The Utilisation of Pholiota nameko, Hypsizygus marmoreus, and Hericium erinaceus Spent Mushroom Substrates in Pleurotus ostreatus Cultivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strain
2.2. Substrate Preparation
2.3. Spawn Preparation, Inoculation, and Incubation
2.4. Cropping, Harvest, Determination of Biological Efficiency and Protein Content
2.5. Statistical Analyses
3. Results
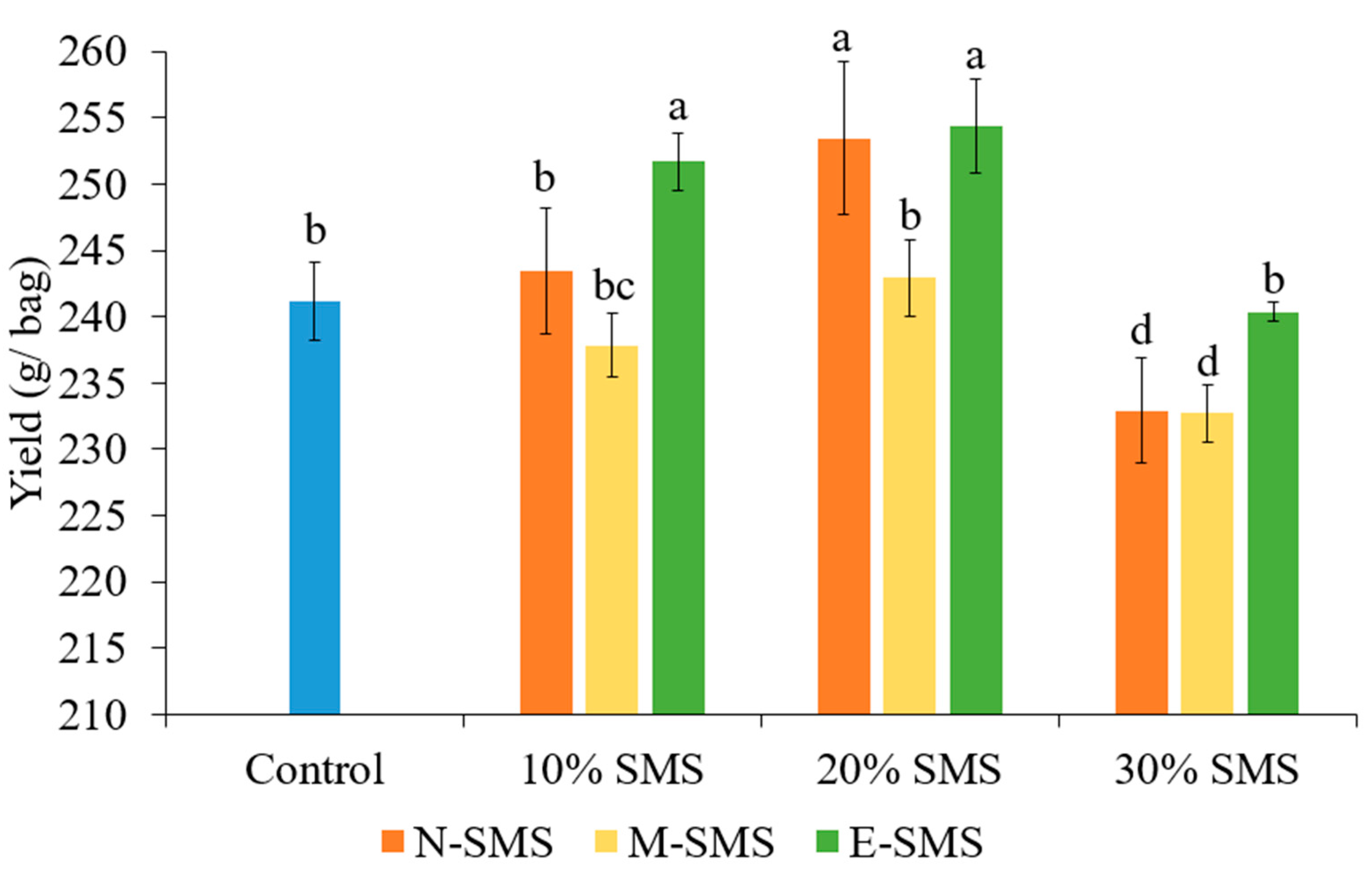
3.1. Yield
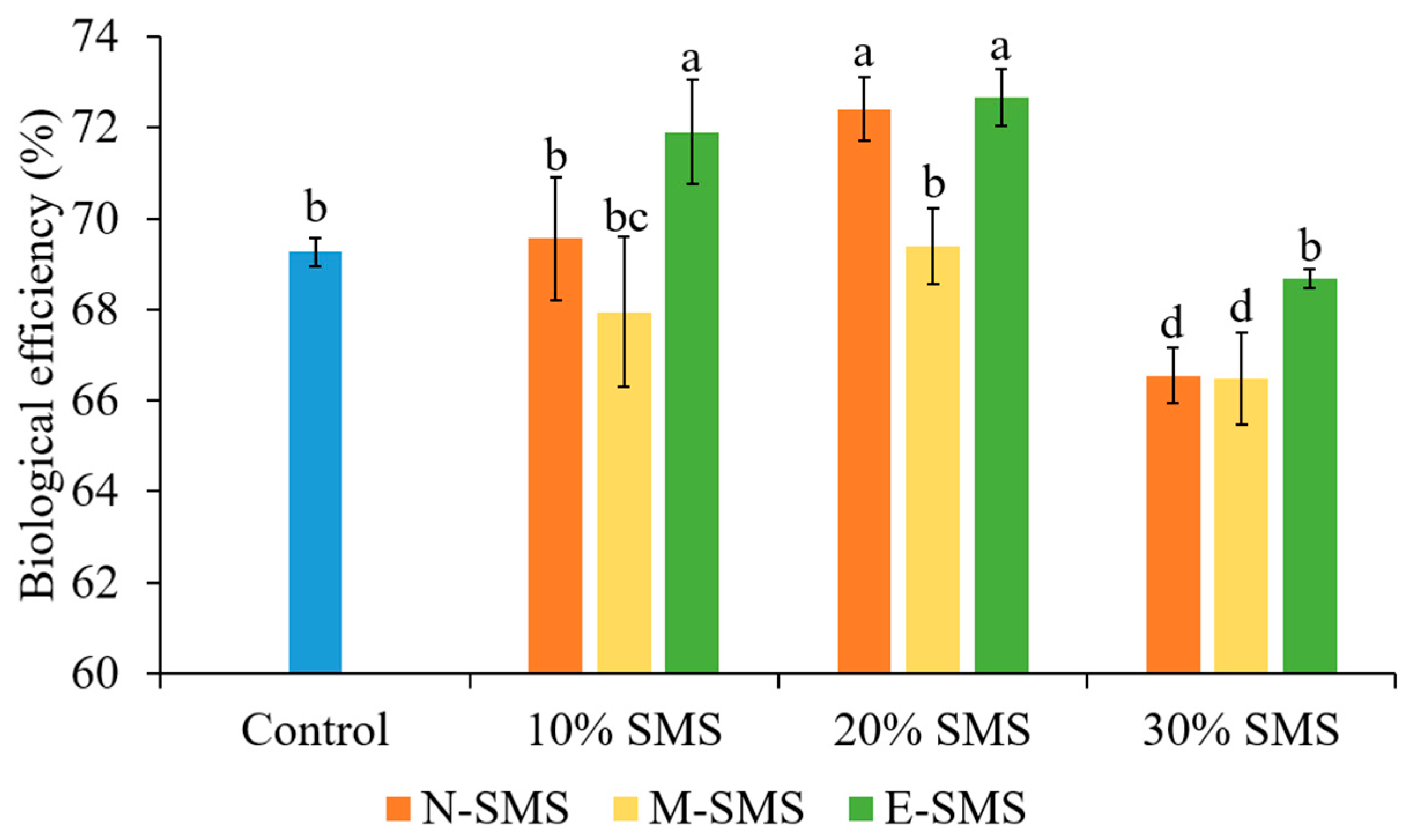
3.2. Biological Efficiency
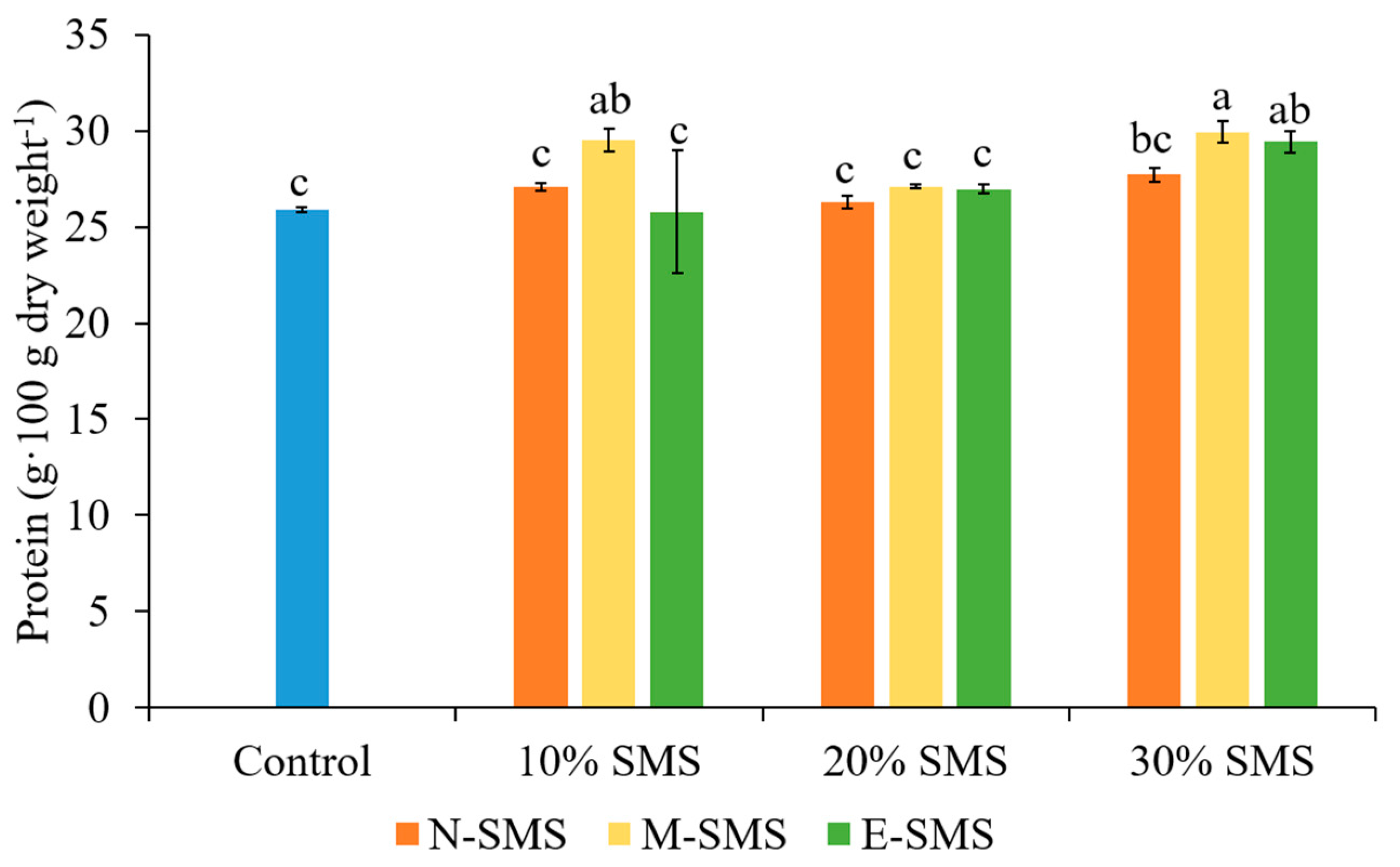
3.3. Protein Content
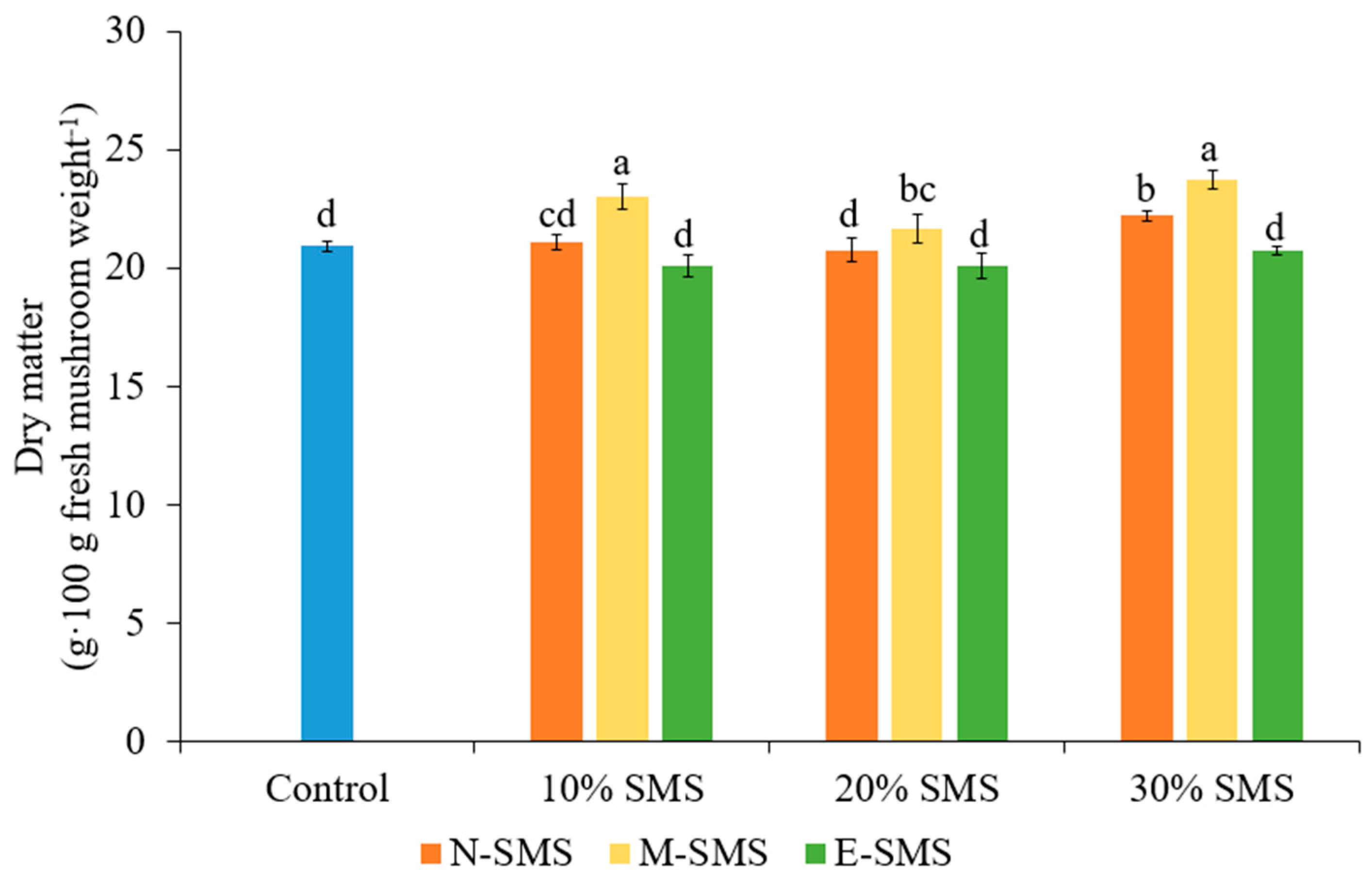
3.4. Dry Matter
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roncero-Ramos, I.; Delgado-Andrade, C. The beneficial role of edible mushrooms in human health. Curr. Opin. Food Sci. 2017, 14, 122–128. [Google Scholar] [CrossRef]
- Chaturvedi, V.K.; Agarwal, S.; Gupta, K.K.; Ramteke, P.W.; Singh, M.P. Medicinal mushroom: Boon for therapeutic applications. 3 Biotech 2018, 8, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Venturella, G.; Ferraro, V.; Cirlincione, F.; Gargano, M.L. Medicinal Mushrooms: Bioactive Compounds, Use, and Clinical Trials. Int. J. Mol. Sci. 2021, 22, 634. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-González, J.A.; Serna-Saldívar, S.O.; Gutiérrez-Uribe, J.A. Nutritional composition and nutraceutical properties of the Pleurotus fruiting bodies: Potential use as food ingredient. J. Food Compos. Anal. 2017, 58, 69–81. [Google Scholar] [CrossRef]
