Abstract
The harvest period of bayberry fruit cultivated in the open field is short and often suffers from continuous cloudy and rainy days, leading to a decrease in yield and a decline in fruit quality. However, cultivating bayberries in greenhouses could avoid the harm due to rain, improve fruit quality and prolong the supply period, thus significantly increasing economic returns. Bayberry fruit quality, assessed by single fruit weight, vertical and horizontal diameters, soluble solids content and sugar-acid ratios, was significantly superior in fruit produced under greenhouse conditions than in fruit produced in the open field. Analysis of the soluble sugar components and the related enzyme activities indicated that the sucrose accumulation and metabolism of bayberry fruit were significantly improved by greenhouse cultivation, possibly owing to differences in sucrose-phosphate synthase and acid invertase activities.
1. Introduction
The Chinese bayberry (Myrica rubra Sieb. et Zucc.) is a characteristic fruit native to China and mainly distributed in the south of the Yangtze River basin, such as Zhejiang, Jiangsu, Fujian and Guangdong provinces. There are many kinds of cultivars, including ‘Dongkui’, ‘Biqi’, ‘DingAo’ and ‘Wandao’. Bayberry is considered as a ‘green and healthy fruit’ because of its special flavor, attractive color and high nutritional value [1]. In south China, bayberry fruits are harvested during the rainy season, characterized by high precipitation, high humidity and little sunshine. As a result, the production and quality of bayberries, as well as the economic benefits, are significantly reduced, and this has restricted the development of the bayberry industry [2]. In order to overcome the disadvantages caused by local climatic conditions, bayberries are usually cultivated in greenhouses, one type of protected cultivation. Protected cultivation has been widely used in fruit industries to prevent damage by rain, diseases and infestations of insect pests, to change the microclimate environment of fruit development and thus improve the color, taste and nutritional composition of fruit, and prolong the storage life [3,4,5]. Fruit of Chinese cherries cultivated in greenhouses contained higher contents of the total soluble solids, ascorbic acid and anthocyanins than did those produced by trees grown in the open fields [6]. Greenhouse cultivation can significantly increase the sucrose content of pear fruits and decrease the content of acids [7]. In addition, Meng et al. (2013) found that greenhouse cultivation could significantly reduce the incidence of grape diseases and delayed the maturation of ‘Cabernet Gernischet’ fruits [8]. Mendoza-Castillo et al. (2017) found that intensive production of figs in hydroponic and greenhouse conditions gave up to 20 times the yield of fresh fruit produced by plants growing in the open field [9]. At present, greenhouse cultivation has been widely used for the production of bayberries.
As one of the most important contributors to fruit flavor, sugar is not only involved in the metabolism of fruit, but is also involved in the synthesis of pigments and other nutrients [10]. There are three main types of sugars, sucrose, glucose and fructose, in the Chinese bayberry, and sucrose is the main component when the fruit is mature. Thus, bayberry is usually defined as a ‘sucrose accumulation type’ fruit [11]. Sugar accumulation in fruits is a complex process controlled by heredity and influenced by environment. Studies have shown that sucrose is the main substance in the transport of leaf photosynthates to fruits. When sucrose is unloaded from the phloem to fruits, it may be broken down into hexoses by sucrose synthase (SS) or invertase (IVR), and these may in turn be converted into sucrose by sucrose-phosphate synthase (SPS). SS plays an important role in plant energy metabolism and the control of the movement of sucrose in various tissues. IVR is an enzyme that hydrolyzes sucrose. There are three forms: neutral invertase (NI) which is soluble in the cytoplasm, and acidic invertases (AI), one form of which is soluble in the cytoplasm and the other is insoluble in the cell walls [12]. Wongmetha et al. (2012) analyzed the change of sucrose metabolism enzyme activities in mango fruits, and the results showed that sucrose accumulation was related to an increase in SPS activity, and AI was the dominant enzyme in the sugar accumulation and quality formation of mango fruits [13]. In addition, Zhang et al. (2019) suggested that NI is the main enzyme responsible for sucrose decomposition in pineapple fruit during development [14]. Fisher et al. (1995) indicated that SS may be an important contributor to sucrose synthesis, and its activity reflects the ability of the sucrose biosynthetic pathway [15]. Similar results have been found in apples, pears, litchi and peaches [16].
In the present study, with the aim to elucidate the impacts of greenhouse cultivation on fruit quality traits, the fruit weight, length and width of bayberries as well as the sugars and the related enzyme activities were analyzed under greenhouse cultivation and open-field cultivation. Our results provided more information and new insights for fruit quality formation under different cultivation systems.
