Effects of C/N Ratio on Lignocellulose Degradation and Enzyme Activities in Aerobic Composting
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Composting Treatments
2.3. Test Sample Collection
2.4. Determination Index and Method
2.5. Calculation Method of Lignocellulose Degradation Rate
2.6. Data Processing
3. Results
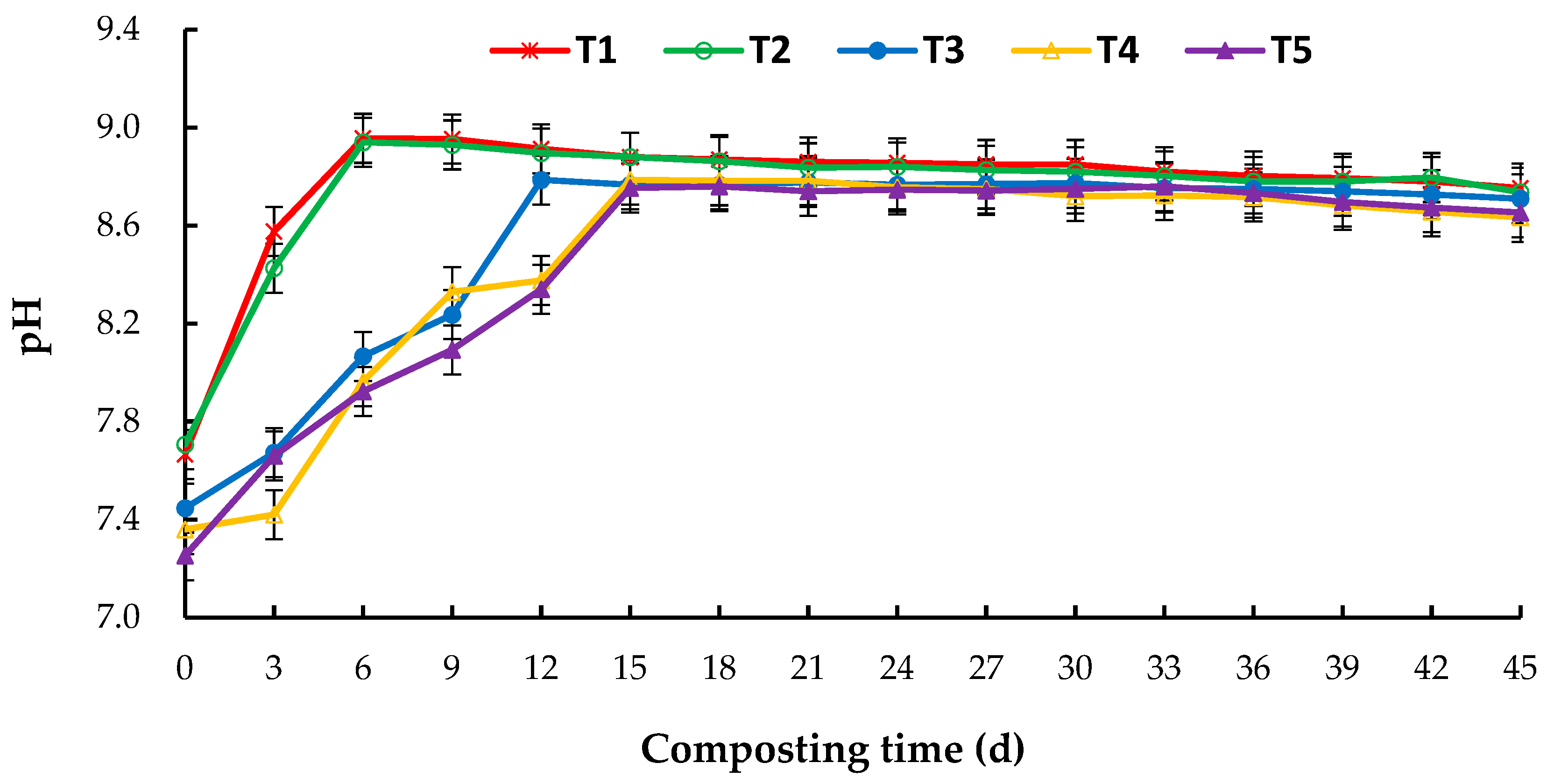
3.1. Changes in Temperature and pH during Aerobic Composting
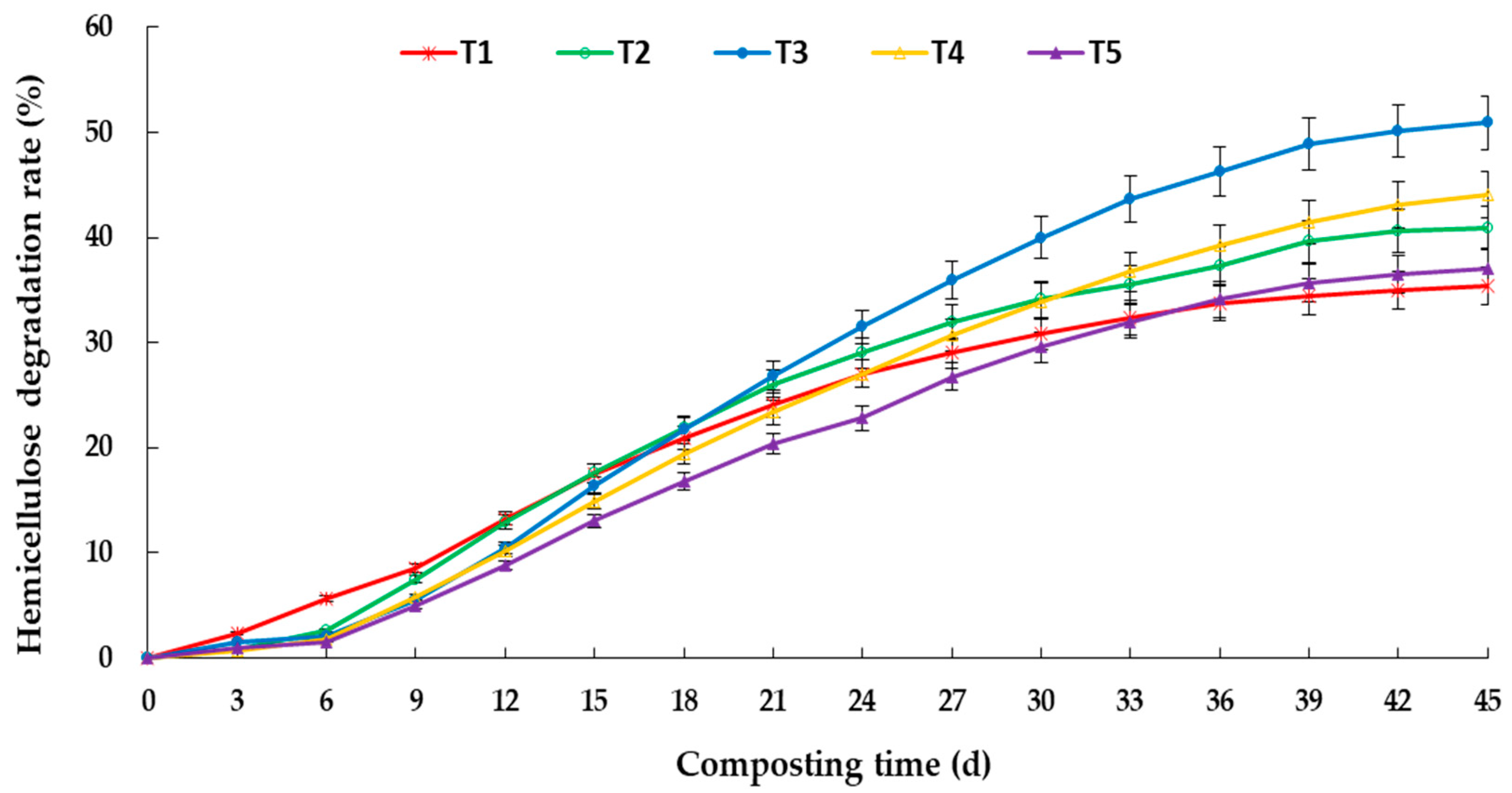
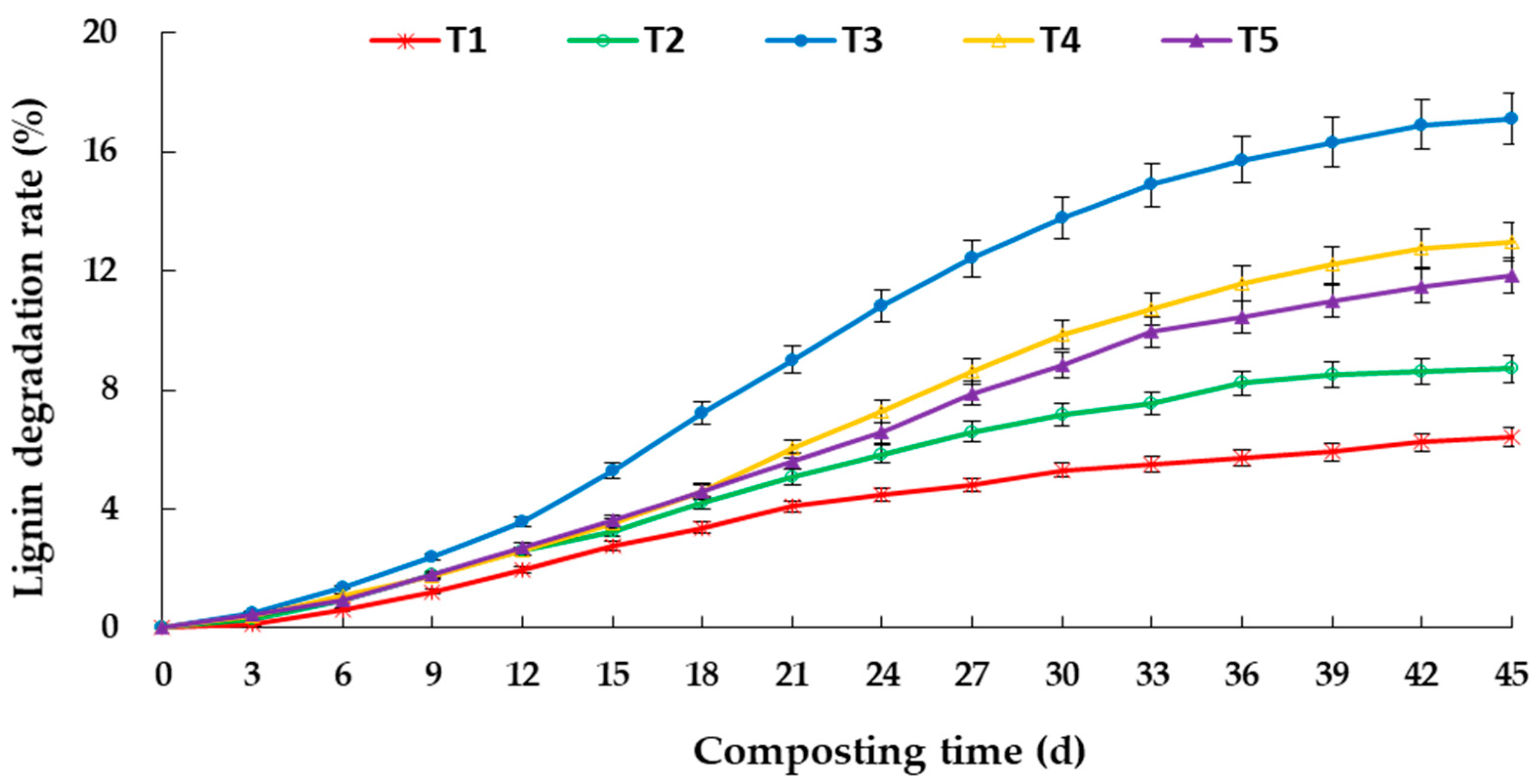
3.2. Degradation of Lignocellulose during Aerobic Composting
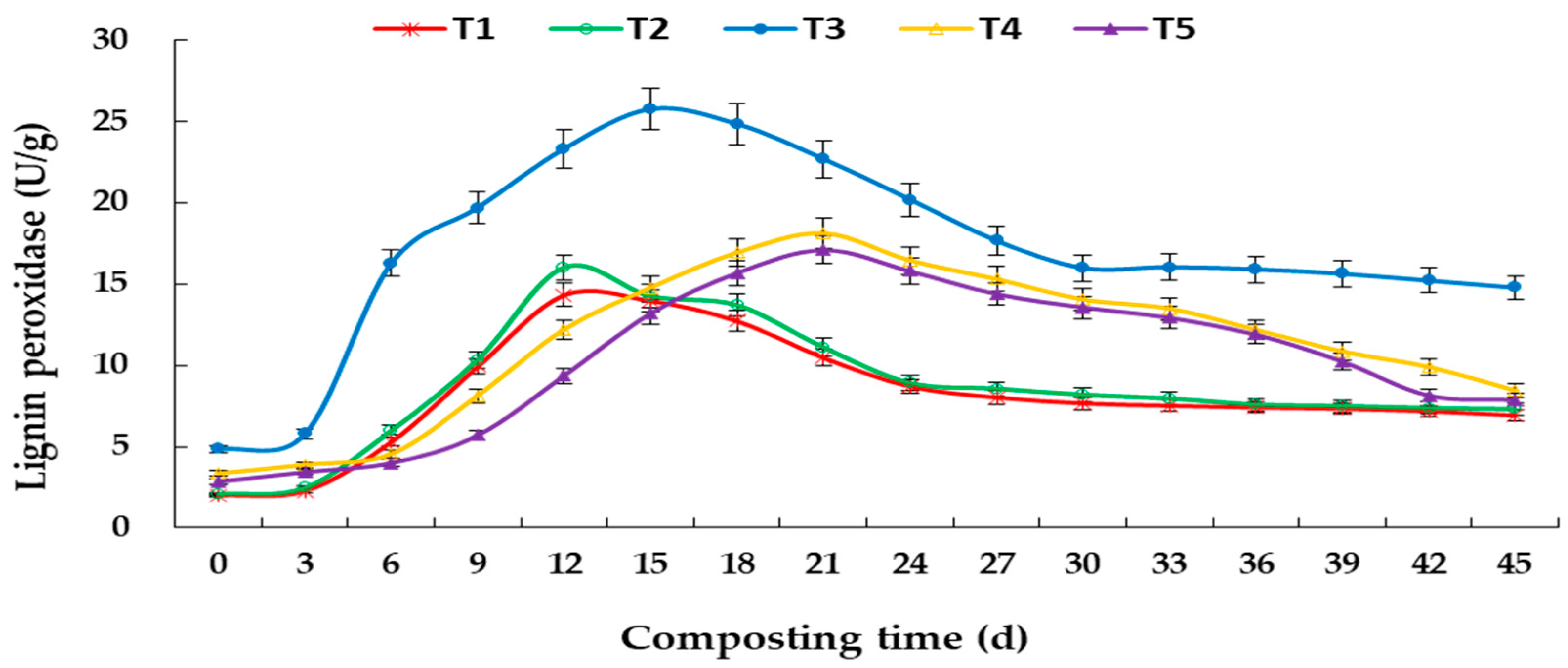
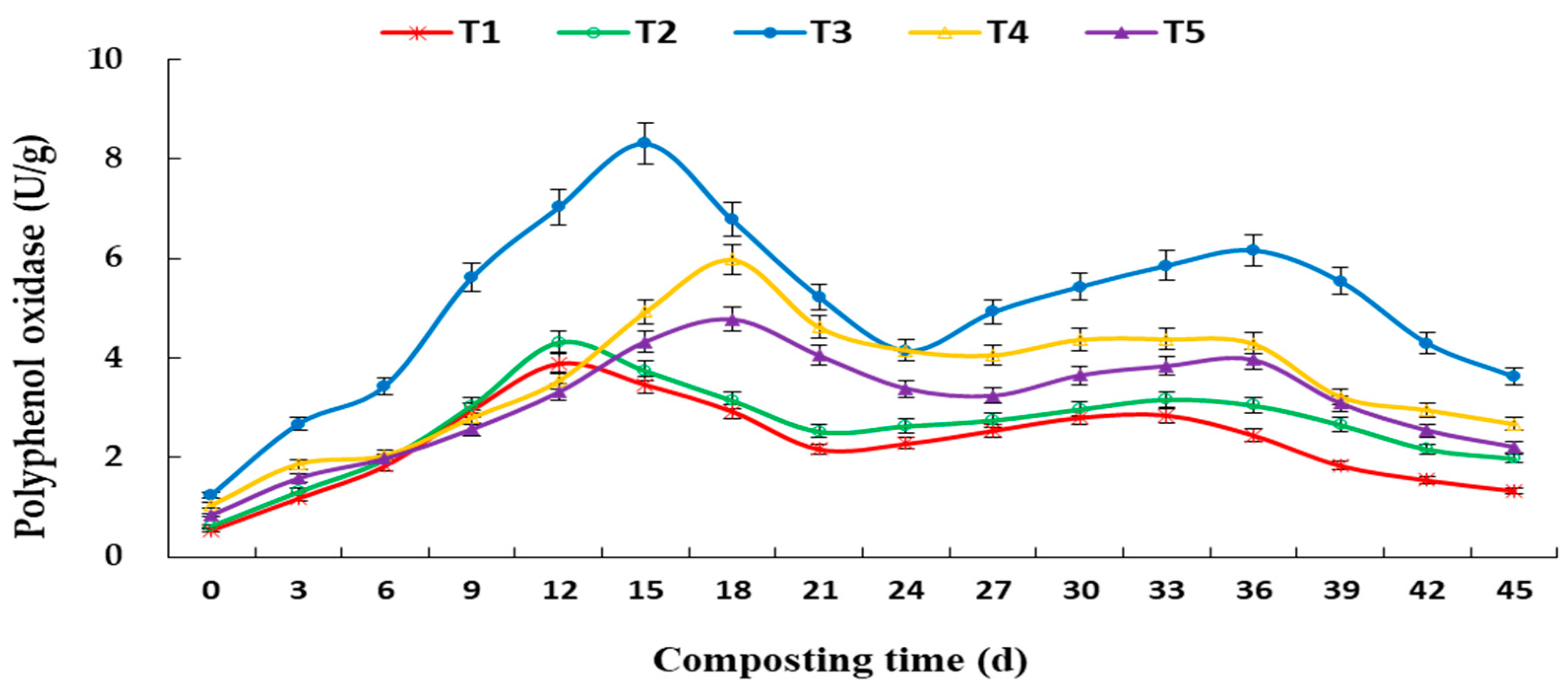
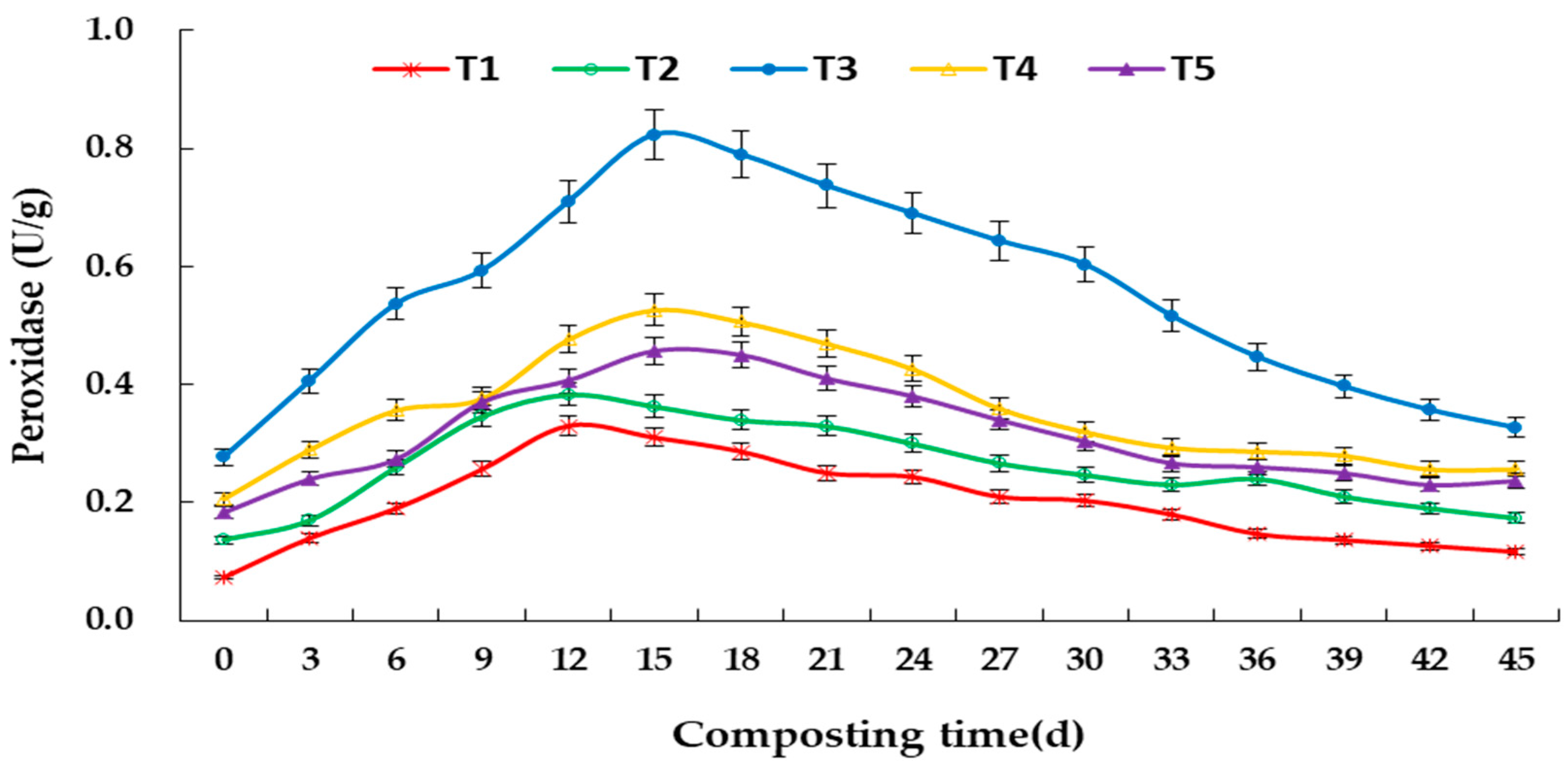
3.3. Changes in Enzyme Activity during Aerobic Composting
3.4. Correlation Analysis
3.4.1. Correlation Analysis between Enzyme Activity and Lignocellulose Degradation
3.4.2. Correlation Analysis between Enzyme Activity, Temperature and pH
4. Discussion
4.1. Effect of C/N Ratio on Temperature and pH in Aerobic Composting
4.2. Effects of C/N Ratio on Degradation of Lignocellulose and Enzyme Activity in Aerobic Composting
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, X.; Zhou, H.; Meng, H.; Ding, J.; Shen, Y.; Cheng, H.; Zhang, X.; Li, R.; Fan, S. Amino acid profile characterization during the co-composting of a livestock manure and maize straw mixture. J. Clean. Prod. 2021, 278, 123494. [Google Scholar] [CrossRef]
