Genotoxicity and Cytotoxicity Induced in Zygophyllum fabago by Low Pb Doses Depends on the Population’s Redox Plasticity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Culture and Exposure to Pb
2.2. Photosynthetic Pigment Quantification
2.3. Pb Quantification in Tissues
2.4. DNA Fragmentation
2.5. Flow Cytometric Analysis
2.6. Quantification of Total Antioxidant Activity and Phenolic Compounds
2.7. Determination of Ascorbate, Dehydroascorbate, Glutathione and Total Soluble Non-Protein Thiols
2.8. Determination of Hydrogen Peroxide, Lipid Peroxidation and Protein Oxidation
2.9. Statistical Analysis
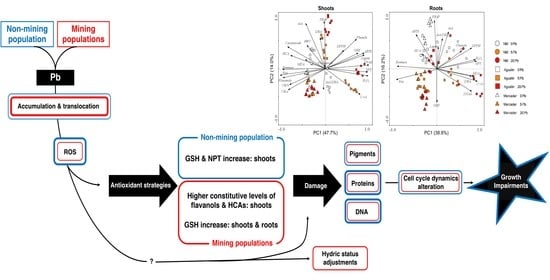
3. Results
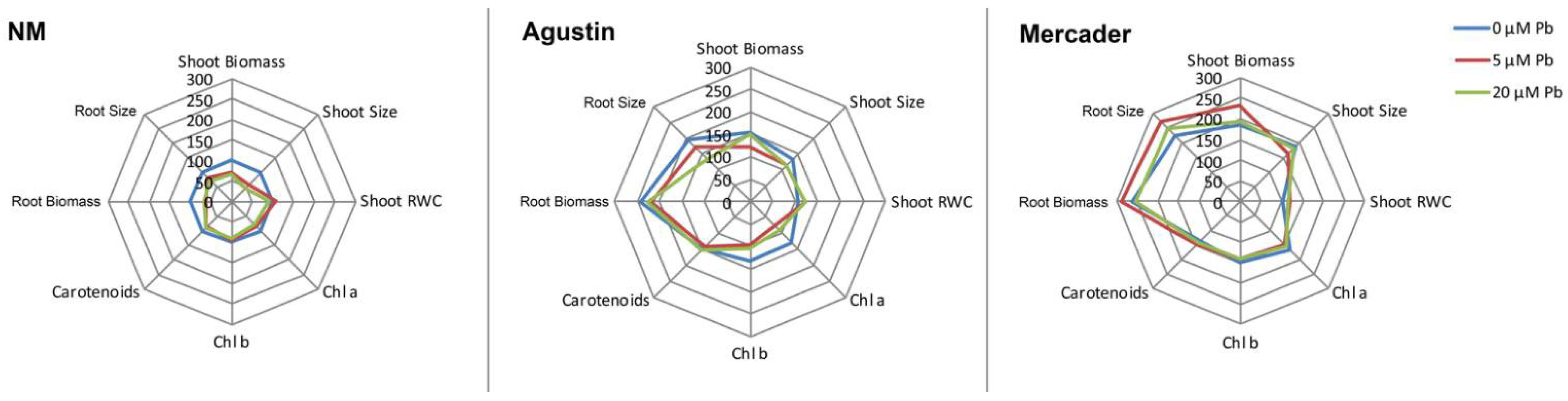
3.1. Comparative Effects of Low Pb Doses on Germination, Seedling Growth and Photosynthetic Pigments
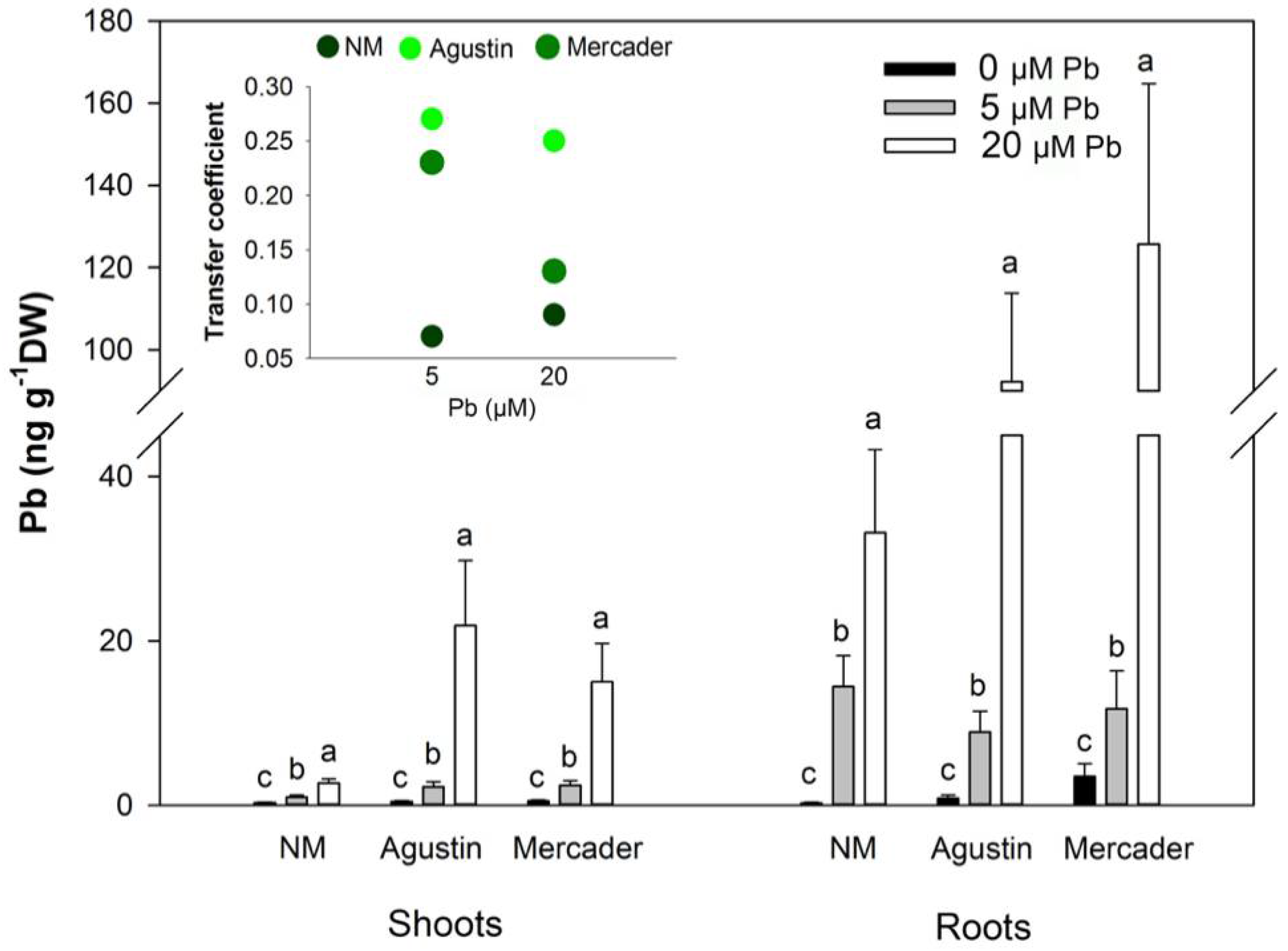
3.2. Pb Content in Shoots and Roots
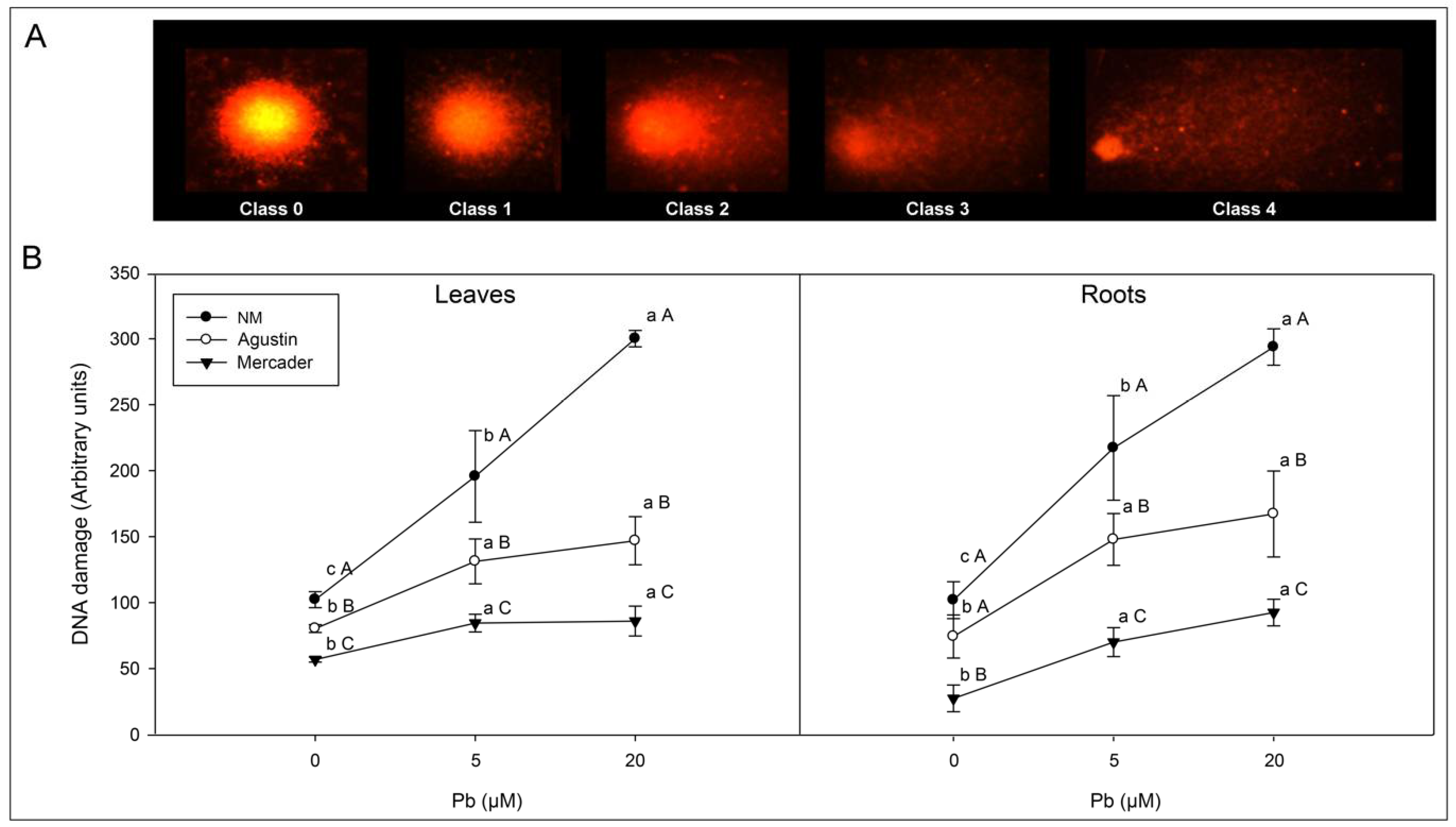
3.3. Genotoxic Effects Induced by Low Pb Doses
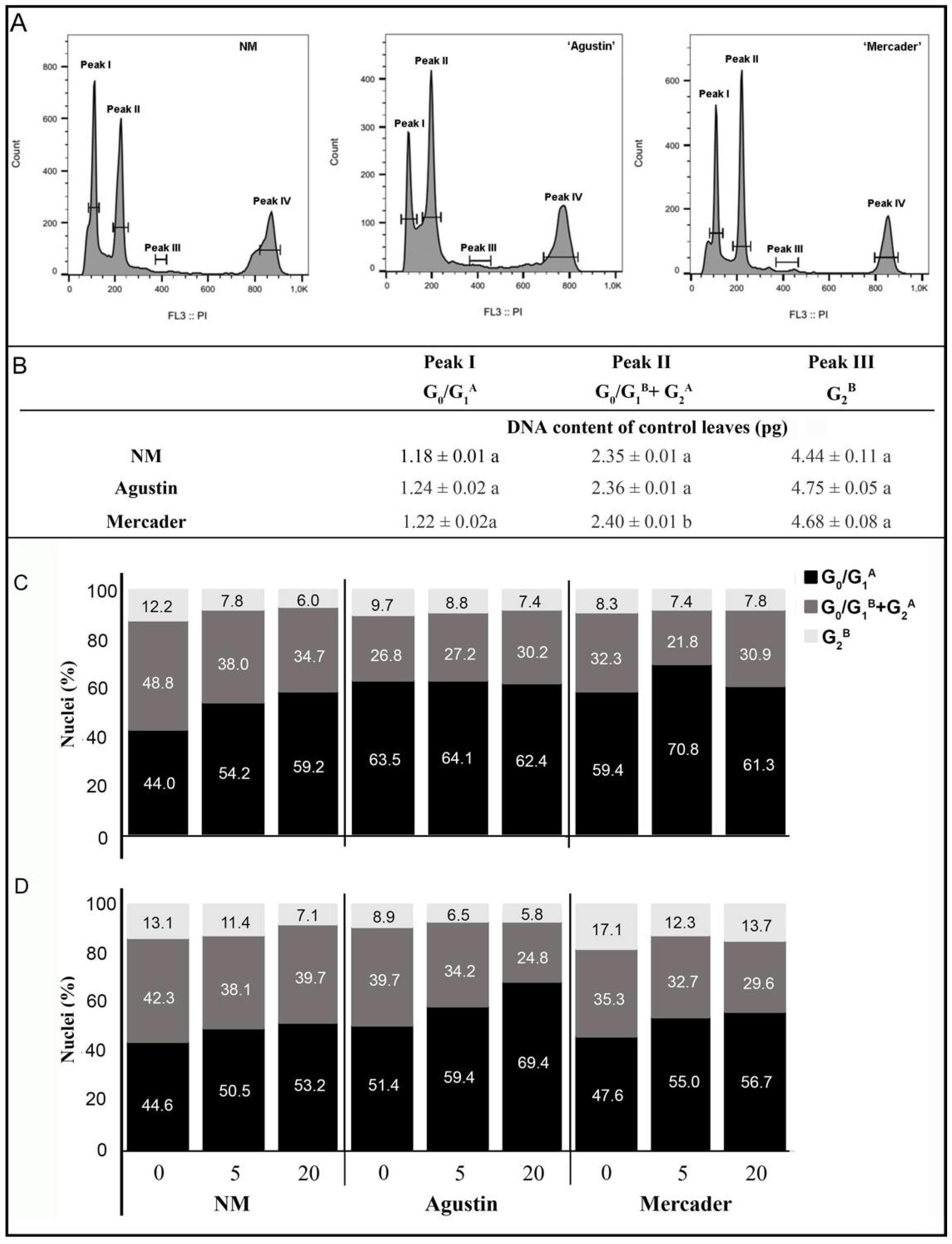
3.4. Cell Cycle Modulation by Low Doses of Pb
