
- Woolf, A.B.; Ferguson, I.B.; Requejo-Tapia, L.C.; Boyd, L.; Laing, W.A.; White, A. Impact of sun exposure on harvest quality of ‘Hass’ avocado fruit. Rev. Chapingo Ser. Hortic 1999, 5, 352–358. [Google Scholar]
- Ferguson, I.B.; Lurie, S.; Bowen, J.H. Protein synthesis and breakdown during heat shock of cultured pear (Pyrus communis L.) cells. Plant Physiol. 1994, 104, 1429–1437. [Google Scholar] [CrossRef] [Green Version]
- Nair, S.; Singh, Z. Pre-storage ethrel dip reduces chilling injury, enhances respiration rate, ethylene production and improves fruit quality of ‘Kensington Pride’ mango. J. Food Agric. Environ. 2003, 1, 93–97. [Google Scholar]
- Shewfelt, R.L.; Del Rosario, B.A. The role of lipid peroxidation in storage disorders of fresh fruits and vegetables. Hortscience 2000, 35, 575–579. [Google Scholar] [CrossRef]
- Nair, S.; Singh, Z. Chilling injury during storage affects respiration rate and fruit quality in ‘Kensington Pride’ mango fruit. Acta Hortic. 2009, 820, 737–743. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Junmatonga, C.; Faiyueb, B.; Rotarayanonta, S.; Uthaibutraa, J.; Boonyakiatc, D.; Saengnila, K. Cold storage in salicylic acid increases enzymatic and non-enzymatic antioxidants of Nam Dok Mai No. 4 mango fruit. Sci. Asia 2015, 41, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Lo’ay, A.A. Chilling Injury in Mangoes. Ph.D. Thesis, Wageningen University, Wageningen, NL, USA, 2005; pp. 1–224. [Google Scholar]
- Lo’ay, A.A.; Dawood, H.D. Tolerance of ‘Baladi’ mandarin fruits to cold storage by postharvest pectin/PVA blend with ascorbic acid treatment. Sci. Hortic. 2019, 256, 108637. [Google Scholar] [CrossRef]
- Peiyan, L.; Xiaolin, Z.; Golam Ferdous, C.M.; Kim, C.; Jeffrey, K.B. Prestorage Application of Oxalic Acid to Alleviate Chilling Injury in Mango Fruit. HortSci. Horts 2015, 50, 1795–1800. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.Y. Reducing chilling injury and maintaining quality of horticultural crops with natural products and their derivatives. Acta Hortic. 2006, 712, 285–290. [Google Scholar] [CrossRef]
- Rodeo, A.J.D.; Esguerra, E.B. Low temperature conditioning alleviates chilling injury in mango (Mangifera indica L. cv. Carabao) fruit. Philipp. J. Crop Ssi. 2013, 38, 24–32. [Google Scholar]
- Pristijono, P.; Golding, J.B.; Bowyer, M.C. Postharvest UV-C Treatment, Followed by Storage in a Continuous Low-Level Ethylene Atmosphere, Maintains the Quality of ‘Kensington Pride’ Mango Fruit Stored at 20 °C. Horticulturae 2019, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Majeed, M.; Jeffery, K.B. Reduction of chilling injury in ‘Tommy Atkins’ mangoes during ripening. Sci. Hortic. 2002, 95, 297–308. [Google Scholar]
- Chaplin, G.R.; Cole, S.R.; Landrin, M.; Nuevo, P.A.; Lam, P.F.; Graham, G. Chilling injury and storage of mango (Mangifera indica L.) held under low temperatures. Acta Hortic. 1991, 291, 461–471. [Google Scholar] [CrossRef]
- Hakim, A.; Purvis, A.C.; Mullinix, B.G. Differences in chilling sensitivity of cucumber varieties depends on storage temperature and the physiological dysfunction evaluated. Postharvest Biol. Technol. 1999, 17, 97–104. [Google Scholar] [CrossRef]
- Wang, Y.S.; Tian, S.P.; Xu, Y. Effects of high oxygen concentration on pro- and antioxidant enzymes in peach fruit during postharvest periods. Food Chem. 2005, 91, 99–104. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, L.; Li, F.; Meng, D.; Sheng, J. Arginase induction by heat treatment contributes to amelioration of chilling injury and activation of antioxidant tenzymes in tomato fruit. Postharvest Biol. Technol. 2013, 79, 1–8. [Google Scholar] [CrossRef]
- Tian, S.P.; Li, B.Q.; Xu, Y. Effects of O2 and CO2 concentration on physiology and quality of litchi fruit in storage. Food Chem. 2005, 91, 659–663. [Google Scholar] [CrossRef]
