Flowering Biology of Rhododendron pulchrum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Site and Materials
2.2. Observation of the Microstructures of Floral Organs
2.2.1. Scanning Electron Microscope
2.2.2. Paraffin Sectioning
2.3. Observation of Morphological Characteristics of Floral Organs and Opening Dynamics of Individual Flowers
2.4. Opening Dynamics of Individual Plants
2.5. Measurement of Pollen Viability in Different Periods
2.6. Measurement of the Stigma Receptivity
- (a)
- Benzidine and hydrogen peroxide method. Stigma collection took place 1 day before flowering and 1, 2, and 3 days after flowering, at which point the flower was no longer receptive. The collected stigmas were placed on concave glass slides containing a benzidine-hydrogen peroxide solution (1% benzidine/3% hydrogen peroxide/water = 4/11/22). Pollen receptivity was observed under a stereomicroscope (Leica KL300 LED, Germany) and confirmed by the generation of bubbles around the material. Changes in the reaction resolution were recorded. If the receptivity was very strong, a blue reaction liquid also appeared around the material.
- (b)
- Pollen germination observation method after artificial pollination. High viability pollen was used to artificially pollinate the stigmas in different flowering periods. Two days after pollination, the collected stigmas were fixed in Carnoy’s fluid (ethanol/acetic acid = 3/1) for 2 days, placed in 70% ethanol, and stored in a refrigerator. The materials were successively rehydrated with 50% and 30% ethanol and ultrapure water, softened for 8 h with 8 