2. Materials and Methods
2.1. Experimental Design
The experiment was conducted at the Lvsheng Bayberry Cooperative Base, located in Jianao Village, Changjie Town, Ninghai County, Zhejiang, China (121°42′36′′ N, 29°18′36′′ E). The soil is gravelly (Table S1). The greenhouse is 50 m long, 16 m wide and 4 m high and is constructed of aluminum alloy window frames, colorless transparent glass and 40 purpose screen windows (Figure 1). Bayberry trees in the greenhouse were grown to 1 m~1.5 m of the roof, and at a planting distance of 3 m × 4 m.

Figure 1.
Bayberries cultivated in greenhouse.
2.1.1. Experimental Design
Organic fertilizer with the proportion of N:P:K is 1:0.2:2.5, was supplied twice a year, in June-July after fruit harvested and October.
2.1.2. Water Management
Drip irrigation and sprinkler irrigation were used for water supply and provided enough water before mulching through drip irrigation every 10 days from February to May; the relative water content of the soil should be 60–65% in the growing stage and 50–60% from the turning stage to the mature stage.
2.1.3. Experimental Design
The test greenhouse is a double roof greenhouse, mainly made of steel reinforcement, concrete, galvanized steel pipe, and PC board (light transmission rate is about 50%). The roof is equipped with a non-powered fan every 5 m, the surrounding walls are made of reinforced concrete and 1.5 m × 1 m windows are opened every 3 m. During the flowering period of the bayberry from March to April, a blower is placed in the shed to assist bayberry pollination. After entering the month of May, shading, ventilation and water spraying should be taken to reduce the temperature in the facility. To reduce the entry of fruit flies and other pests, a 40-mesh pest control net should be set up in the ventilation area of the greenhouse to reduce the spread of diseases and keep the garden clean and tidy.
2.2. Sampling
‘Dongkui’ (DK) bayberry fruits were collected in the greenhouse and from the open field at 51, 58, 65, 72 and 79 days after flowering. The fruit dimensions were measured. The fruits were then frozen in liquid nitrogen and stored at −80 °C for further analysis.
2.3. Measurements
2.3.1. Environmental Conditions
Environmental conditions in the greenhouse and the open field were recorded every 20 min during the development of the fruit: temperature and relative humidty by six automatic temperature and humidity recorders (TH20R-EX-F, Luge Technology Co., LTD., Hangzhou, China), light intensity by three illumination recorders (L99-LX, Luge Technology Co., LTD., Hangzhou, China), and carbon dioxide concentrations by three carbon dioxide recorders (L99-CO2, Luge Technologies Co., LTD., Hangzhou, China).
2.3.2. Bayberry Fruit Development Index
The horizontal and longitudinal dimensions of fruit were measured with a vernier caliper. The weight of individual fruits was measured by an analytical balance. The soluble solids content of fruit was determined by the PR-101 digital refractometer (Konica Minolta Holdings, Inc., Tokyo, Japan). Three biological replicates were taken, with 10 fruits for each replicate.
2.3.3. Soluble Sugar Content
The soluble sugar components of bayberry fruit were determined by HPLC according to Komatsu et al. (1999) [17] and Chen et al. (2002) [18]. 1 g of ground bayberry fruit was mixed with 5 mL extraction solution (methyl alcohol: chloroform: water 12:5:3) and vortexed at full speed, then centrifuged at 4500× g for 15 min. The extraction was repeated three times. The extraction solutions were combined, filtered, and separated by addition of double distilled water, and the chloroform layer was removed. The supernatant was made up to 25 mL, a 4 mL sample of which was dried with a rotary evaporator in a water bath at 55 °C, and then 1 mL pure water was added to dissolve the residue. A total of 0.5mL was taken and carried on an Oasis HLB column (3 cc/60 mg, hydrophilic and lipophilic polymer filler, Waters, Palo Alto, USA) which was used for solid-phase extraction (SPE). The eluate was stored after sterilization. High-performance liquid chromatography (Agilent 1100, Palo Alto, USA) was used for detection. HPLC detection conditions: Sugar-PakTM 1 (6.5 mm × 300 mm, Waters); column temperature: 80 °C; mobile phase: double distilled water; flow rate: 0.6 mL min−1; detector: differential refractive index detector.
2.3.4. Enzyme Activity
Enzyme activity was measured according to Lowell et al. (1989) [19] and Hubbard et al. (1991) [20] with some modifications.
Invertase Extraction
A total of 0.5 g mixed samples were accurately weighed and 5 mL of buffer solution was added (potassium phosphate-buffered solution 200 mmol L−1, pH 7.5, containing 5 mmol L−1 MgCl2, 0.1% β-mercaptoethanol, 0.05% Triton X-100, 0.05% BSA, 2% PVPP).