- Reis, F.S.; Martins, A.; Vasconcelos, M.H.; Morales, P.; Ferreira, I.C.F.R. Functional foods based on extracts or compounds derived from mushrooms. Trends Food Sci. Technol. 2017, 66, 48–62. [Google Scholar] [CrossRef]
- Ma, G.; Yang, W.; Zhao, L.; Pei, F.; Fang, D.; Hu, Q. A critical review on the health promoting effects of mushrooms nutraceuticals. Food Sci. Hum. Wellness. 2018, 7, 125–133. [Google Scholar] [CrossRef]
- Raghavendra, V.B.; Venkitasamy, C.; Pan, Z.; Nayak, C. Functional Foods from Mushroom. In Microbial Functional Foods and Nutraceuticals; Gupta, V.K., Treichel, H.V., Shapaval, V., Antonio de Oliveira, L., Tuohy, M.G., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 65–91. [Google Scholar] [CrossRef]
- Taofiq, O.; González-Paramás, A.M.; Martins, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Mushrooms extracts and compounds in cosmetics, cosmeceuticals and nutricosmetics—A review. Ind. Crops Prod. 2016, 90, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Choi, M.H.; Li, J.; Yang, H.; Shin, H.J. Mushroom Cosmetics: The Present and Future. Cosmetics 2016, 3, 22. [Google Scholar] [CrossRef]
- Mushroom Cultivation Market by Type (Button Mushroom, Oyster Mushroom, Shiitake Mushroom, Other Types), By Phase, By Region (North America, Europe, Asia Pacific, South America, Rest of the World)—Global Forecast to 2025 Mushroom Cultivation Market by Type (Button Mushroom, Oyster Mushroom, Shiitake Mushroom, Other Types), By Phase, By Region (North America, Europe, Asia Pacific, South America, Rest of the World)—Global Forecast to 2025. Available online: https://www.researchandmarkets.com/reports/5018601/mushroom-cultivation-market-by-type-button (accessed on 20 July 2021).