mol/L NaOH solution and kept overnight in ultrapure water; then, they were stained for 3–4 h by adding 0.1% aniline blue solution (0.1% aniline blue and 0.15 mol/L dipotassium phosphate) [23,24]. The materials were pressed into a tablet using standard procedures and observed under a fluorescence microscope (Leica DM3000, Germany) to determine whether the pollen on the stigmas germinated; the results were recorded, and photographs of the findings were taken. The receptivity was determined by the presence or absence of germinated pollen attached to the stigmas and was positively correlated with the amount of germinated pollen attached to the stigmas [25].
2.7. Estimation of the P/O Ratio
2.8. Estimation of OutCrossing Index (OCI)
3. Results
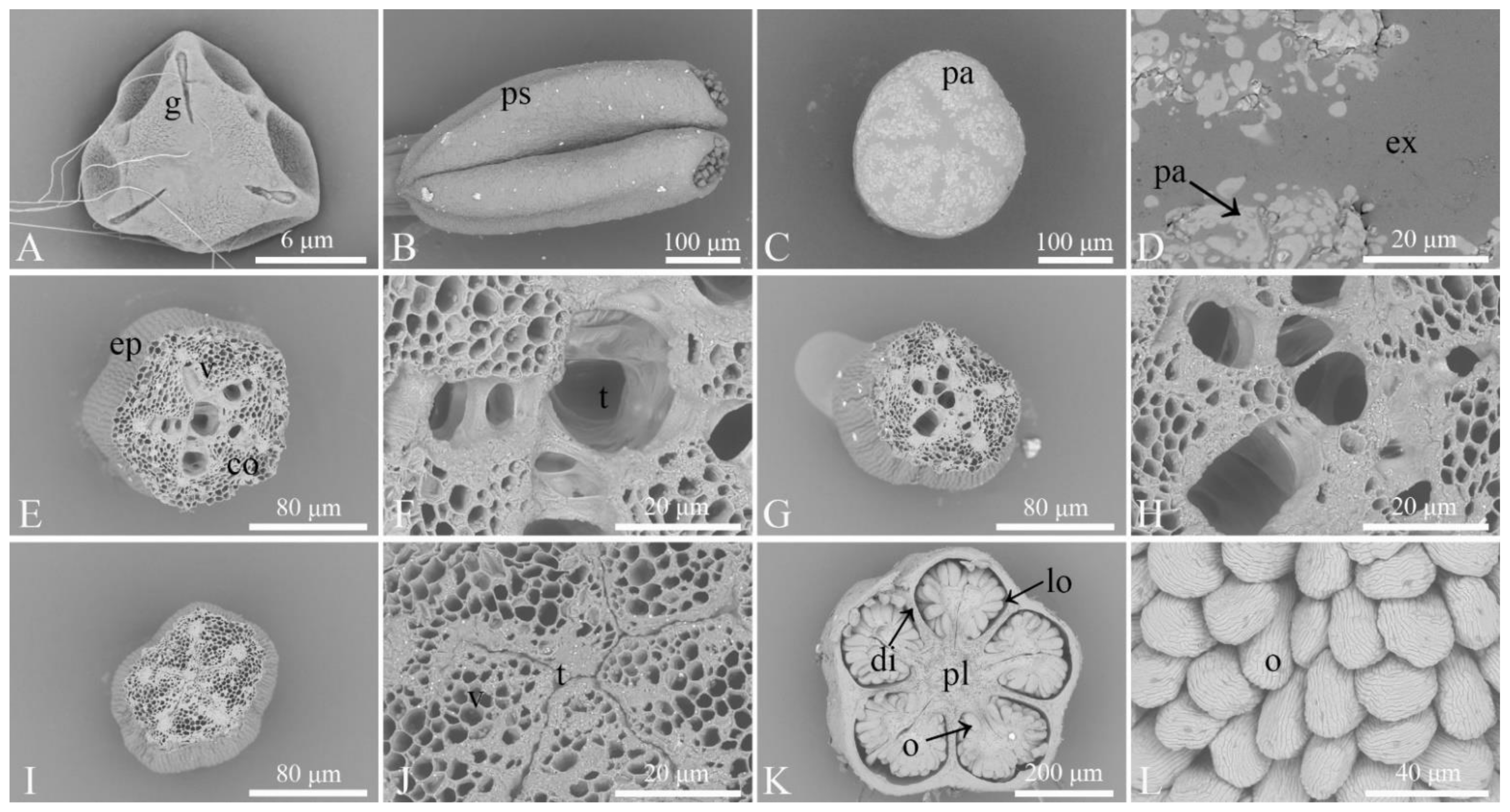
3.1. Observation of the Microstructures of Floral Organs
3.2. Observation of the Morphological Characteristics of Floral Organs and Opening Dynamics of Single Flowers
3.3. Opening Dynamics of Individual Plants
3.4. Measurement of Pollen Vitality in Different Periods
3.5. Detection of Stigma Receptivity
- (1)
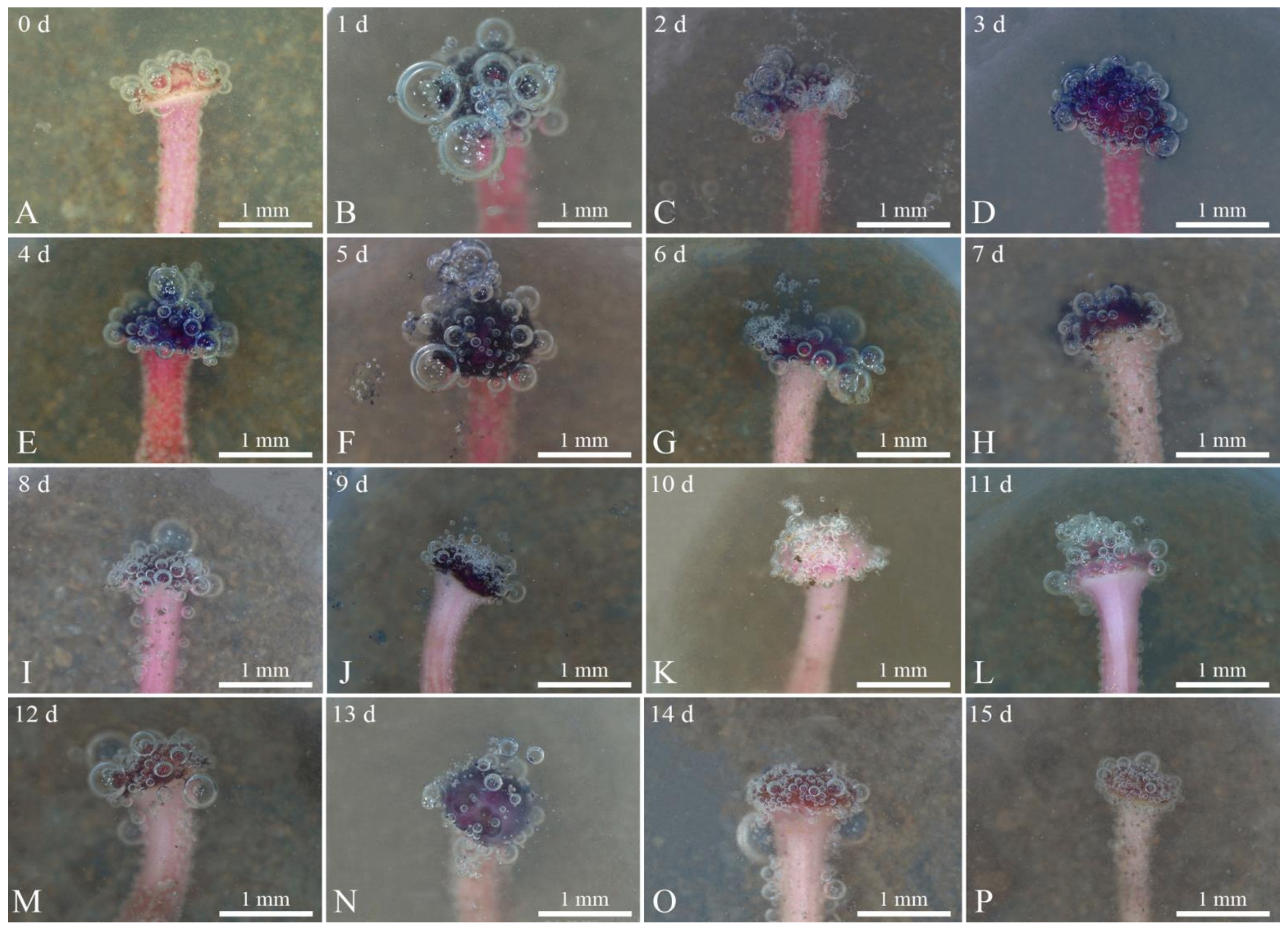
- Benzidine-hydrogen peroxide method (Table 3 and Figure 7). Benzidine-hydrogen peroxide solution was used to test the receptivity of R. pulchrum stigmas. Observation under a stereomicroscope revealed that the reactions of stigmas was not intense on the day before flowering (Figure 7A), with only a few bubbles on the stigma, indicating that receptivity was not strong. After 1–2 days (Figure 7B,C) of flowering, the receptivity gradually increased, and