The extraction of sucrose-phosphate synthase and sucrose synthase was similar to that of invertase. Enzyme extraction buffer (pH 7.5): Hepes-NaOH 200 mmol L−1, containing 5 mmol L−1 MgCl2, 0.1% β-mercaptoethanol, 0.05% TritonX-100, 0.05% BSA, 1 mmol L−1 EDTA, 1 mmol L−1 EGTA, 10 mmol L−1 sodium ascorbate, 10 mmol L−1 cysteine-hydrochloric acid, 2% glycerol, 2% PVPP. Enzyme desalting buffer solution (pH 7.5): Hepes-NaOH 20 mmol L−1 containing 0.25 mmol L−1 MgCl2, 0.01% β-mercaptoethanol, 0.05% BSA, 1 mmol L−1 EDTA, 1 mmol L−1 EGTA, 0.2% glycerol.
Invertase Activity
The reaction system, 490 μL, was made up of 100 μL of a purified enzyme and 390 μL reaction solution (80 mmol L−1 potassium acetate and phosphate buffer (AI, pH 4.5; NI, pH 7.5), 100 mmol L−1 sucrose); water bath at 37 °C for 30 min; 490 μL DNS were then added to a boiling water bath for 5 min; after cooling to room temperature, the absorbance value of A540 was measured by a microplate reader. The control contained cooled enzyme solution.
Sucrose-Phosphate Synthase Activity
The volume of the reaction system, 70 μL, was made up of 24.5 μL of purified enzyme and 45.5 μL reaction solution (containing 50 mmol L−1 HEPES-NaOH (pH 7.5), 15 mmol L−1 MgCl2, 1 mmol L−1 EDTA, 5 mmol L−1 NaF, 16 mmol L−1 UDPG, 4 mmol L−1 F-6-P and 20 mmol L−1 G-6-P); the reaction system was held in a water bath at 30 °C for 30 min, then 70 μL 5 mmol L−1 NaOH was added to stop the reaction and held in a boiling water bath for 10 min. After cooling to room temperature, 1 mL 0.14% anthracnose (dissolved in 13.8 mmol L−1 H2SO4) was added and kept at 40 °C for 20 min. After cooling, the absorbance value of A620 was determined by a micrometer. The control contained no Fru-6-P and Glc-6-P.
Sucrose Synthase Activity
(1) Decomposition direction:
A total of 210 μL of purified enzyme solution was added to 280 μL enzymatic reaction solution (containing 80 mmol L−1 MES buffer (pH 5.5), 5 mmol L−1 NaF, 100 mmol L−1 sucrose and 5 mmol L−1 UDP). After being held in a 30 °C water bath for 30 min, 490 μL DNS was rapidly added to terminate the reaction. After then being held in a boiling water bath for 5 min, the A540 absorbance value was determined by an enzyme marker after cooling. The control contained killed the enzyme solution and no sucrose or UDP were found.
(2) Synthesis direction:
A total of 40 μL of purified enzyme solution (containing 80 mmol L−1 HEPES-NaOH (pH 8.5), 5 mmol L−1 KCN, 5 mmol L−1 NaF, 100 mmol L−1 fructose and 15 mmol L−1 UDPG) was added to 30 μL of purified enzyme solution, held in a 30 °C water bath for 30 min, and 70 μL 5 mmol L−1 NaOH was added to terminate the reaction. The solution was placed in a boiling water bath for 10 min, and then 1 mL 0.14% anthracnonse (dissolved in 13.8 mmol L−1 H2SO4) was added after cooling. The mixture was next held in a water bath at 40 °C for 20 min. After cooling, the absorbance value of A620 was measured with a microplate reader. In the control, fructose and UDPG were not added to the reaction system.
2.3.5. Titratable Acid and Vitamin C Content
The content of titratable acid was measured by NaOH titration. The content of vitamin C was determined by 2, 6-dichlorophenol indophenol titration according to Ge et al. (2013) [21].
2.3.6. Effective Accumulated Temperature
Effective accumulated temperature (GDD) indicates that the accumulated thermal effect required to complete a certain stage of bayberry development is constant, and the relationship between GDD and average daily temperature (T):
T is the average daily temperature (°C), and Tb and Tm are the lower and upper limit temperatures of bayberry development, respectively, Tb = 3.4 °C, Tm = 32 °C [22]. The daily thermal effect increases e−β and e−γ.
2.4. Statistical Analysis
The data chart was made with Origin Pro 2017 (Origin Lab Corporation, Northampton, MA, USA) software. SPSS 21.0 (SPSS Inc., Chicago, IL, USA) software was applied for the canonical correlation analysis and to analyze the data by ANOVA, and Duncan’s multiple comparisons were used to detect the significant difference between the groups with a significance level of 0.05.