- Medina, J.; Monreal, C.M.; Antilen, M.; Calabi-Floody, M.; Velasco-Molina, M.; Meier, S.; Borie, F.; Cornejo, P.; Knicker, H. Influence of inorganic additives on wheat straw composting: Characterization and structural composition of organic matter derived from the process. J. Environ. Manag. 2020, 260, 110137. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Q.; Tang, Z.; Zhang, W.; Yu, G.; Shen, Q.; Zhao, F.-J. Heavy metal concentrations and arsenic speciation in animal manure composts in China. Waste Manag. 2017, 64, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.P.; Alburquerque, J.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef] [PubMed]
- Ayilara, M.S.; Olanrewaju, O.S.; Babalola, O.O.; Odeyemi, O. Waste management through composting: Challenges and potentials. Sustainability 2020, 12, 4456. [Google Scholar] [CrossRef]
- Bi, S.; Hong, X.; Yang, H.; Yu, X.; Fang, S.; Bai, Y.; Liu, J.; Gao, Y.; Yan, L.; Wang, W.; et al. Effect of hydraulic retention time on anaerobic co-digestion of cattle manure and food waste. Renew. Energy 2019, 150, 213–220. [Google Scholar] [CrossRef]
- Ghanney, P.; Kugbe, J.X.; Anning, D.K. Role of Microbial Biomechanics in Composting with Special Reference to Lignocellulose Biomass Digestion. Asian J. Biotechnol. Bioresour. Technol. 2021, 7, 30–46. [Google Scholar] [CrossRef]