- Iturbe-Ormaetxe, I.; Escuredo, P.R.; Arrese-Igor, C.; Becana, M. Oxidative damage in pea plants exposed to water deficit or paraquat. Plant Physiol. 1998, 116, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Levine, R.L.; Williams, J.A.; Stadtman, E.R.; Schacter, E. Carbonyl assay for determination of oxidatively modified proteins. Methods Enzym. 1994, 233, 346–357. [Google Scholar]
- Yang, H.; Wu, F.; Cheng, J. Reduced chilling injury in cucumber by nitric oxide and the antioxidant response. Food Chem. 2011, 127, 1237–1242. [Google Scholar] [CrossRef]
- Xu, M.; Dong, J.; Zhang, M.; Xu, X.; Sun, L. Cold-induced endogenous nitric oxide generation plays a role in chilling tolerance of loquat fruit during postharvest storage. Postharvest Biol. Technol. 2012, 65, 5–12. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Leshem, Y.Y. Plant Membranes: A Biophysical Approach to Structure, Development and Senescence; Kluwer Acadamic Publishers: Dordrecht, The Netherlands, 1992; pp. 1–266. ISBN 0792313534. [Google Scholar]
- Foyer, C.H.; Ruban, A.V.; Noctor, G. Viewing oxidative stress through the lens of oxidative signaling rather than damage. Biochem. J. 2017, 474, 877–883. [Google Scholar] [CrossRef] [Green Version]
- Lo’ay, A.A.; Dawood, H.D. Chilling injury, fruit color maturity stages, and antioxidant enzyme activities of lemon ’baladi CV’ fruits under cold storage stress. Sci. Hortic. 2019, 257, 108676. [Google Scholar] [CrossRef]
- Galletta, G.J.; Bringhurst, R.S. Strawberry management. In Small Fruit Crop Management; Galletta, G.J., Bringhurst, R.S., Eds.; Prentice-Hall: Englewood Cliffs, NJ, USA, 1990; pp. 83–156. [Google Scholar]
- McKeon, A.W.; Warland, J.; McDonald, M.R. Long-term climate and weather patterns in reation to crop yield: A minireview. Can. J. Bot. 2006, 84, 1031–1036. [Google Scholar] [CrossRef]
- Usha, K.; Thakre, M.; Goswami, A.K.; Nayan, D.G. Fundamental of Fruit Production; Division of Fruits and Horticultural Technology; Indian Agricultural Research Institute: New Delhi, Indian, 2015; pp. 1–245. [Google Scholar]
- Lo’ay, A.A.; Doaa, M.H. The potential of vine rootstocks impacts on ‘Flame Seedless’ bunches behavior under cold storage and antioxidant enzyme activity performance. Sci. Hortic. 2020, 260, 108844. [Google Scholar] [CrossRef]
- Hodges, D.M. Overview: Oxidative stress and postharvest produce. In Postharvesst Oxidative Stress in Horticultural Crops; Hodges, D.M., Ed.; Food Products Press: New York, NY, USA, 2003; pp. 1–12. [Google Scholar]
- Lo’ay, A.A.; El-Khateeb, A.Y. Antioxidant enzyme activities and exogenous ascorbic acid treatment of ‘Williams’ banana during long-term cold storage stress. Sci. Hortic. 2018, 234, 210–219. [Google Scholar] [CrossRef]
- Hodges, D.M.; Lester, G.E.; Munro, K.D.; Toivonen, P.M.A. Oxidative stress: Importance for postharvest quality. HortScience 2004, 39, 924–929. [Google Scholar] [CrossRef] [Green Version]
- Lo’ay, A.A.; Taher, M.A. Effectiveness salicylic acid blending in chitosan/PVP biopolymer coating on antioxidant enzyme activities under low storage temperature stress of ‘Banati’ guava fruit. Sci. Hortic. 2018, 238, 343–349. [Google Scholar] [CrossRef]
- Hicks, J.R.; Manzano-Mendez, J.; Masters, J.F. Temperature extremes and tomato ripening. In Proceedings of the Fourth Tomato Quality Workshop, Miami, FL, USA, 7–10 March 1983. [Google Scholar]
- Foyer, C.H.; Noctor, G. Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol. Plant 2003, 119, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Tang, T.; Huang, D.-W.; Zhou, C.-Q.; Li, X.; Xie, Q.-J.; Liu, F.-S. Molecular cloning and expression patterns of copper/zinc superoxide dismutase and manganese superoxide dismutase in Musca domestica. Gene 2012, 505, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Lo’ay, A.A.; Taha, N.A.; El-Khateeb, Y.A. Storability of ‘Thompson Seedless’ grapes: Using biopolymer coating chitosan and polyvinyl alcohol blending with salicylic acid and antioxidant enzymes activities during cold storage. Sci. Hortic. 2019, 249, 314–321. [Google Scholar] [CrossRef]