3. Results
3.1. Environmental Factors
The average temperature, relative humidity, light intensity and carbon dioxide concentration were measured during bayberry fruit development under the two different cultivation conditions (Figure 2). Our results showed that the temperature in the greenhouse was significantly higher than in the open field (Figure S1A). The average temperature in the greenhouse was 23.8 °C from April to June, including a 16-day high-temperature period, and the total effective accumulated temperature from the young fruit stage to the fruit ripening stage was 1163.4 °C d. The average temperature during fruit development in the open field was 23.2 °C, and the total effective accumulated temperature from the young fruit stage to fruit maturity was 1110.5 °C d. The relative humidity in the greenhouse was higher than that in the open field at the beginning and consistent with each other until the late stages (Figure S1B). The average light intensity of greenhouse was much lower than that of open fields (Figure S1C). The average daily illumination intensity in the greenhouse was 176.6 μmol m−2 s−1, half of the intensity, 353.1 μmol m−2 s−1, in the open field. The CO2 concentration in the greenhouse was also lower than that of open field (Figure S1D). From April to June, the average CO2 concentration in the greenhouse was 386.5 μL L−1, about 85% of that in the open field.

Figure 2.
Development stages of ‘Dongkui’ under different cultivation. Numbers of mean fruits at 51, 58, 65, 72, 79 days after flowering.
3.2. Fruit Weight and Diameter
The weight and dimensions of bayberries were greater when grown in the greenhouse, but the overall patterns of fruit growth did not change. Fruit weight initially increased slowly and then increased dramatically in both cultivations, and significantly higher weights in the greenhouse, the difference being greatest at fruit maturity (Figure 3A). At maturity, ‘DK’ fruit from the greenhouse weighed 30.9 g, significantly more (13.8 g) than fruit in the open field. In terms of fruit diameter, the longitudinal diameter was dominant in the early stage of development, and the transverse diameter was dominant in the middle and late stages. Therefore, the fruit shape index showed a downward trend, from 1.2~1.4 when young to 0.95~1.0 at maturity. Under both cultivation conditions, the length increased rapidly early in development, followed by slow growth period from 50 to 60 days after flowering, and began to grow rapidly at 60 days after flowering (Figure 3B). However, the transverse diameter of fruit grew slowly before 60 days after flowering, and then entered a period of rapid growth (Figure 3C). At maturity, the length and transverse diameter of DK fruit in the open field were 32.4 mm and 30.9 mm, significantly less than those of fruit produced in the greenhouse (36.0 mm and 38.3 mm, respectively).
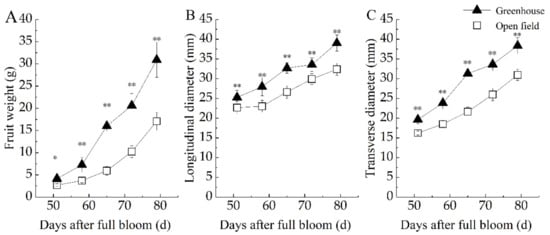
Figure 3.
Changes in fruit weight and diameter of bayberry grown in a greenhouse and in the open field. (A) Fruits weight during bayberry developmental stages. (B) Longitudinal diameter of fruits during bayberry developmental stages. (C) Transverse diameter of fruits during bayberry developmental stages. Graphics marked ** are significantly different (p < 0.01), * is significantly different (p < 0.05) based on ANOVA.
3.3. Quality Indexes
Greenhouse cultivation fruit yields significantly increased compared with the open-filed option (Table S2). The soluble solids content of mature bayberry fruit in the greenhouse was 13.5%, significantly higher than that 10.9% in fruit produced in the open field (Table S3). The content of vitamin C in fruit under the two cultivation conditions gradually decreased as the fruit developed, although the content of vitamin C content of mature fruit grown under greenhouse conditions was still relatively high (20.23 mg kg−1 FW).
The fruit type index of mature fruit produced either in the greenhouse or in the open field was close to 1, the fruit were round, indicating that the conditions of cultivation had almost no influence on fruit shape.
The soluble solids: acid ratios in ripe fruit from the greenhouse and the open field were 14.69 and 9.73, respectively. The sugar: acid ratio of mature fruit from the greenhouse cultivation was 10.27, 1.4 times higher than that of fruit from open-field cultivation.