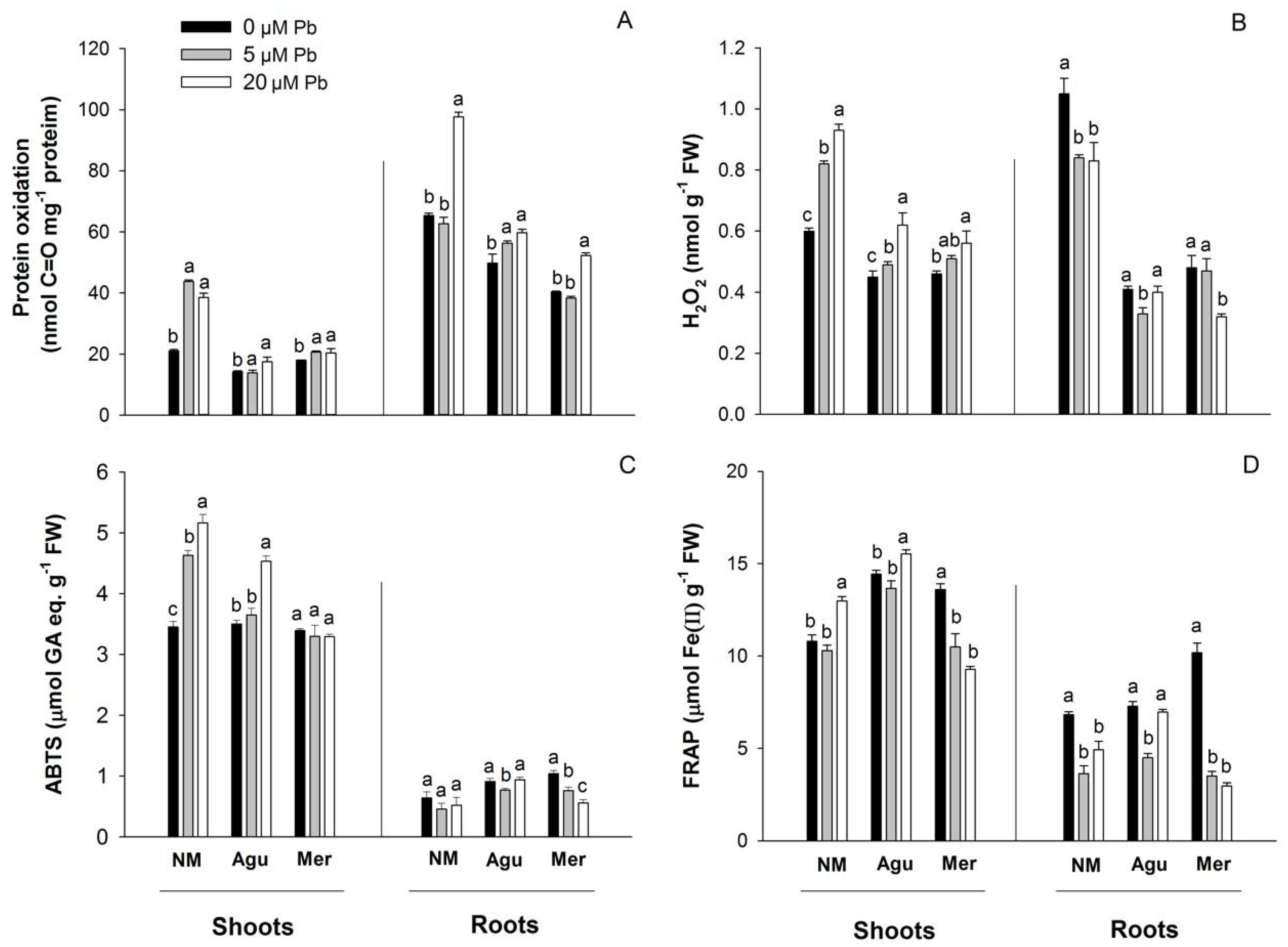
3.5. Redox Status Modulation by Low Doses of Pb
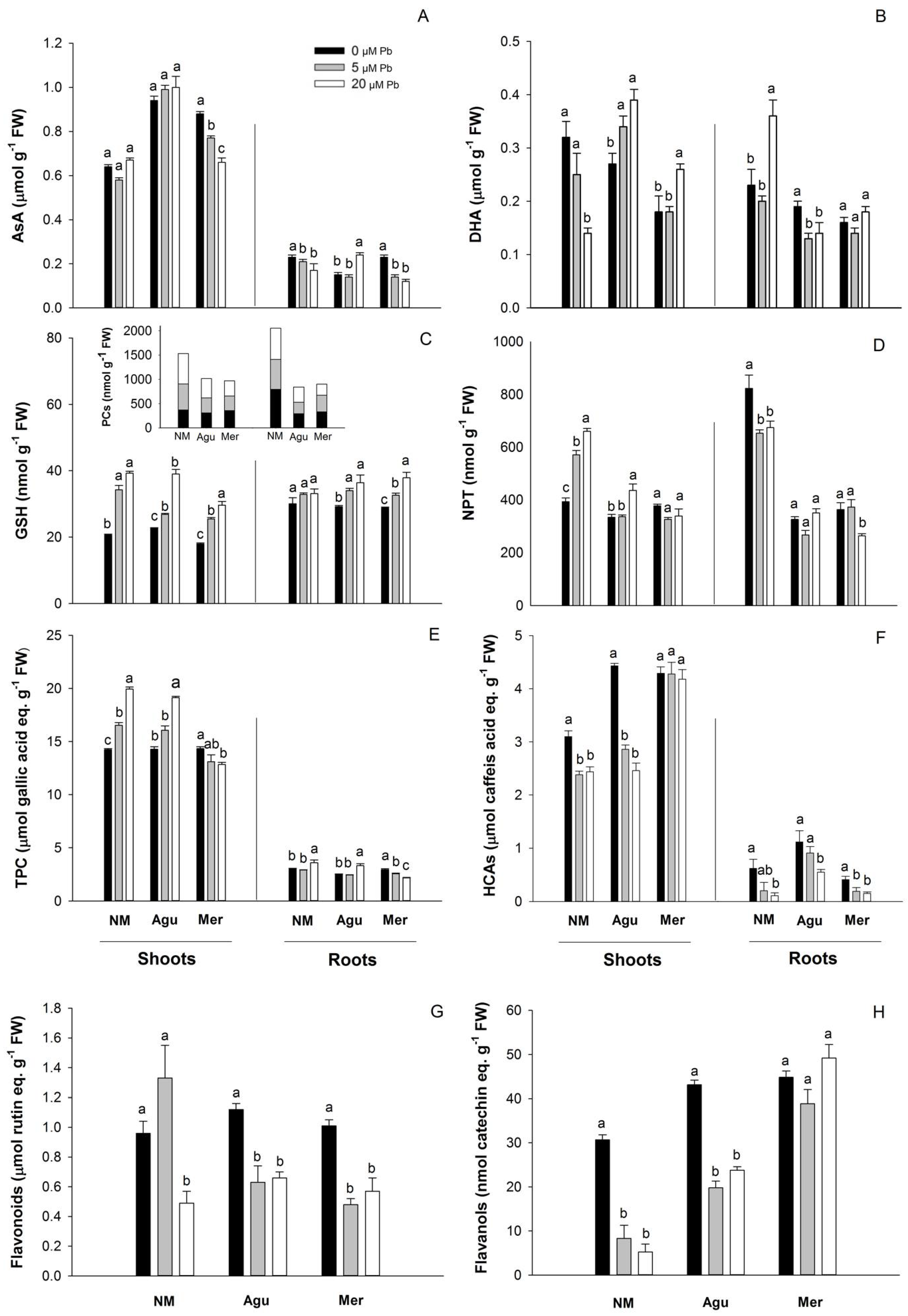
3.5.1. Ascorbate and Thiols
3.5.2. Phenolic Compounds
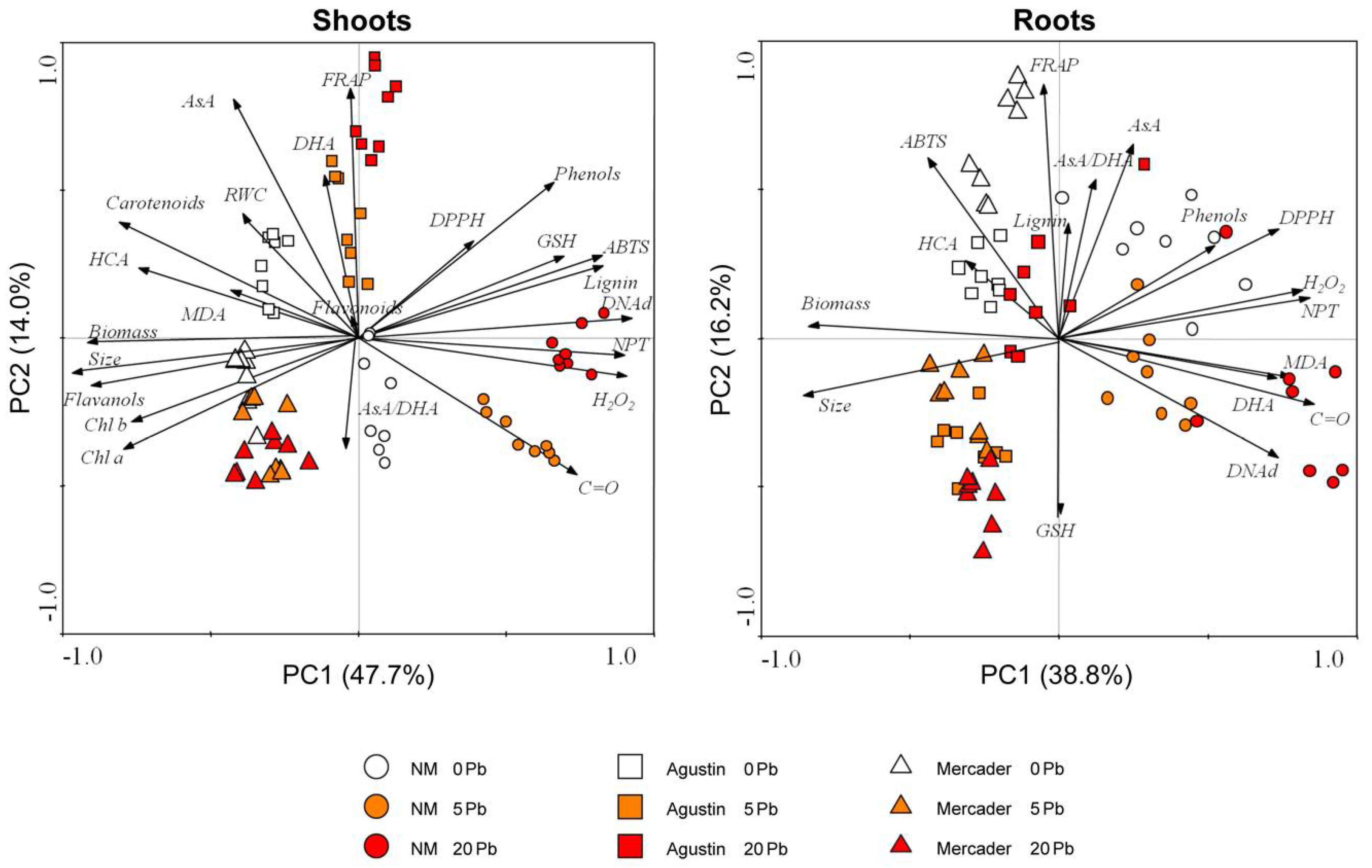
3.6. General Overview of Z. fabago Response to Pb
4. Discussion
4.1. Pb Uptake and Genotoxicity
4.2. Cell Cycle Progression
4.3. Oxidative Stress and Antioxidant Response
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mallhi, Z.I.; Rizwan, M.; Mansha, A.; Ali, Q.; Asim, S.; Ali, S.; Hussain, A.; Alrokayan, S.H.; Khan, H.A.; Alam, P.; et al. Citric acid enhances plant growth, photosynthesis, and phytoextraction of lead by alleviating the oxidative stress in castor beans. Plants 2019, 8, 525. [Google Scholar] [CrossRef] [Green Version]
- Egendorf, S.P.; Groffman, P.; Moore, G.; Cheng, Z. The limits of lead (Pb) phytoextraction and possibilities of phytostabilization in contaminated soil: A critical review. Int. J. Phytoremed. 2020, 22, 916–930. [Google Scholar] [CrossRef]