- Royse, D.J.; Baars, J.; Tan, Q. Current Overview of Mushroom Production in the World. In Edible and Medicinal Mushrooms; Wiley: Hoboken, NJ, USA, 2017; pp. 5–13. [Google Scholar] [CrossRef]
- Market Data Forecast 2021, Mushroom Cultivation Market by Type (Button Mushroom, Shiitake Mushroom, Oyster Mushroom, and Others), By Region (North America, Europe, Asia Pacific, South America, Rest of the World)—Forecast to 2026. Available online: https://www.marketdataforecast.com/market-reports/mushroom-cultivation-market (accessed on 20 July 2021).
- Philippoussis, A.; Zervakis, G.; Diamantopoulou, P. Bioconversion of agricultural lignocellulosic wastes through the cultivation of the edible mushrooms Agrocybe aegerita, Volvariella volvacea and Pleurotus spp. World J. Microbiol. Biotechnol. 2001, 17, 191–200. [Google Scholar] [CrossRef]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef]
- Philippoussis, A.N. Production of Mushrooms Using Agro-Industrial Residues as Substrates. In Biotechnology for Agro-Industrial Residues Utilisation; Springer: Dordrecht, The Netherlands, 2009; pp. 163–196. [Google Scholar] [CrossRef]
- Barshteyn, V.; Krupodorova, T. Utilization of agro-industrial waste by higher mushrooms: Modern view and trends. J. Microbiol. Biotechnol. Food Sci. 2016, 5, 563–577. [Google Scholar] [CrossRef] [Green Version]
- Kamthan, R.; Tiwari, I. Agricultural Wastes—Potential Substrates for Mushroom Cultivation. Eur. J. Exp. Biol. 2017, 7, 31. [Google Scholar] [CrossRef]