3.4. Sugar Accumulation
The total sugar content of the fruit began to increase around 60 days after flowering, as did the contents of the individual sugars, sucrose, glucose and fructose. Sucrose predominated, accounting for about 50% of the total sugar, glucose 25% and fructose 24% (Figure 4). At maturity, the sucrose, fructose and glucose contents of bayberries produced under greenhouse conditions were about a third higher than in fruit produced in the open field: sucrose 42.7 mg g−1 (Figure 4B), 30.7% higher; fructose 23.9 mg g−1 (Figure 4C), 33.4% higher; and glucose 25.7 mg g−1 (Figure 4D), 30.1% higher.
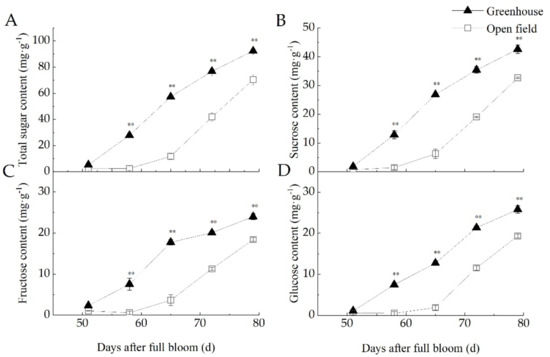
Figure 4.
Sugar content of bayberry fruit cultivated in a greenhouse or in the open field. (A) Total sugar content of the fruits during bayberry developmental stages. (B) Sucrose content of the fruits during bayberry developmental stages. (C) Fructose content of the fruits during bayberry developmental stages. (D) Glucose content of the fruits during bayberry developmental stages. Graphics marked ** are significantly different (p < 0.01) based on ANOVA.
3.5. Sucrose Metabolism-Related Enzymes Activities
Based on their optimum pH, invertases are classified into acid invertases (AI) and neutral invertases (NI). Both invertase activities gradually decreased as the fruit developed fluctuating slightly at maturity (Figure 5A,B). AI activities were generally higher in fruit grown in the open field, whereas there were no significant differences in NI activities.

Figure 5.
Sucrose metabolizing enzymes in bayberry fruit under different cultivation conditions. (A) Acid invertase activities of the fruits during bayberry developmental stages. (B) Neutral invertase activities of the fruits during bayberry developmental stages. (C,D) SS activities of the fruits during bayberry developmental stages. (E) SPS activities of the fruits during bayberry developmental stages. Different lowercase letters indicate significant difference (p < 0.05).
3.5.1. Sucrose Synthase
Sucrose synthase in the synthesis direction (SSs) reached a maximum late in fruit development (Figure 5C), and after day 60 from full bloom there was no significant differences between fruit in greenhouse cultivation and in the open field. The very high activities at day 50 in open-field-cultivated fruit may be anomalous, but to check this, activities in much younger fruit need to be checked.
Sucrose synthase in the direction of decomposition (SSc) varied comparatively little in greenhouse-cultivated fruit with greater fluctuations in open-field cultivated fruit (Figure 5D).
3.5.2. Sucrose-Phosphate Synthase
Sucrose-phosphate synthase (SPS) was low during the early stages of fruit development (Figure 5E) but then gradually increased as the fruit developed. SPS activities were always higher in the fruit grown under greenhouse conditions. The activity of SPS in greenhouse cultivation was the highest at 6.5 μmol h−1 g−1 FW, 1.1 μmol h−1 g−1 FW higher than in the open field.
4. Discussion
Greenhouse cultivation is an important measure to avoid climatic extremes in order to improve fruit yields and quality [23,24,25,26,27,28]. We found that greenhouse cultivation can accelerate the growth of bayberry fruit by raising the ambient temperature; the accumulation of effective accumulated temperature was also gradually increased. The average relative humidity in the greenhouse increased by 3~6% compared with that in the open field; similar results were found in loquat and tomato fruits [24,29], suggesting that appropriate higher temperature and humidity may be positively associated with fruit yields and qualities. The light intensity had a significant effect on the photosynthesis of fruit trees, our studies indicated that light intensity was significantly lower in the greenhouse than that of open-field cultivation; these maybe due to the reflection and absorption of sunlight by PC board in greenhouse. However, some studies suggested that partial shading can promote the growth and improve the photosynthetic capacity of bayberries [26]; these may be also the reason why greenhouse cultivation could increase fruit yield and qualities.