- Orroñoa, D.I.; Schindlera, V.; Lavadoab, R.S. Heavy metal availability in Pelargonium hortorum rhizosphere: Interactions, uptake and plant accumulation. J. Plant Nutr. 2012, 35, 1374–1386. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, M.N.V. Plant-lead interactions: Transport, toxicity, tolerance, and detoxification mechanisms. Ecotoxicol. Environ. Saf. 2018, 166, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead Uptake, Toxicity, and Detoxification in Plants. Rev. Environ. Contam. Toxicol. 2011, 213, 113–136. [Google Scholar] [CrossRef] [Green Version]
- Rabęda, I.; Bilski, H.; Mellerowicz, E.J.; Napieralska, A.; Suski, S.; Woźny, A.; Krzesłowska, M. Colocalization of low-methylesterified pectins and Pb deposits in the apoplast of aspen roots exposed to lead. Environ. Pollut. 2015, 205, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Dietz, K.-J. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2009, 14, 43–50. [Google Scholar] [CrossRef]
- Zine, H.; Midhat, L.; Hakkou, R.; El Adnani, M.; Ouhammou, A. Guidelines for a phytomanagement plan by the phytostabilization of mining wastes. Sci. Afr. 2020, 10, e00654. [Google Scholar] [CrossRef]