- Kumla, J.; Suwannarach, N.; Sujarit, K.; Penkhrue, W.; Kakumyan, P.; Jatuwong, K.; Vadthanarat, S.; Lumyong, S. Cultivation of Mushrooms and Their Lignocellulolytic Enzyme Production through the Utilization of Agro-Industrial Waste. Molecules 2020, 25, 2811. [Google Scholar] [CrossRef] [PubMed]
- Poppe, J. Use of agricultural waste materials in the cultivation of mushrooms. Mushroom Sci. 2000, 15, 3–22. [Google Scholar]
- Ma, Y.; Wang, Q.; Sun, X.; Wang, X.; Su, W.; Song, N. A Study on Recycling of Spent Mushroom Substrate to Prepare Chars and Activated Carbon. BioResources 2014, 9, 3939–3954. [Google Scholar] [CrossRef] [Green Version]
- Hanafi, F.H.; Rezania, S.; Taib, S.M.; Din, M.F.; Yamauchi, M.; Sakamoto, M.; Hara, H.; Park, J.; Ebrahimi, S.S. Environmentally sustainable applications of agro-based spent mushroom substrate (SMS): An overview. J. Mater. Cycles Waste Manag. 2018, 20, 1383–1396. [Google Scholar] [CrossRef]
- Phan, C.W.; Sabaratnam, V. Potential uses of spent mushroom substrate and its associated lignocellulosic enzymes. Appl. Microbiol. Biotechnol. 2012, 96, 863–873. [Google Scholar] [CrossRef]
- Rinker, D.L. Spent Mushroom Substrate Uses. In Edible and Medicinal Mushrooms; Diego, C.Z., Pardo-Giménez, A., Eds.; Wiley-Blackwell: West Sussex, UK, 2017; pp. 427–454. [Google Scholar] [CrossRef]
- Food and Agricultural Organization of the United Nations (FAO). FAO Statistics Division. 2019. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 20 July 2021).