Greenhouse cultivation resulted in larger and heavier bayberry fruits than open-field cultivation. This is consistent with previous research which showed that greenhouse cultivation could significantly increase fruit weight [29,30]. Research in pears showed that the appearance of fruit was improved, the soluble solids and total soluble sugars were increased and the titratable acid content and fruit firmness were decreased significantly [31]. Our results showed that the soluble solids content, solids-to-acid ratio and sugar-acid ratios in bayberry fruit from greenhouse cultivation were significantly higher than those in the open field. However, there was no significant differences in vitamin C content and fruit shape. Thus, we conclude that bayberry quality, especially sugar content, can be improved by greenhouse cultivation. The sucrose, fructose and glucose contents of bayberry fruit grown in the greenhouse were significantly higher than those of fruit grown in the open field. Similar results have been found in pears: the soluble sugar, fructose, glucose and sorbitol contents under greenhouse cultivation were significantly higher than those under open cultivation [31]. In bayberries, during the period of fruit ripening, sucrose accounted for more than half the total sugar, indicating that the accumulation of sucrose was dominant in bayberry. This is consistent with the results of Shi et al. (2014) [32].
Sucrose accumulation in fruit is determined by the balance of sugar biosynthesis, consumption and transportation. Hence, the correlation analysis of three sugars and enzymes related to sucrose metabolism were analyzed and the results showed that AI and NI were significantly negatively correlated with sucrose content, while SPS and sucrose content were significantly positively correlated during fruit development (Table 1). In addition, the activity of SPS under greenhouse cultivation was higher than that in open field, whereas the activity of AI was relatively lower (Figure 5). It has been reported that the high activity of sucrose synthase in the synthesis direction (SSs) and sucrose-phosphate synthase (SPS) were the main driving forces of sucrose accumulation in ripe fruit of strawberry [33], and numerous studies have documented that SPS and AI are important factors to promote the increase in sucrose content [32,34,35]. Thus, our results suggest that the higher sucrose content in bayberry fruit under greenhouse cultivation may be due to the higher SPS activity and lower AI activity.

Table 1.
Canonical correlation analysis between activity of sucrose metabolizing enzymes and sugar accumulation in bayberry fruit. acidic invertases (AI), neutral invertase (NI), sucrose-phosphate synthase (SPS), sucrose synthase in the synthesis direction (SSs), sucrose synthase in the direction of decomposition (SSc). Graphics marked * are significantly different (p < 0.05) based on ANOVA.
5. Conclusions
In summary, our results showed that the weight, width and soluble solids of bayberry fruit cultivated in the greenhouse were all higher than those in open field cultivation, indicating that cultivation in a greenhouse could significantly improve the fruit quality. Sucrose is the main sugar in bayberry fruit, and the results indicate that the high sucrose content in fruit grown under greenhouse conditions may be due to the higher SPS activity and low AI activity. These results may provide new clues for fruit quality formation for bayberry fruit, especially increased sucrose accumulation in greenhouse cultivation.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/horticulturae7110429/s1, Table S1. Physical and chemical properties of the soil. Table S2. Comparison of fruit yield between open field and greenhouse cultivation. Table S3. Effects of different cultivation on the fruit quality of ‘Dongkui’ in mature stage. Figure S1. Environmental factors under different cultivation.
Author Contributions
K.X. conceived and designed the experiments. B.-P.W. and C.Z. performed the experiments. B.-P.W. wrote the draft manuscript. Y.-B.G. and W.-W.Z. discussed and improved the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Zhejiang province (LQ21C150001), the National Natural Science Foundation of China (30871718), and the Scientific Research and Development Foundation of Zhejiang A & F University (2019FR046).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used in this study i can be found in this published article and it’s Supplementary Materials.
Acknowledgments
We would like to thank Allan Ross Ferguson and Shaojia Li for suggestions and comments to the manuscript.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Correction Statement
This article has been republished with a minor correction to the Funding statement. This change does not affect the scientific content of the article.