- Zúñiga-Arias, G.; Ruben, R.; van Boekel, M. Managing quality heterogeneity in the mango supply chain: Evidence from Costa Rica. Trends Food Sci. Technol. 2009, 20, 168–179. [Google Scholar] [CrossRef]
- Sudhakar Rao, D.V.; Gopalakrishna Rao, K.P. Controlled atmosphere storage of mango cultivars ‘Alphonso’ and ‘Banganapalli’ to extend storage-life and maintain quality. J. Hortic. Sci. Biotechnol. 2008, 83, 351–359. [Google Scholar] [CrossRef]
- Nakamura, N.; Rao, D.V.S.; Shiina, T.; Nawa, Y. Respiration Properties of Tree-Ripe Mango under CA Condition. Jpn. Agric. Res. Q. JARQ 2004, 38, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Singh, Z.; Singh, R.K.; Sane, V.A.; Nath, P. Mango—Postharvest Biology and Biotechnology. Crit. Rev. Plant Sci. 2013, 32, 217–236. [Google Scholar] [CrossRef]
- Montefiori, M.; Costa, G.; McGhie, T.; Ferguson, A.R. Effects of light and temperature on colour changes in ripening fruit of actinidia macrosperma. Acta Hortic. 2005, 682, 185–190. [Google Scholar] [CrossRef]
- Woolf, A.B.; Wexler, A.; Prusky, D.; Kobiler, E.; Lurie, S. Direct Sunlight Influences Postharvest Temperature Responses and Ripening of Five Avocado Cultivars. J. Am. Soc. Hortic. Sci. 2000, 125, 370–376. [Google Scholar] [CrossRef] [Green Version]
- Prusky, D.; Keen, W.T. Inducible preformed compounds andtheir involvement in the resistance of plant pathogens. In Novel Approaches to Integrated Pest Management; Reuveni, R., Ed.; Lewis Publ. Inc.: Chelsa, MI, USA, 1993; pp. 139–152. [Google Scholar]



| CI Index | CAT | APX | POD | SOD | MDA | PCG | C2H4 | CO2 | EL% | O2.- | H2O2 | DPPH* | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CI Index | * 1.0000 | −0.5837 | −0.5055 | 0.0045 | −0.4578 | 0.9335 | 0.9760 | −0.1403 | 0.0454 | 0.7455 | 0.8132 | 0.8591 | −0.9048 |
| CAT | 1.0000 | 0.8762 | 0.7312 | 0.9587 | −0.6569 | −0.6216 | −0.0676 | −0.0532 | −0.3995 | −0.4844 | −0.4750 | 0.5245 | |
| APX | 1.0000 | 0.7137 | 0.8118 | −0.6062 | −0.5816 | −0.1083 | −0.0964 | −0.3611 | −0.4469 | −0.3470 | 0.4091 | ||
| POD | 1.0000 | 0.7752 | −0.0800 | −0.0430 | −0.2241 | 0.0154 | 0.1769 | 0.0965 | 0.1565 | −0.1157 | |||
| SOD | 1.0000 | −0.5620 | −0.4815 | −0.2003 | −0.0710 | −0.2486 | −0.3476 | −0.3943 | 0.4395 | ||||
| MDA | 1.0000 | 0.9351 | −0.0482 | 0.1194 | 0.7732 | 0.8432 | 0.8927 | −0.9171 | |||||
| PCG | 1.0000 | −0.1398 | 0.0242 | 0.7569 | 0.8149 | 0.8195 | −0.8829 | ||||||
| C2H4 | 1.0000 | 0.3164 | −0.4597 | −0.3990 | −0.1712 | 0.0640 | |||||||
| CO2 | 1.0000 | −0.0778 | −0.0558 | 0.0736 | −0.1278 | ||||||||
| EL% | 1.0000 | 0.9763 | 0.8715 | −0.7805 | |||||||||
| O2.- | 1.0000 | 0.8968 | −0.8247 | ||||||||||
| H2O2 | 1.0000 | −0.9351 | |||||||||||
| DPPH* | 1.0000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo’ay, A.A.; Mostafa, N.A.; Al-Qahtani, S.M.; Al-Harbi, N.A.; Hassan, S.; Abdein, M.A. Influence of the Position of Mango Fruit on the Tree (Mangifera indica L. CV. ‘Zibda’) on Chilling Sensitivity and Antioxidant Enzyme Activity. Horticulturae 2021, 7, 515. https://doi.org/10.3390/horticulturae7120515
Lo’ay AA, Mostafa NA, Al-Qahtani SM, Al-Harbi NA, Hassan S, Abdein MA. Influence of the Position of Mango Fruit on the Tree (Mangifera indica L. CV. ‘Zibda’) on Chilling Sensitivity and Antioxidant Enzyme Activity. Horticulturae. 2021; 7(12):515. https://doi.org/10.3390/horticulturae7120515
Chicago/Turabian StyleLo’ay, A. A., Nada A. Mostafa, Salem Mesfir Al-Qahtani, Nadi Awad Al-Harbi, Sabry Hassan, and Mohamed A. Abdein. 2021. "Influence of the Position of Mango Fruit on the Tree (Mangifera indica L. CV. ‘Zibda’) on Chilling Sensitivity and Antioxidant Enzyme Activity" Horticulturae 7, no. 12: 515. https://doi.org/10.3390/horticulturae7120515
APA StyleLo’ay, A. A., Mostafa, N. A., Al-Qahtani, S. M., Al-Harbi, N. A., Hassan, S., & Abdein, M. A. (2021). Influence of the Position of Mango Fruit on the Tree (Mangifera indica L. CV. ‘Zibda’) on Chilling Sensitivity and Antioxidant Enzyme Activity. Horticulturae, 7(12), 515. https://doi.org/10.3390/horticulturae7120515