- Geissdoerfer, M.; Savaget, P.; Bocken, N.M.P.; Hultink, E.J. The Circular Economy—A new sustainability paradigm? J. Clean. Prod. 2017, 143, 757–768. [Google Scholar] [CrossRef] [Green Version]
- Zied, D.C.; Sánchez, J.E.; Noble, R.; Pardo-Giménez, A. Use of Spent Mushroom Substrate in New Mushroom Crops to Promote the Transition towards A Circular Economy. Agronomy 2020, 10, 1239. [Google Scholar] [CrossRef]
- Rao, J.R.; Nelson, D.; Lafferty, N.; Moore, J.E.; Millar, B.C.; Xu, J.; Watabe, M. Biohazards and ecotoxicological considerations of landspreading of spent compost wastes. Commun. Agric. Appl. Biol. Sci. 2003, 68, 885–892. [Google Scholar]
- Lou, Z.; Sun, Y.; Bian, S.; Ali Baig, S.; Hu, B.; Xu, X. Nutrient conservation during spent mushroom compost application using spent mushroom substrate derived biochar. Chemosphere 2017, 169, 23–31. [Google Scholar] [CrossRef]
- Kumbhar, A.; Gade, R.; Shitole, A.; Bandgar, M. Nutritional value of spent mushroom substrate of Agaricus bisporus. J. Mycopathol. Res. 2014, 52, 65–68. [Google Scholar]
- Catal, S.; Peksen, A. Physical, chemical and biological properties of spent mushroom substrates of different mushroom species. Acta Hortic. 2020, 1287, 353–360. [Google Scholar] [CrossRef]
- Antunes, F.; Marçal, S.; Taofiq, O.; Morais, A.; Freitas, A.C.; Ferreira, I.; Pintado, M. Valorization of Mushroom by-Products as a Source of Value-Added Compounds and Potential Applications. Molecules 2020, 25, 2672. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Wösten, H.A.B. Mushroom cultivation in the circular economy. Appl. Microbiol. Biotechnol. 2018, 102, 7795–7803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasińska, A. Spent mushroom compost (SMC)—Retrieved added value product closing loop in agricultural production. Acta Agrar. Debr. 2018, 150, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Rajan, S.; Sarsaiya, S.; Jain, S.K. Mushroom for the National Circular Economy. Int. J. Sci. 2020, 7, 58–66. [Google Scholar]
- Mamiro, D.P.; Royse, D.J.; Beelman, R.B. Yield, size, and mushroom solids content of Agaricus bisporus produced on non-composted substrate and spent mushroom compost. World J. Microbiol. Biotechnol. 2007, 23, 1289–1296. [Google Scholar] [CrossRef]
- Mamiro, D.P.; Royse, D.J. The influence of spawn type and strain on yield, size and mushroom solids content of Agaricus bisporus produced on non-composted and spent mushroom compost. Bioresour. Technol. 2008, 99, 3205–3212. [Google Scholar] [CrossRef]
- Gern, R.M.M.; Libardi Junior, N.; Patrício, G.N.; Wisbeck, E.; Chaves, M.B.; Furlan, S.A. Cultivation of Agaricus blazei on Pleurotus spp. spent substrate. Braz. Arch. Biol. Technol. 2010, 53, 939–944. [Google Scholar] [CrossRef] [Green Version]
- González Matute, R.; Figlas, D.; Curvetto, N. Agaricus blazei production on non-composted substrates based on sunflower seed hulls and spent oyster mushroom substrate. World J. Microbiol. Biotechnol. 2011, 27, 1331–1339. [Google Scholar] [CrossRef]
- Ashrafi, R.; Mian, M.H.; Rahman, M.; Jahiruddin, M. Recycling of Spent Mushroom Substrate for the Production of Oyster Mushroom. Res. Biotechnol. 2014, 5, 13–21. [Google Scholar]
- Noonsong, V.; Puttakun, N.; Tinsirisuk, M.; Seephueak, P. Recycling of spent Pleurotus compost for production of the Agrocybe cylindracea. Mycosphere 2016, 7, 36–43. [Google Scholar] [CrossRef]
- Zeng, X.; Han, F.; Ye, J.; Zhong, Y. Recycling spent Pleurotus eryngii substrate supplemented with Tenebrio molitor feces for cultivation of Agrocybe chaxingu. Int. J. Recycl. Org. Waste Agric. 2017, 6, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, C. Cultivation of Pleurotus ostreatus and other edible mushrooms. Appl. Microbiol. Biotechnol. 2010, 85, 1321–1337. [Google Scholar] [CrossRef] [PubMed]
- Ritota, M.; Manzi, P. Pleurotus spp. Cultivation on Different Agri-Food By-Products: Example of Biotechnological Application. Sustainability 2019, 11, 5049. [Google Scholar] [CrossRef] [Green Version]
- Patel, Y.; Naraian, R.; Singh, V.K. Medicinal properties of Pleurotus Species (Oyster Mushroom): A Review. WJFPB 2012, 3, 1–12. [Google Scholar]
- Deepalakshmi, K.; Mirunalini, S. Pleurotus ostreatus: An oyster mushroom with nutritional and medicinal properties. J. Biochem. Tech. 2014, 5, 718–726. [Google Scholar]