- Ernst, W.H.; Nelissen, H.J.; Bookum, W.M.T. Combination toxicology of metal-enriched soils: Physiological responses of a Zn- and Cd-resistant ecotype of Silene vulgaris on polymetallic soils. Environ. Exp. Bot. 2000, 43, 55–71. [Google Scholar] [CrossRef]
- Hego, E.; Vilain, S.; Barre, A.; Claverol, S.; Dupuy, J.-W.; Lalanne, C.; Bonneu, M.; Plomion, C.; Mench, M. Copper stress-induced changes in leaf soluble proteome of Cu-sensitive and tolerant Agrostis capillaris L. populations. Proteomics 2016, 16, 1386–1397. [Google Scholar] [CrossRef]
- Lefèvre, I.; Vogel-Mikuš, K.; Arčon, I.; Lutts, S. How do roots of the metal-resistant perennial bush Zygophyllum fabago cope with cadmium and zinc toxicities? Plant Soil 2016, 404, 193–207. [Google Scholar] [CrossRef]
- López-Orenes, A.; Bueso, M.C.; Conesa, H.M.; Calderón, A.; Ferrer, M.A. Seasonal changes in antioxidative/oxidative profile of mining and non-mining populations of Syrian beancaper as determined by soil conditions. Sci. Total Environ. 2017, 575, 437–447. [Google Scholar] [CrossRef]