- Lu, Q.; Zhao, Y.; Gao, X.; Wu, J.; Zhou, H.; Tang, P.; Wei, Q.; Wei, Z. Effect of tricarboxylic acid cycle regulator on carbon retention and organic component transformation during food waste composting. Bioresour. Technol. 2018, 256, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Xu, X.H.; Wang, J.; Liu, J. Effect of inoculating lignin degradation strains on enzymic activities in composting. J. Agro-Environ. Sci. 2012, 31, 843–847. [Google Scholar]
- Wan, W.; Wang, Y.; Tan, J.; Qin, Y.; Zuo, W.; Wu, H.; He, H.; He, D. Alkaline phosphatase—Harboring bacterial community and multiple enzyme activity contribute to phosphorus transformation during vegetable waste and chicken manure composting. Bioresour. Technol. 2020, 297, 122406. [Google Scholar] [CrossRef]
- Huang, G.F.; Wong, J.W.C.; Wu, Q.T.; Nagar, B.B. Effect of C/N on composting of pig manure with sawdust. Waste Manag. 2004, 24, 805–813. [Google Scholar] [CrossRef]
- Iqbal, M.K.; Nadeem, A.; Sherazi, F.; Khan, R.A. Optimization of process parameters for kitchen waste composting by response surface methodology. Int. J. Environ. Sci. Technol. 2015, 12, 1759–1768. [Google Scholar] [CrossRef]
- Yang, Y.; Du, W.; Ren, X.; Cui, Z.; Zhou, W.; Lv, J. Effect of bean dregs amendment on the organic matter degradation, humification, maturity and stability of pig manure composting. Sci. Total Environ. 2020, 708, 134623. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Xiaofen, W.; Wanbin, Z.; Hongyan, Y.; Cheng, X.; Zongjun, C. Degradation of corn stalk by the composite microbial system of MC1. J. Environ. Sci. 2008, 20, 109–114. [Google Scholar]