- Ahmed, M.; Abdullah, N.; Nuruddin, N.N. Yield and nutritional composition of oyster mushrooms: An alternative nutritional source for rural people. Sains Malays. 2016, 45, 1609–1615. [Google Scholar] [CrossRef] [Green Version]
- Piska, K.; Sułkowska-Ziaja, K.; Muszyńska, B. Edible mushroom Pleurotus ostreatus (Oyster mushroom)—Its dietary significance and biological activity. Acta Sci. Pol. Hort. Cult. 2017, 16, 151–161. [Google Scholar]
- Sałata, A.; Lemieszek, M.; Parzymies, M. The nutritional and health properties of an oyster mushroom (Pleurotus ostreatus (Jacq. Fr) P. Kumm.). Acta Sci. Pol. Hortorum Cultus 2018, 17, 185–197. [Google Scholar] [CrossRef]
- Waktola, G.; Temesgen, T. Pharmacological activities of Oyster mushroom (Pleurotus ostreatus). Novel Res. Microbiol. J. 2020, 4, 688–695. [Google Scholar] [CrossRef]
- Raman, J.; Jang, K.Y.; Oh, Y.L.; Oh, M.; Im, J.H.; Lakshmanan, H.; Sabaratnam, V. Cultivation and Nutritional Value of Prominent Pleurotus spp.: An Overview. Mycobiology 2021, 49, 1–14. [Google Scholar] [CrossRef]
- Cohen, L.; Persky, Y.; Hadar, R. Biotechnological applications and potential of wood-degrading mushrooms of the genus Pleurotus. Appl. Microbiol. Biotechnol. 2002, 58, 582–594. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Mączka, W.; Wińska, K.; Uklańska-Pusz, C.M. Mushrooms of the Pleurotus genus—Properties and application. Biotechnol. Food Sci. 2019, 83, 13–30. [Google Scholar]
- Sekan, A.S.; Myronycheva, O.S.; Karlsson, O.; Gryganskyi, A.P.; Blume, Y.B. Green potential of Pleurotus spp. in biotechnology. PeerJ. 2019, 7, e6664. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, K.; Sharma, A.; Tejwan, N.; Bhardwaj, S.; Bhardwaj, P.; Nepovimova, E.; Shami, A.; Kalia, A.; Kumar, A.; Abd-Elsalam, K.A.; et al. Pleurotus Macrofungi-Assisted Nanoparticle Synthesis and Its Potential Applications: A Review. J. Fungi 2020, 6, 351. [Google Scholar] [CrossRef] [PubMed]
- Kües, U.; Liu, Y. Fruiting body production in basidiomycetes. Appl. Microbiol. Biotechnol. 2000, 54, 141–152. [Google Scholar] [CrossRef]
- Patil, S.S.; Ahmed, S.A.; Telang, S.M.; Baig, M.M.V. The nutritional value of Pleurotus ostreatus (Jacq.:Fr.) Kumm cultivated on different lignocellulosic agro-wastes. Innov. Rom. Food Biotechnol. 2010, 7, 66–76. [Google Scholar]
- Carvalho, C.S.; Sales-Campos, C.; Andrade, C.N. Mushrooms of the Pleurotus Genus: A review of cultivation techniques. Interciencia 2010, 35, 177–182. [Google Scholar]
- Hoa, H.T.; Wang, C.L.; Wang, C.H. The Effects of Different Substrates on the Growth, Yield, and Nutritional Composition of Two Oyster Mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2015, 43, 423–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besufekad, Y.; Mekonnen, A.; Girma, B.; Daniel, R.; Tassema, G.; Melkamu, J.; Asefa, M.; Fikiru, T.; Denboba, L. Selection of appropriate substrate for production of oyster mushroom (Pleurotus ostreatus). J. Yeast Fungal Res. 2020, 11, 15–25. [Google Scholar] [CrossRef]
- Obodai, M.; Cleland-Okine, J.; Vowotor, K.A. Comparative study on the growth and yield of Pleurotus ostreatus mushroom on different lignocellulosic by-products. J. Ind. Microbiol. Biotechnol. 2003, 30, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Estrada, A.E.; Royse, D.J. Yield, size and bacterial blotch resistance of Pleurotus eryngii grown on cottonseed hulls/oak sawdust supplemented with manganese, copper and whole ground soybean. Bioresour. Technol. 2007, 98, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, B.E.; Earnshaw, D.M.; Masarirambi, M.T. Growth and yield response of Oyster Mushroom (Pleurotus ostreatus) grown on different locally available substrates. Curr. Res. J. Biol. Sci. 2012, 4, 623–629. [Google Scholar]
- Mintesnot, B.; Ayalew, A.; Kebede, A. Evaluation of Biomass of Some Invasive Weed Species as Substrate for Oyster Mushroom (Pleurotus spp.) Cultivation. Pak. J. Biol. Sci. 2014, 17, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Kinge, T.R.; Djidjou, T.A.M.; Nji, T.M.; Ache, N.A.; Mih, A.M. Effect of local substrates on the growth and yield of Pleurotus ostreatus K. in the North West Region. Cameroon Curr. Res. Environ. 2016, 6, 11–19. [Google Scholar] [CrossRef]