the stigma reaction was the most intense on the third and fourth days (Figure 7D,E) after flowering; at this time, there were numerous bubbles of a large radius, and the blue reaction liquid reached its peak, indicating that receptivity was strongest at this time. There was still a small amount of blue reaction liquid on the fifth to seventh day of flowering (Figure 7F–H), indicating that flowers were still receptive during this period, although the reaction was weaker than on the third and fourth days of flowering. The stigma began to turn black from the 8th to the 15th day (Figure 7I–P), and no blue reaction liquid was observed in this time. During this period, the receptivity further declined, with only minute bubbles, showing basically no receptivity. Therefore, we inferred that the period in which the stigma of R. pulchrum were most receptive was the third to fourth day of flowering.
- (2)
- Pollen germination observation method (Figure 8). After regular artificial pollination, we took images with a fluorescence microscope and found that on the day before flowering (Figure 8A), the pollen was attached to the stigma. Large quantities of germinated pollen grains were found attached to the stigma until the ninth day of flowering (Figure 8B–J). On the 10th day, the number of pollen grains attached to the stigma decreased significantly (Figure 8H). Clearly, the stigma had only weak receptivity at this time. There was basically no pollen germination from the 11th day to the 15th day (Figure 8L–P). The results of the two experimental methods were basically consistent.