- Ferrer, M.A.; Cimini, S.; López-Orenes, A.; Calderón, A.; De Gara, L. Differential Pb tolerance in metallicolous and non-metallicolous Zygophyllum fabago populations involves the strengthening of the antioxidative pathways. Environ. Exp. Bot. 2018, 150, 141–151. [Google Scholar] [CrossRef]
- López-Orenes, A.; Martínez-Pérez, A.; Calderón, A.A.; Ferrer, M.A. Pb-induced responses in Zygophyllum fabago plants are organ-dependent and modulated by salicylic acid. Plant Physiol. Biochem. 2014, 84, 57–66. [Google Scholar] [CrossRef] [PubMed]
- López-Orenes, A.; Dias, M.C.; Ferrer, M.A.; Calderón, A.; Moutinho-Pereira, J.; Correia, C.; Santos, C. Different mechanisms of the metalliferous Zygophyllum fabago shoots and roots to cope with Pb toxicity. Environ. Sci. Pollut. Res. 2018, 25, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Gichner, T.; Žnidar, I.; Száková, J. Evaluation of DNA damage and mutagenicity induced by lead in tobacco plants. Mutat. Res. Toxicol. Environ. Mutagen. 2008, 652, 186–190. [Google Scholar] [CrossRef]
- Shahid, M.; Pinelli, E.; Pourrut, B.; Silvestre, J.; Dumat, C. Lead-induced genotoxicity to Vicia faba L. roots in relation with metal cell uptake and initial speciation. Ecotoxicol. Environ. Saf. 2011, 74, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.; Silva, P.; Oliveira, H.; Gaivão, I.; Matos, M.; Pinto-Carnide, O.; Santos, C. Pb low doses induced genotoxicity in Lactuca sativa plants. Plant Physiol. Biochem. 2017, 112, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, U.; Farooq, M.; Hussain, S.; Maqsood, M.; Hussain, M.; Ishfaq, M.; Ahmad, M.; Anjum, M.Z. Lead toxicity in plants: Impacts and remediation. J. Environ. Manag. 2019, 250, 109557. [Google Scholar] [CrossRef]
- Fischer, S.; Kühnlenz, T.; Thieme, M.; Schmidt, H.; Clemens, S. Analysis of plant Pb tolerance at realistic submicromolar concentrations demonstrates the role of phytochelatin synthesis for Pb detoxification. Environ. Sci. Technol. 2014, 48, 7552–7559. [Google Scholar] [CrossRef]
- Guidelines for Water Reuse 600/R-12/618; Environmental Protection Agency (EPA): Washington, DC, USA, 2012.