- Atila, F. Evaluation of suitability of various agro-wastes for productivity of Pleurotus djamor, Pleurotus citrinopileatus and Pleurotus eryngii mushrooms. J. Exp. Agric. Int. 2017, 17, 1–11. [Google Scholar] [CrossRef]
- Fernandes, Â.; Barros, L.; Martins, A.; Herbert, P.; Ferreira, I.C.F.R. Nutritional characterisation of Pleurotus ostreatus (Jacq. ex Fr.) P. Kumm. produced using paper scraps as substrate. Food Chem. 2015, 169, 396–400. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhang, Z.; Li, M.; Li, X.; Sun, Z. Yield, size, nutritional value, and antioxidant activity of oyster mushrooms grown on perilla stalks. Saudi J. Biol. Sci. 2017, 24, 347–354. [Google Scholar] [CrossRef] [Green Version]
- Adebayo, E.A.; Martínez-Carrera, D. Oyster mushrooms (Pleurotus) are useful for utilizing lignocellulosic biomass. Afr. J. Biotechnol. 2015, 14, 52–67. [Google Scholar] [CrossRef]
- Grimm, A.; Eilertsen, L.; Chen, F.; Huang, R.; Atterhem, L.; Xiong, S. Cultivation of Pleurotus ostreatus Mushroom on Substrates Made of Cellulose Fibre Rejects: Product Quality and Spent Substrate Fuel Properties. Waste Biomass Valor. 2021, 12, 4331–4340. [Google Scholar] [CrossRef]
- Mahari, W.A.; Peng, W.; Nam, W.L.; Yang, H.; Lee, X.Y.; Lee, Y.K.; Liew, R.K.; Ma, N.L.; Mohammad, A.; Sonne, C.; et al. A review on valorization of oyster mushroom and waste generated in the mushroom cultivation industry. J. Hazard. Mater. 2020, 400, 123156. [Google Scholar] [CrossRef]
- Bellettini, M.B.; Fiorda, F.A.; Maieves, H.A.; Teixeira, G.L.; Ávila, S.; Hornung, P.S.; Júnior, A.M.; Ribani, R.H. Factors affecting mushroom Pleurotus spp. Saudi J. Biol. Sci. 2019, 26, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Sakoda, A.; Suzuki, M. Biological efficiency and nutritional value of Pleurotus ostreatus cultivated on spent beer grain. Bioresour. Technol. 2001, 78, 293–300. [Google Scholar] [CrossRef]
- Bhattacharjya, D.K.; Paul, R.K.; Miah, M.N.; Ahmed, K.U. Comparative Study on Nutritional Composition of Oyster Mushroom (Pleurotus ostreatus Fr.) Cultivated on Different Sawdust Substrates. Biores. Commun. 2015, 1, 93–98. [Google Scholar]
- Garuba, T.; Abdukkareem, K.A.; Ibrahim, I.A.; Oyebamiji, O.I.; Shoyooye, O.A.; Ajibade, T.D. Influence of substrates on the nutritional quality of Pleurotus pulmonarius and Pleurotus ostreatus. Ceylon J. Sci. 2017, 46, 67. [Google Scholar] [CrossRef] [Green Version]
- Koutrotsios, G.; Danezis, G.P.; Georgiou, C.A.; Zervakis, G.I. Rare earth elements concentration in mushroom cultivation substrates affects the production process and fruit-bodies content of Pleurotus ostreatus and Cyclocybe cylindracea. J. Sci. Food Agric. 2018, 98, 5418–5427. [Google Scholar] [CrossRef] [PubMed]
- Masevhe, M.R.; Soundy, P.; Taylor, N.J. Alternative substrates for cultivating oyster mushrooms (Pleurotus ostreatus). S. Afr. J. Plant Soil. 2015, 33, 97–103. [Google Scholar] [CrossRef]
- Daba, A.S.; El Nakieb, F.; Hafez, E.E. The effect of Recycling Different Wastes (as a substrate) on Mushroom (Pleurotus ostreatus) Fruit Bodies, Morphologically, Genetically and its Metabolites. Biosci. Res. 2018, 15, 2286–2294. [Google Scholar]
- Girmay, Z.; Gorems, W.; Birhanu, G.; Zewdie, S. Growth and yield performance of Pleurotus ostreatus (Jacq. Fr.) Kumm (oyster mushroom) on different substrates. AMB Express 2016, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Tesfay, T.; Godifey, T.; Mesfin, R.; Kalayu, G. Evaluation of waste paper for cultivation of oyster mushroom (Pleurotus ostreatus) with some added Supplementary Materials. AMB Express 2020, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Jo, W.-S.; Kim, J.S.; Cho, D.H.; Park, S.D.; Jung, H.Y. Fruitbody Development of Pleurotus ostreatusvia Bottle Cultivation Using Recycled Substrate. Mycobiology 2008, 36, 157. [Google Scholar] [CrossRef] [Green Version]
- Pardo-Giménez, A.; Picornell Buendía, M.R.; de Juan Valero, J.A.; Pardo-González, J.E.; Cunha Zied, D. Cultivation of Pleurotus ostreatus using supplemented spent oyster mushroom substrate. Acta Hortic. 2012, 933, 267–272. [Google Scholar] [CrossRef]
- Wang, S.; Xu, F.; Li, Z.; Zhao, S.; Song, S.; Rong, C.; Geng, X.; Liu, Y. The spent mushroom substrates of Hypsizigus marmoreus can be an effective component for growing the oyster mushroom Pleurotus ostreatus. Sci. Hortic. 2015, 186, 217–222. [Google Scholar] [CrossRef]