3.6. Estimation of the P/O Ratio
3.7. Estimation of the OCI
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitchell, R.J.; Karron, J.D.; Holmquist, K.G.; Bell, J.M. The influence of Mimulus ringens floral display size on pollinator visitation patterns. Func. Ecol. 2004, 18, 116–124. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, H.; Zhou, Q.; Zhao, R.; Sheng, Q.; Zhu, Z. Flowering characteristics and reproductive biology of Nymphaea hybrid, a precious water lily. Sci. Hortic. 2021, 287, 110268. [Google Scholar] [CrossRef]
- Grant, V. Plant Speciation; Columbia University Press: New York, NY, USA; Chichester, UK, 1981. [Google Scholar]
- Xian, C. Study on Reproductive Biology of Lilium Davidii Varunicolor Salish; Shenyang Agricultural University: Shenyang, China, 2019; (In Chinese with English Abstract). [Google Scholar]
- Hirst, P.M. Advances in Understanding Flowering and Pollination in Apple Tree; Achieving Sustainable Cultivation of Apples; Burleigh Dodds Science Publishing: Cambridgeshire, Great Britain, 2017; pp. 109–126. [Google Scholar]
- Christopher, D.A.; Karron, J.D.; Semski, W.R.; Smallwood, P.A.; Trapnell, D.W.; Mitchell, R.J. Selfing rates vary with floral display, pollinator visitation and plant density in natural populations of Mimulus ringens. J. Evol. Biol. 2021, 34, 803–815. [Google Scholar] [CrossRef]
- Tao, R. Reproductive Ecology of Rhododendron Polylepis in Huanxi Sub-Alpine Botanical Garden; Sichuan Agricultural University: Chengdu, China, 2016; (In Chinese with English Abstract). [Google Scholar]
- Bowers, C.G. Rhododendrons and Azaleas: Their Origins, Cultivation and Development; Macmillan Co.: New York, NY, USA, 1960. [Google Scholar]
- Stevenson, J.B. The Species of Rhododendron; Royal Horticultural Society: London, UK, 1947. [Google Scholar]
- Leppik, E.E. Evolutionary interactions between Rhodo-dendrons, pollinating insects and rust fungi. Quart. Bull. Am. Rhod. Soc. 1974, 28, 70–89. [Google Scholar]
- Stevens, P.F. The altitudinal and geographical distribution in flower types in Rhododendron section Vireya, especially in the Papuasian species, and their significance. Bot. J. Linn. Soc. 1976, 72, 1–33. [Google Scholar] [CrossRef]
- Argent, G.C.G. Notes from the Royal Botanic Garden Edinburgh, Vireya rhododendrons in Borneo. Notes R. Bot. Gard. Edinb. 1985, 43, 53–61. [Google Scholar]
- Robinson, T. The Organic Constituents of Higher Plants; Cordus Press: North Amherts, MA, USA, 1991. [Google Scholar]
- Ng, S.C.; Corlett, R.T. Comparative reproductive biology of the six species of Rhododendron (Ericaceae) in Hong Kong, South China. Can. J. Bot. 2000, 78, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Shirasawa, K.; Kobayashi, N.; Nakatsuka, A.; Ohta, H.; Isobe, S. Whole-genome sequencing and analysis of two azaleas, Rhododendron ripense and Rhododendron kiyosumense. DNA Res. 2021, 25, dsab010. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.S.; Li, X.Q.; Zhu, X.T.; Huang, X.L.; Jin, S.H. Complete chloroplast genome of Rhododendron pulchrum, an ornamental medicinal and food tree. Mitochondrial. DNA B 2019, 4, 3527–3528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Li, X.; Zhu, X.; Huang, X.; Jin, S. The complete plastid genome of Rhododendron pulchrum and comparative genetic analysis of Ericaceae Species. Forests 2020, 11, 158. [Google Scholar] [CrossRef] [Green Version]
- Si, G.; Zhang, Y.; Gu, X.; Wang, Y.Q.; Zhao, B. Study on cutting propagation technology of Qinling wild Rhododendron calophytum. North. Hortic. 2012, 3, 7. [Google Scholar]
- Chen, Z.; Yang, X.; Xu, J.; Jiao, B.; Li, Y.; Xu, Y.; Dai, T. First report of Phytopythium helicoides causing crown and root rot on Rhododendron pulchrum in China. Plant Dis. 2021, 105, 713. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, X.; Li, Y.X.; Chen, Z.P.; Dai, T.T. First report of Phytophthora pini causing foliage blight and shoot dieback of Rhododendron Pulchrum in China. Plant Dis. 2021, 105, 1229. [Google Scholar] [CrossRef]