- Párraga-Aguado, I.; González-Alcaraz, M.; López-Orenes, A.; Ferrer-Ayala, M.; Conesa, H. Evaluation of the environmental plasticity in the xerohalophyte Zygophyllum fabago L. for the phytomanagement of mine tailings in semiarid areas. Chemosphere 2016, 161, 259–265. [Google Scholar] [CrossRef]
- Gichner, T.; Patková, Z.; Száková, J.; Demnerová, K. Cadmium induces DNA damage in tobacco roots, but no DNA damage, somatic mutations or homologous recombination in tobacco leaves. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2004, 559, 49–57. [Google Scholar] [CrossRef]
- Rodriguez, E.; Sousa, M.; Gomes, A.; Azevedo, R.; Mariz-Ponte, N.; Sario, S.; Mendes, R.J.; Santos, C. Genotoxic endpoints in a Pb-accumulating pea cultivar: Insights into Pb2+ contamination limits. Environ. Sci. Pollut. Res. 2019, 26, 32368–32373. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Santos, C.; Matos, M.; Pinto-Carnide, O. Al toxicity mechanism in tolerant and sensitive rye genotypes. Environ. Exp. Bot. 2012, 75, 89–97. [Google Scholar] [CrossRef]
- Loureiro, J.; Rodriguez, E.; Dolezel, J.; Santos, C. Two new nuclear isolation buffers for plant dna flow cytometry: A Test with 37 Species. Ann. Bot. 2007, 100, 875–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Tortosa, V.; López-Orenes, A.; Martínez-Pérez, A.; Ferrer, M.A.; Calderón, A.A. Antioxidant activity and rosmarinic acid changes in salicylic acid-treated Thymus membranaceus shoots. Food Chem. 2012, 130, 362–369. [Google Scholar] [CrossRef]
- López-Orenes, A.; Bueso, M.C.; Conesa, H.; Calderón, A.A.; Ferrer, M.A. Seasonal ionomic and metabolic changes in Aleppo pines growing on mine tailings under Mediterranean semi-arid climate. Sci. Total Environ. 2018, 637-638, 625–635. [Google Scholar] [CrossRef]
- Queval, G.; Noctor, G. A plate reader method for the measurement of NAD, NADP, glutathione, and ascorbate in tissue extracts: Application to redox profiling during Arabidopsis rosette development. Anal. Biochem. 2007, 363, 58–69. [Google Scholar] [CrossRef]
- Hartley-Whitaker, J.; Ainsworth, G.; Vooijs, R.; Bookum, W.T.; Schat, H.; Meharg, A.A. Phytochelatins are involved in differential arsenate tolerance in Holcus lanatus. Plant Physiol. 2001, 126, 299–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, J.A.; Ferrer, M.A.; Jiménez, A.; Barceló, A.R.; Sevilla, F. Antioxidant Systems and O2−/H2O2 Production in the apoplast of pea leaves. Its relation with salt-induced necrotic lesions in Minor Veins. Plant Physiol. 2001, 127, 817–831. [Google Scholar] [CrossRef]
- Ernst, W.H.O.; Verkleij, J.A.C.; Schat, H. Metal tolerance in plants. Acta Bot. Neerl. 1992, 41, 229–248. [Google Scholar] [CrossRef]
- Efahr, M.; Elaplaze, L.; Ebendaou, N.; Ehocher, V.; Mzibri, M.E.; Ebogusz, D.; Esmouni, A. Effect of lead on root growth. Front. Plant Sci. 2013, 4, 175. [Google Scholar] [CrossRef] [Green Version]
- Mohtadi, A.; Ghaderian, S.M.; Schat, H. A comparison of lead accumulation and tolerance among heavy metal hyperaccumulating and non-hyperaccumulating metallophytes. Plant Soil 2012, 352, 267–276. [Google Scholar] [CrossRef]
- Salazar, M.J.; Wannaz, E.D.; Blanco, A.; Pazcel, E.M.M.; Pignata, M.L. Pb tolerance and accumulation capabilities of Bidens pilosa L. growing in polluted soils depend on the history of exposure. Chemosphere 2021, 269, 128732. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.; Azevedo, R.; Fernandes, P.; Santos, C. Cr(VI) induces dna damage, cell cycle arrest and polyploidization: A flow cytometric and comet assay study in Pisum sativum. Chem. Res. Toxicol. 2011, 24, 1040–1047. [Google Scholar] [CrossRef]
- Cimini, S.; Gualtieri, C.; Macovei, A.; Balestrazzi, A.; De Gara, L.; Locato, V. Redox balance-DDR-miRNA triangle: Relevance in genome stability and stress responses in plants. Front. Plant Sci. 2019, 10, 989. [Google Scholar] [CrossRef]
- Amini-Chermahini, F.; Ebrahimi, M.; Farajpour, M.; Bordbar, Z.T. Karyotype analysis and new chromosome number reports in Zygophyllum species. Caryologia 2014, 67, 321–324. [Google Scholar] [CrossRef]