References
- Li, X.; He, Y. Non-destructive measurement of acidity of Chinese bayberry using Vis/NIRS techniques. Eur. Food Res. Technol. 2006, 223, 731–736. [Google Scholar] [CrossRef]
- Liang, S.M.; Zhu, T.T.; Zhang, S.W.; Zheng, X.L.; Qi, X.J. Effects of transparent film of different colors on photosynthetic characteristics and fruit quality in Chinese bayberry. J. Fruit Sci. 2019, 36, 1049–1057. [Google Scholar]
- Jin, L.F.; Guo, D.Y.; Ning, D.Y.; Hussain, S.B.; Liu, Y.Z. Covering the trees of Kinokuni tangerine with plastic film during fruit ripening improves sweetness and alters the metabolism of cell wall components. Acta Physiol. Plant. 2018, 40, 182. [Google Scholar] [CrossRef]
- Jat, R.; Singh, V.P.; Kumar, V. Greenhouse cultivation of fruit crops with special reference to India: An overview. J. Appl. Polym. Sci. 2020, 12, 252–260. [Google Scholar] [CrossRef]
- Gruda, N.; Bisbisa, M.; Tanny, J. Impacts of protected vegetable cultivation on climate change and adaptation strategies for cleaner production–A review. J. Clean. 2019, 225, 324–339. [Google Scholar] [CrossRef]
- Tian, T.; Qiao, G.; Deng, B.; Wen, Z.; Hong, Y.; Wen, X.P. The effects of rain shelter coverings on the vegetative growth and fruit characteristics of Chinese cherry (Prunus pseudocerasus Lindl.). Sci. Hort. 2019, 254, 228–235. [Google Scholar] [CrossRef]
- Zhang, Q.M.; Pang, X.M.; Chen, X.M.; Ye, J.H.; Lin, S.X.; Jia, X.L. Rain-shelter cultivation influence rhizosphere bacterial community structure in pear and its relationship with fruit quality of pear and soil chemical properties. Sci. Hortic. 2020, 269, 109419. [Google Scholar] [CrossRef]
- Meng, J.F.; Ning, P.F.; Xu, T.F.; Zhang, Z.W. Effect of rain-shelter cultivation of Vitis vinifera cv. Cabernet Gernischet on the phenolic profile of berry skins and the incidence of grape diseases. Molecules 2013, 18, 381–397. [Google Scholar] [CrossRef]
- Mendoza-Castillo, V.M.; Vargas-Canales, J.M.; Calderón-Zavala, G.; Mendoza-Castillo, M.d.C.; Santacruz-Varela, A. Intensive production systems of fig (Ficus carica L.) under greenhouse conditions. Expl. Agric. 2017, 53, 339–350. [Google Scholar] [CrossRef]
- Cheng, J.H.; Xie, M.; Jiang, G.H.; Xu, K. The signaling role of hexokinase in plants. Chin. J. Cell Biol. 2004, 26, 594–598. [Google Scholar]
- Qian, J.B.; Chen, Z.M.; Chen, J.W.; Xie, M.; Qin, Q.Q.; Yang, R.X.; Wu, J.; Gui, H. The characteristics of fruit development and the accumulation of major quality composition in developing red bayberry (Myrica rubra Sieb. et Zucc.) fruit. Acta Agric. Zhejiangensis 2006, 3, 151–154. [Google Scholar]
- Wan, H.J.; Wu, L.; Yang, Y.L.; Zhou, G.Z.; Ruan, Y.L. Evolution of Sucrose Metabolism: The Dichotomy of Invertases and Beyond. Trends Plant Sci. 2018, 23, 163–177. [Google Scholar] [CrossRef]
- Wongmetha, O.; Ke, L.S.; Liang, Y.S. Sucrose metabolism and physiological changes during mango cv. Irwin growth and development. Hortic. Environ. Biotechnol. 2012, 5, 373–377. [Google Scholar] [CrossRef]
- Zhang, X.M.; Liu, S.H.; Du, L.Q.; Yao, Y.L.; Wu, J.Y. Activities, transcript levels, and subcellular localizations of sucrose phosphate synthase, sucrose synthase, and neutral invertase and change in sucrose content during fruit development in pineapple (Ananas comosus). J. Hortic. Sci. Biotechnol. 2019, 94, 573–579. [Google Scholar] [CrossRef]
- Fisher, D.B.; Wang, N. Sucrose concentration gradients along the post phloem transport pathway in the maternal tissues of developing wheat grains. Plant Physiol. 1995, 109, 587–592. [Google Scholar] [CrossRef]
- Tanase, K.; Shiratake, K.; Mori, H.; Yamaki, S. Changes in the phosphorylation state of sucrose synthase during development of Japanese pear fruit. Physiol. Plant. 2002, 114, 21–26. [Google Scholar] [CrossRef]
- Komatsu, A.; Takanokura, Y.; Moriguchi, T.; Omura, M.; Akihama, T. Differential expression of three sucrose-phosphate synthase isoforms during sucrose accumulation in citrus fruits (Citrus unshiu Marc.). Plant Sci. 1999, 140, 169–178. [Google Scholar] [CrossRef]