- Yang, L.; Gao, C.; Wei, H.L.; Hu, Y. Screening experiment of pollination combination of four improved Camellia oleifera varieties. Seed 2020, 39, 66–69, 74, (In Chinese with English Abstract). [Google Scholar]
- Gao, C.; Rui, Y.; Guo, Q.Q.; Yuan, D.Y. Microstructure and ultrastructure characteristics of stigma and style of Camellia Oleifera. For. Res. 2019, 32, 1–7. [Google Scholar]
- Burke, J.J. Moisture sensitivity of cotton pollen. Agron. J. 2002, 94, 883–888. [Google Scholar]
- Hirasuka, S.; Zhang, S.L.; Nakagawa, E.; Kawai, Y. Selective inhibition of the growth of incompatible pollen tubes by S-protein in the Japanese pear. Sex Plant Reprod. 2001, 13, 209–215. [Google Scholar] [CrossRef]
- Li, Y.H.; Kang, X.Y. Stigma receptivity and its detection methods of white poplars. Acta Bot. Boreali-Occident. Sin. 2007, 2007, 864–870, (In Chinese with English Abstract). [Google Scholar]
- Cruden, R.W. Pollen-ovule ratios: A conservative indicator of breeding systems in flowering plants. Evolution 1977, 31, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.Y.; Tan, X.F.; Hu, Q.S.; Zou, F. Study on Camellia pollen characteristics and the vitality under different storage conditions. J. Zhejiang For. Sci. Technol. 2008, 5, 66–69, (In Chinese with English Abstract). [Google Scholar]
- Heiduk, A.; Pramanik, D.; Spaans, M.; Gast, L.; Dorst, N.; van Heuven, B.J.; Gravendeel, B. Pitfall flower development and organ identity of Ceropegia sandersonii (Apocynaceae-Asclepiadoideae). Plants 2020, 9, 1767. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.S.; Silva, R.J.; Paulino-Neto, H.F.; Pereira, M.J.B. Beetle pollination and flowering rhythm of Annona coriacea Mart.(Annonaceae) in Brazilian cerrado:Behavioral features of its principal pollinators. PLoS ONE 2017, 12, e0171092. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.L. Research on Reproductive Biology of Rhododendron Excellens Hemsl. et. Wils; Nanjing Forestry University: Nanjing, China, 2011; (In Chinese with English Abstract). [Google Scholar]
- Song, Y.P.; Huang, Z.H.; Huang, S.Q. Pollen aggregation by viscin threads in Rhododendron varies with pollinator. New Phytol. 2019, 221, 1150–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.Q. Reproductive Biology of Rhododendron Moulmainense Hook f. and Its Adaptability of Introduction at Low Altitude Areas; Beijing Forestry University: Beijing, China, 2017; (In Chinese with English Abstract). [Google Scholar]
- Li, Y.; Xu, J.; Shi, L.; Li, F. Examination of pollen germination and vitality of different cultivars of Ziziphus mauritiana in greenhouse. J. Fruit Sci. 2005, 6. Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-GSKK200506030.htm (accessed on 2 November 2021).
- Hufford, K.M.; Hamrick, J.L. Viability selection at three early life stages of the tropical tree, Platypodium elegans (Fabaceae, Papilionoideae). Evolution 2003, 57, 518–526. [Google Scholar] [CrossRef]
- Wada, N.; Uemura, S. Size-dependent flowering behavior and heat production of a sequential hermaphrodite, Symplocarpus renifolius (Araceae). Am. J. Bot. 2000, 87, 1489–1494. [Google Scholar] [CrossRef]
- Wang, X.R.; Ding, G.J. Reproductive biology characteristic of Jatropha curcas (Euphorbiaceae). Rev. De Biol. Trop. 2012, 60, 1525–1533. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Liu, X.; Li, Z.; Ma, H.; Wan, Y.; Liu, X.; Fu, L. Study on reproductive biology of Rhododendron longipedicellatum: A newly discovered and special threatened plant surviving in limestone habitat in Southeast Yunnan, China. Front. Plant Sci 2018, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Barrett, S.C.H. The evolution of mating strategies in flowering plants. Trends Plant Sci. 1998, 3, 335–341. [Google Scholar] [CrossRef]