- Amini-Chermahini, F.; Ebrahimi, M.; Farajpour, M. Karyological studies in Zygophyllum fabago L. (Syrian bean caper) in Iran. Caryologia 2017, 70, 289–294. [Google Scholar] [CrossRef]
- Laport, R.G.; Minckley, R.L.; Ramsey, J. Phylogeny and cytogeography of the north american creosote bush (Larrea tridentata, Zygophyllaceae). Syst. Bot. 2012, 37, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H.; Noctor, G. redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxid. Redox Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef]
- Niyogi, K.K. Safety valves for photosynthesis. Curr. Opin. Plant Biol. 2000, 3, 455–460. [Google Scholar] [CrossRef]
- Chen, F.; Wang, F.; Wu, F.; Mao, W.; Zhang, G.; Zhou, M. Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol. Biochem. 2010, 48, 663–672. [Google Scholar] [CrossRef]
- Kumar, G.H.; Kumari, J.P. Heavy metal lead influative toxicity and its assessment in phytoremediating plants—A Review. Water Air Soil Pollut. 2015, 226, 324. [Google Scholar] [CrossRef]
- Sečenji, M.; Hideg, É.; Bebes, A.; Györgyey, J. Transcriptional differences in gene families of the ascorbate–glutathione cycle in wheat during mild water deficit. Plant Cell Rep. 2010, 29, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. glutathione is a key player in metal-induced oxidative stress defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef] [Green Version]
- Hernández, L.E.; Plata, J.S.; Montero-Palmero, M.B.; Carrasco-Gil, S.; Flores-Cáceres, M.L.; Ortega-Villasante, C.; Escobar, C. Contribution of glutathione to the control of cellular redox homeostasis under toxic metal and metalloid stress. J. Exp. Bot. 2015, 66, 2901–2911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Ye, Z.H.; Wang, X.R.; Wong, M.H. Increase of glutathione in mine population of Sedum alfredii: A Zn hyperaccumulator and Pb accumulator. Phytochemistry 2005, 66, 2549–2556. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Huang, H.; Yang, X.; Razafindrabe, B.; Inouhe, M. The detoxification of lead in Sedum alfredii H. is not related to phytochelatins but the glutathione. J. Hazard. Mater. 2010, 177, 437–444. [Google Scholar] [CrossRef]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Andjelkovic, M.; Vancamp, J.; Demeulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006, 98, 23–31. [Google Scholar] [CrossRef]
- Mouradov, A.; Spangenberg, G. Flavonoids: A metabolic network mediating plants adaptation to their real estate. Front. Plant Sci. 2014, 5, 620. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Orenes, A.; Alba, J.M.; Kant, M.R.; Calderón, A.A.; Ferrer, M.A. OPDA and ABA accumulation in Pb-stressed Zygophyllum fabago can be primed by salicylic acid and coincides with organ-specific differences in accumulation of phenolics. Plant Physiol. Biochem. 2020, 154, 612–621. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Orenes, A.; Santos, C.; Dias, M.C.; Oliveira, H.; Ferrer, M.Á.; Calderón, A.A.; Silva, S. Genotoxicity and Cytotoxicity Induced in Zygophyllum fabago by Low Pb Doses Depends on the Population’s Redox Plasticity. Horticulturae 2021, 7, 455. https://doi.org/10.3390/horticulturae7110455
López-Orenes A, Santos C, Dias MC, Oliveira H, Ferrer MÁ, Calderón AA, Silva S. Genotoxicity and Cytotoxicity Induced in Zygophyllum fabago by Low Pb Doses Depends on the Population’s Redox Plasticity. Horticulturae. 2021; 7(11):455. https://doi.org/10.3390/horticulturae7110455
Chicago/Turabian StyleLópez-Orenes, Antonio, Conceição Santos, Maria Celeste Dias, Helena Oliveira, María Á. Ferrer, Antonio A. Calderón, and Sónia Silva. 2021. "Genotoxicity and Cytotoxicity Induced in Zygophyllum fabago by Low Pb Doses Depends on the Population’s Redox Plasticity" Horticulturae 7, no. 11: 455. https://doi.org/10.3390/horticulturae7110455
APA StyleLópez-Orenes, A., Santos, C., Dias, M. C., Oliveira, H., Ferrer, M. Á., Calderón, A. A., & Silva, S. (2021). Genotoxicity and Cytotoxicity Induced in Zygophyllum fabago by Low Pb Doses Depends on the Population’s Redox Plasticity. Horticulturae, 7(11), 455. https://doi.org/10.3390/horticulturae7110455