- Zhao, M.; Zeng, G.; Huang, D.; Feng, C.; Huang, C.; Hu, S.; Su, F.; Lai, C.; Wei, Z. Research on sorption and transport characteristics of ligninolytic enzymes in different compost substances. Environ. Sci. 2010, 31, 1647–1654. [Google Scholar]
- Pickard, M.; Roman, R.; Tinoco, R.; Vazquez-Duhalt, R. Polycyclic aromatic hydrocarbon metabolism by white rot fungi and oxidation by coriolopsis gallica UAMH 8260 Laccase. Appl. Environ. Microbiol. 1999, 65, 3805–3809. [Google Scholar] [CrossRef]
- Aquino-Bolaños, E.N.; Mercado-Silva, E. Effects of polyphenol oxidase and peroxidase activity, phenolics and lignin content on the browning of cut jicama. Postharvest Biol. Technol. 2004, 33, 275–283. [Google Scholar] [CrossRef]
- Yu, H.; Zeng, G.; Huang, H.; Xi, X.; Wang, R.; Huang, D.; Huang, G.; Li, J. Microbial community succession and lignocellulose degradation during agricultural waste composting. Biodegradation 2007, 18, 793–802. [Google Scholar] [CrossRef]
- Cárdenas-González, B.; Ergas, S.J.; Switzenbaum, M.S. Characterization of compost biofiltration media. J. Air Waste Manag. Assoc. 1999, 49, 784–793. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.; Sun, X. Influence of bulking agents on physical, chemical, and microbiological properties during the two-stage composting of green waste. Waste Manag. 2016, 48, 115–126. [Google Scholar] [CrossRef]