- Bai, T.; Guan, W.L.; Song, J.; Xie, W.J.; Li, S.F. Flowering characteristics and breeding system of Rhododendron siderophyllum. J. West China For. Sci. 2014, 43, 47–53. [Google Scholar]
- Webb, C.J.; Lloyd, D.G. The avoidance of interference between the presentation of pollen and stigmas in angiosperms II. Herkogamy. N. Z. J. Bot. 1986, 24, 163–178. [Google Scholar] [CrossRef]
- Torres-Díaz, C.; Gomez-Gonzalez, S.; Stotz, G.C.; Torres-Morales, P.; Paredes, B.; Pérez-Millaqueo, M.; Gianoli, E. Extremely long-lived stigmas allow extended cross-pollination opportunities in a high Andean plant. PLoS ONE 2011, 6, e19497. [Google Scholar] [CrossRef] [PubMed]
- Rambuda, T.D.; Johnson, S.D. Breeding systems of invasive alien plants in South Africa: Does Baker’s rule apply? Divers. Distrib. 2004, 10, 409–416. [Google Scholar] [CrossRef]






| Measurement Parameter | Minimum (mm) | Maximum (mm) | Mean (mm) | SD | Number of Samples (Flowers) |
|---|---|---|---|---|---|
| Filament length | 36.46 | 57.67 | 47.22 | 5.02 | 30 |
| Filament width | 0.06 | 0.48 | 0.27 | 0.12 | 30 |
| Style length | 44.97 | 64.23 | 53.55 | 4.71 | 30 |
| Style width | 0.28 | 0.78 | 0.47 | 0.11 | 30 |
| Sepal length | 6.39 | 19.28 | 12.79 | 3.53 | 30 |
| Sepal width | 2.29 | 5.09 | 3.83 | 0.58 | 30 |
| Pedicel length | 9.75 | 17.6 | 14.82 | 1.87 | 30 |
| Pedicel width | 1.28 | 1.99 | 1.69 | 0.17 | 30 |
| Maximum diameter of the open flower | 61.37 | 71.98 | 67.63 | 2.70 | 30 |
| Minimum diameter of the open flower | 56.94 | 72.30 | 65.62 | 3.88 | 30 |
| Flowering Period | SN | Number of Stained Pollen Tetrads | Total Number of Pollen Tetrads | Proportion of the Stained Pollen Tetrads | Mean |
|---|---|---|---|---|---|
| Initial flowering period | 1 | 94 | 103 | 91.26% | 88.98% |
| 2 | 54 | 61 | 88.52% | ||
| 3 | 95 | 109 | 87.16% | ||
| Blooming period | 1 | 89 | 106 | 83.96% | 77.93% |
| 2 | 80 | 108 | 74.07% | ||
| 3 | 100 | 132 | 75.76% | ||
| End of flowering period | 1 | 24 | 104 | 23.08% | 17.62% |
| 2 | 10 | 78 | 12.82% | ||
| 3 | 20 | 118 | 16.95% |
| Time (Day of Flowering) | Number of Bubbles | Mucus Secretion | Receptivity |
|---|---|---|---|
| The day before flowering | + | + | + |
| 1st | ++ | ++ | ++ |
| 2nd | ++ | ++ | ++ |
| 3rd | +++ | +++ | +++ |
| 4th | +++ | +++ | +++ |
| 5th | +++ | ++ | ++ |
| 6th | ++ | ++ | ++ |
| 7th | + | + | + |
| 8th | + | + | + |
| 9th | + | + | + |
| 10th | + | - | +/- |
| 11th | + | - | +/- |
| 12th | + | - | +/- |
| 13th | - | - | - |
| 14th | - | - | - |
| 15th | - | - | - |
| Observed Factor | Min | Max | Mean | SD |
|---|---|---|---|---|
| Number of pollen tatrads per flower | 499,600.00 | 572,200.00 | 532,400.00 | 22,593.93 |
| Ovule number per flower | 1584.00 | 1902.00 | 1768.87 | 92.64 |
| P/O ratio | 266.00 | 328.00 | 301.47 | 18.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, J.; Gao, C.; Wei, H.; Wang, B.; Hu, Y.; Guo, Z.; Long, L.; Yang, L.; Li, H. Flowering Biology of Rhododendron pulchrum. Horticulturae 2021, 7, 508. https://doi.org/10.3390/horticulturae7110508
Qiu J, Gao C, Wei H, Wang B, Hu Y, Guo Z, Long L, Yang L, Li H. Flowering Biology of Rhododendron pulchrum. Horticulturae. 2021; 7(11):508. https://doi.org/10.3390/horticulturae7110508
Chicago/Turabian StyleQiu, Jie, Chao Gao, Hongli Wei, Biao Wang, Yang Hu, Zhiyan Guo, Li Long, Lu Yang, and Huie Li. 2021. "Flowering Biology of Rhododendron pulchrum" Horticulturae 7, no. 11: 508. https://doi.org/10.3390/horticulturae7110508
APA StyleQiu, J., Gao, C., Wei, H., Wang, B., Hu, Y., Guo, Z., Long, L., Yang, L., & Li, H. (2021). Flowering Biology of Rhododendron pulchrum. Horticulturae, 7(11), 508. https://doi.org/10.3390/horticulturae7110508