- Picornell, M.R.; Pardo, A.; De Juan-Valerot, J.A. Reuse of degraded Pleurotus ostreatus substrate through supplementation with wheat bran and Calprozime® quantitative parameters. Agron. Colomb. 2015, 33, 261–270. [Google Scholar] [CrossRef]
- Picornell-Buendía, M.R.; Pardo-Gimenéz, A.; de Juan-Valero, J.A. Agronomic assessment of spent mushroom substrates for mushroom cultivation. Biotechnol. Agron. Soc. Environ. 2016, 20, 363–374. [Google Scholar]
- Economou, C.N.; Philippoussis, A.N.; Diamantopoulou, P.A. Spent mushroom substrate for a second cultivation cycle of Pleurotus mushrooms and dephenolization of agro-industrial wastewaters. FEMS Microbiol. Lett. 2020, 367, fnaa060. [Google Scholar] [CrossRef]
- Gąsecka, M.; Mleczek, M.; Siwulski, M.; Niedzielski, P. Phenolic composition and antioxidant properties of Pleurotus ostreatus and Pleurotus eryngii enriched with selenium and zinc. Eur. Food Res. Technol. 2015, 242, 723–732. [Google Scholar] [CrossRef] [Green Version]
- Stamets, P. Growing Gourmet and Medicinal Mushrooms, 3rd ed.; Ten Speed Press: Berkley, CA, USA, 2000. [Google Scholar]
- Mleczek, M.; Gąsecka, M.; Budka, A.; Niedzielski, P.; Siwulski, M.; Kalač, P.; Mleczek, P.; Rzymski, P. Changes in mineral composition of six strains of Pleurotus after substrate modifications with different share of nitrogen forms. Eur. Food Res. Technol. 2021, 247, 245–257. [Google Scholar] [CrossRef]
- Yang, W.; Guo, F.; Wan, Z. Yield and size of oyster mushroom grown on rice/wheat straw basal substrate supplemented with cotton seed hull. Saudi J. Biol. Sci. 2013, 20, 333–338. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.T.; Miles, P.G. Edible Mushrooms and Their Cultivation; CRC Press: Boca Raton, FL, USA, 1989. [Google Scholar]
- Siqueira, O.A.P.A.; Zanon, A.R.; Martins, O.G.; De Andrade, M.C.N. New substrates for the cultivation of Pleurotus ostreatus using exhausted compost. Afr. J. Agric. Res. 2016, 11, 2295–2301. [Google Scholar] [CrossRef] [Green Version]
- Jafarpour, M. High protein complementation with high fiber substrates for oyster mushroom cultures. Afr. J. Biotechnol. 2012, 11, 3284–3289. [Google Scholar] [CrossRef]
- Bonatti, M.; Karnopp, P.; Soares, H.; Furlan, S. Evaluation of Pleurotus ostreatus and Pleurotus sajor-caju nutritional characteristics when cultivated in different lignocellulosic wastes. Food Chem. 2004, 88, 425–428. [Google Scholar] [CrossRef]
- Jin, Z.; Li, Y.; Ren, J.; Qin, N. Yield, Nutritional Content, and Antioxidant Activity of Pleurotus ostreatuson Corncobs Supplemented with Herb Residues. Mycobiology 2018, 46, 24–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meera, P.; Kumaran, S.; Vasudeo, G. Making mushroom production process a zero waste enterprise. Int. J. Environ. Sci. 2014, 5, 236–242. [Google Scholar] [CrossRef]
| Substrates | Substrate Composition (SMS:Wheat Straw-Based Substrate) |
|---|---|
| Wheat straw-based substrate (control) | 0:100 |
| Pholiota nameko (N-SMS) | 10:90 |
| 20:80 | |
| 30:70 | |
| Hypsizygus marmoreus (M-SMS) | 10:90 |
| 20:80 | |
| 30:70 | |
| Hericium erinaceus (E-SMS) | 10:90 |
| 20:80 | |
| 30:70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisiecka, J.; Prasad, R.; Jasinska, A. The Utilisation of Pholiota nameko, Hypsizygus marmoreus, and Hericium erinaceus Spent Mushroom Substrates in Pleurotus ostreatus Cultivation. Horticulturae 2021, 7, 396. https://doi.org/10.3390/horticulturae7100396
Lisiecka J, Prasad R, Jasinska A. The Utilisation of Pholiota nameko, Hypsizygus marmoreus, and Hericium erinaceus Spent Mushroom Substrates in Pleurotus ostreatus Cultivation. Horticulturae. 2021; 7(10):396. https://doi.org/10.3390/horticulturae7100396
Chicago/Turabian StyleLisiecka, Jolanta, Raghavendra Prasad, and Agnieszka Jasinska. 2021. "The Utilisation of Pholiota nameko, Hypsizygus marmoreus, and Hericium erinaceus Spent Mushroom Substrates in Pleurotus ostreatus Cultivation" Horticulturae 7, no. 10: 396. https://doi.org/10.3390/horticulturae7100396
APA StyleLisiecka, J., Prasad, R., & Jasinska, A. (2021). The Utilisation of Pholiota nameko, Hypsizygus marmoreus, and Hericium erinaceus Spent Mushroom Substrates in Pleurotus ostreatus Cultivation. Horticulturae, 7(10), 396. https://doi.org/10.3390/horticulturae7100396