- Ren, L.M.; Schuchardt, F.; Shen, Y.J.; Li, G.X.; Li, C.P. Impact of struvite crystallization on nitrogen losses during composting of pig manure and cornstalk. Waste Manag. 2010, 30, 885–892. [Google Scholar] [CrossRef]
- Wang, Q.; Awasthi, M.K.; Ren, X.; Zhao, J.; Li, R.; Wang, Z.; Chen, H.; Wang, M.; Zhang, Z. Comparison of biochar, zeolite and their mixture amendment for aiding organic matter transformation and nitrogen conservation during pig manure composting. Bioresour. Technol. 2017, 245, 300–308. [Google Scholar] [CrossRef]
- Hua, D.; Liu, F.; Li, G.; Jiang, T. Effect of turning and covering techniques on pig manure—Straw composting property. Trans. Chin. Soc. Agric. Eng. 2011, 27, 210–216. [Google Scholar]
- Wang, L.; Li, Y.; Prasher, S.O.; Yan, B.; Ou, Y.; Cui, H.; Cui, Y. Organic matter, a critical factor to immobilize phosphorus, copper, and zinc during composting under various initial C/N ratios. Bioresour. Technol. 2019, 289, 121745. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, H.; Zhang, J.; Chen, Y.; Zeng, G.; Yuan, Y.; Cao, W.; Fang, W.; Hou, K.; Wang, B.; et al. Influence of FeONPs amendment on nitrogen conservation and microbial community succession during composting of agricultural waste: Relative contributions of ammonia-oxidizing bacteria and archaea to nitrogen conservation. Bioresour. Technol. 2019, 287, 121463. [Google Scholar] [CrossRef]
- Dashtban, M.; Schraft, H.; Qin, W. Fungal bioconversion of lignocellulosic residues; opportunities & perspectives. Int. J. Biol. Sci. 2009, 5, 578–595. [Google Scholar]