- Chen, J.W.; Zhang, S.L.; Zhang, L.C.; Zhao, Z.Z.; Xu, J.G. Fruit photosynthesis and assimilate translocation and partitioning: Their characteristics and role in sugar accumulation in developing citrus unshiu fruit. J. Integr. Plant Biol. 2002, 44, 158–163. [Google Scholar]
- Lowell, C.A.; Tomlinson, P.T.; Koch, K.E. Sucrose-metabolizing enzymes in transport tissues and adjacent sink structures in developing citrus fruit. Plant Physiol. 1989, 90, 1394–1402. [Google Scholar] [CrossRef]
- Hubbard, N.L.; Pharr, D.M.; Huber, S.C. Sucrose phosphate synthase and other sucrose metabolizing enzymes in fruits of various species. Physiol. Plant. 1991, 82, 191–196. [Google Scholar] [CrossRef]
- Ge, C.L.; Liu, K.P.; Qu, X.Y.; Xu, X.B.; Huang, C.H. Variation of Sugar, Acid and Vitamin C Contents in Fruit Development in Different Types of Kiwifruit. Agric. Sci. Technol. 2013, 14, 1772–174, 1778. [Google Scholar]
- Yang, Z.Q.; Huang, H.J.; Jin, Z.F.; Li, Y.X.; Huang, C.R.; Fei, Y.J. Development and validation of a photo-thermal effectiveness based simulation model for development of Myrica rubra. Acta Hortic. Sinica 2011, 38, 1259–1266. [Google Scholar]
- Xie, M.; Chen, J.W.; Cheng, J.H.; Qin, Q.Q.; Jiang, G.H.; Wang, L.H.; Qi, X.J. Studies on the fruit development and its relationship with sugar accumulation in bayberry fruit. J. Fruit Sci. 2005, 22, 634–638. [Google Scholar]
- Polat, A.A.; Durgac, C.; Caliskan, O. Effect of protected cultivation on the precocity, yield and fruit quality in loquat. Sci. Hortic. 2005, 104, 189–198. [Google Scholar] [CrossRef]
- Kamiloğlu, Ö.; Polat, A.A.; Durgaç, C. Comparison of open field and protected cultivation of five early table grape cultivars under Mediterranean conditions. Turk. J. Agric. For. 2011, 35, 491–499. [Google Scholar]
- Zeng, G.H.; Guo, Y.G.; Xu, J.X.; Hu, M.Y.; Zheng, J.; Wu, Z.W. Partial shade optimizes photosynthesis and growth in bayberry (Myrica rubra ) trees. Hortic. Environ. Biotechnol. 2017, 58, 203–211. [Google Scholar] [CrossRef]
- Li, X.X.; He, F.; Wang, J.; Li, Z.; Pan, Q.H. Simple rain-shelter cultivation prolongs accumulation period of anthocyanins in wine grape berries. Molecules 2014, 19, 14843–14861. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.B.; Zheng, W.W.; Zhang, C.; Zhang, L.L.; Xu, K. High temperature and high light intensity induced photoinhibition of bayberry (Myrica rubra Sieb. et Zucc.) by disruption of D1 turnover in photosystem II. Sci. Hortic. 2019, 248, 132–137. [Google Scholar] [CrossRef]
- Harel, D.; Fadida, H.; Slepoy, A.; Gantz, S.; Shilo, K. The effect of mean daily temperature and relative humidity on pollen, fruit set and yield of tomato grown in commercial protected cultivation. Agronomy 2014, 4, 167–177. [Google Scholar] [CrossRef]
- Chouinard, G.; Veilleux, J.; Pelletier, F.; Larose, M.; Philion, V.; Cormier, D. Impact of exclusion netting row covers on arthropod presence and crop damage to ‘Honeycrisp’ apple trees in North America: A five-year study. Crop Prot. 2017, 98, 248–254. [Google Scholar] [CrossRef]
- Li, G.B.; Fan, J.D.; Zhang, T.; Zhang, M.; Han, J.L.; Yang, F. Changes of rain shelter environment and the effects on fruit quality of early-maturing pear. J. Northw. AF Univ. 2020, 48, 77–85. [Google Scholar]
- Shi, L.Y.; Cao, S.F.; Shao, J.R.; Chen, W.; Yang, Z.F.; Zheng, Y.H. Relationship between sucrose metabolism and anthocyanin biosynthesis during ripening in Chinese bayberry fruit. J. Agric. Food Chem. 2014, 62, 10522–10528. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.W.; Qing, Q.P.; Xie, M.; Jing, G.H.; Xe, H.X.; Cheng, J.H.; Wu, J.; Sun, C.B. Characteristics of sucrose and hexose metabolism in relation to sugar accumulation in developing strawberry fruit. J. Fruit Sci. 2007, 24, 49–54. [Google Scholar]
- Burger, Y.; Schaffer, A.A. The Contribution of Sucrose Metabolism Enzymes to Sucrose Accumulation in Cucumis melo. J. Am. Soc. Hortic. Sci. 2007, 132, 704–712. [Google Scholar] [CrossRef]
- Shi, L.Y.; Cao, S.F.; Shao, J.R.; Chen, W.; Yang, Z.F.; Zheng, Y.H. Chinese bayberry fruit treated with blue light after harvest exhibit enhanced sugar production and expression of cryptochrome genes. Postharvest Biol. Technol. 2016, 111, 197–204. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).