- Deng, H.; Wang, C.; Lu, H.; Wang, F.; Tu, Q.; Wu, W. Research progress in succession of actinomycetal communities and their capacity of degrading lignocellulose during composting process. Chin. J. Appl. Environ. Biol. 2013, 19, 581–586. [Google Scholar] [CrossRef]
- Xi, B.D.; Liu, H.L.; Bai, Q.Z.; Huang, G.H.; Zeng, G.M.; Li, Y.J. Study on current status of lignin and cellulose biodegradation in composting process. Technol. Equip. Environ. Pol. Cont. 2002, 3, 19–23. [Google Scholar]
- Eiland, F.; Klamer, M.; Lind, A.M.; Leth, M.; Bååth, E. Influence of initial C/N ratio on chemical and microbial composition during long term composting of straw. Microb. Ecol. 2001, 41, 272–280. [Google Scholar] [CrossRef]
- Barrington, S.F.; El Moueddeb, K.; Porter, B. Improving small—Scale composting of apple waste. Canad. Agric. Eng. 1997, 39, 9–16. [Google Scholar]
- Mathur, S.P. Composting processes. In Bioconversion of Waste Materials to Industrial Products, 1st ed.; Martin, A.M., Ed.; Springer Publishing: New York, NY, USA, 1991; pp. 147–183. [Google Scholar]
- Kumar, M.; Ou, Y.L.; Lin, J.G. Co-composting of green waste and food waste at low C/N ratio. Waste Manag. 2010, 30, 602–609. [Google Scholar] [CrossRef]
- Pedersen, M.; Meyer, A.S. Lignocellulose pretreatment severity—Relating pH to biomatrix opening. New Biotechnol. 2010, 27, 739–750. [Google Scholar] [CrossRef]
- Shi, M.; Zhao, Y.; Zhu, L.; Song, X.; Tang, Y.; Qi, H.; Cao, H.; Wei, Z. Denitrification during composting: Biochemistry, implication and perspective. Int. Biodeterior. Biodegrad. 2020, 153, 105043. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z. Three important enzymes for lignin degradation. J. Biol. 2003, 20, 9–11. [Google Scholar]
- Reddy, C.A. An overview of the recent advances on the physiology and molecular biology of lignin peroxidases of Phanerochaete chrysosporium. J. Biotechnol. 1993, 30, 91–107. [Google Scholar] [CrossRef]



| Raw Material | Moisture Content (%) | Total Organic Carbon (%) | Total Nitrogen (%) | Cellulose (%) | Hemicellulose (%) | Lignin (%) | C/N Ratio |
|---|---|---|---|---|---|---|---|
| Cow dung | 66.57 | 44.26 | 2.44 | 22.98 | 21.37 | 9.88 | 18.14 |
| Corn straw | 4.28 | 49.76 | 1.01 | 31.43 | 26.65 | 15.3 | 49.27 |
| Treatment | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|
| Cow dung (kg) | 1000 | 1000 | 1000 | 1000 | 1000 |
| Corn straw (kg) | 3.70 | 53.29 | 233.5 | 512.4 | 979.6 |
| C/N ratio | 15 * | 20 | 25 | 30 | 35 |
| Cellulose | Hemicellulose | Lignin | |
|---|---|---|---|
| Lignin peroxidase (LIP) | 0.442 ** | 0.288 ** | 0.403 ** |
| Polyphenol oxidase (PPO) | 0.436 ** | 0.293 ** | 0.432 ** |
| Manganese peroxidase (MNP) | 0.290 | 0.111 | 0.219 ** |
| Peroxidase (POD | 0.122 | −0.036 | 0.163 |
| Lignin Peroxidase (LIP) | Polyphenol Oxidase (PPO) | Manganese Peroxidase (MNP) | Peroxidase (POD) | |
|---|---|---|---|---|
| Lignin peroxidase (LIP) | – | |||
| Polyphenol oxidase (PPO) | 0.916 ** | – | ||
| Manganese peroxidase (MNP) | 0.902 ** | 0.856 ** | – | |
| Peroxidase (POD) | 0.850 ** | 0.843 ** | 0.855 ** | – |
| Temperature (T) | 0.282 * | 0.272 * | 0.472 ** | 0.524 ** |
| pH | 0.486 ** | 0.435 ** | 0.450 ** | 0.147 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Zhang, H.; Qiu, H.; Anning, D.K.; Li, M.; Wang, Y.; Zhang, C. Effects of C/N Ratio on Lignocellulose Degradation and Enzyme Activities in Aerobic Composting. Horticulturae 2021, 7, 482. https://doi.org/10.3390/horticulturae7110482
Yang H, Zhang H, Qiu H, Anning DK, Li M, Wang Y, Zhang C. Effects of C/N Ratio on Lignocellulose Degradation and Enzyme Activities in Aerobic Composting. Horticulturae. 2021; 7(11):482. https://doi.org/10.3390/horticulturae7110482
Chicago/Turabian StyleYang, Huizhen, He Zhang, Huizhen Qiu, Dominic Kwadwo Anning, Mengchan Li, Youling Wang, and Chunhong Zhang. 2021. "Effects of C/N Ratio on Lignocellulose Degradation and Enzyme Activities in Aerobic Composting" Horticulturae 7, no. 11: 482. https://doi.org/10.3390/horticulturae7110482
APA StyleYang, H., Zhang, H., Qiu, H., Anning, D. K., Li, M., Wang, Y., & Zhang, C. (2021). Effects of C/N Ratio on Lignocellulose Degradation and Enzyme Activities in Aerobic Composting. Horticulturae, 7(11), 482. https://doi.org/10.3390/horticulturae7110482






