Advances in Watermelon Grafting to Increase Efficiency and Automation
Abstract
1. Introduction
2. Justification
3. Traditional Watermelon Grafting Techniques
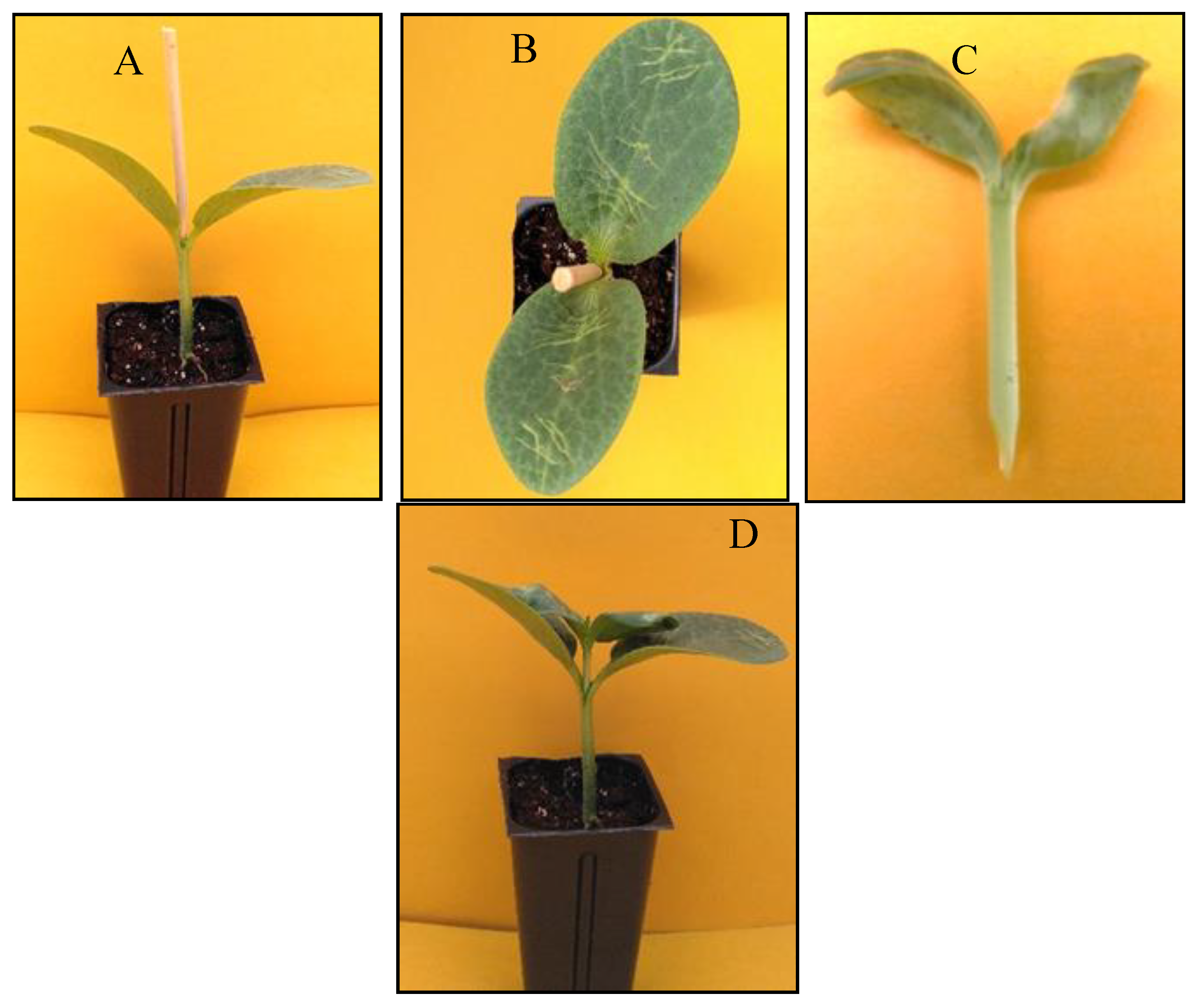
Rootstock Regrowth
4. Splice Grafting Increases Grafting Efficiency
5. Graft Union Formation of Watermelon
5.1. Role of Cotyledons
5.2. Role of Carbohydrates
5.3. Role of Abscisic Acid
5.4. Role of Auxin
6. Limitations in Watermelon Grafting
6.1. Survival
6.2. Cost
6.3. Grafting Automation
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cohen, R.; Burger, Y.; Horev, C.; Koren, A. Introducing grafted cucurbits to modern agriculture: The Israeli experience. Plant Dis. 2007, 91, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.R.; Perkins- Veazie, P.; Sakata, Y.; López- Galarza, S.; Maroto, J.V.; Lee, S.G.; Huh, Y.C.; Miguel, A.; King, S.R.; Cohen, R.; et al. Cucurbit grafting. Crit. Rev. Plant Sci. 2008, 27, 50–74. [Google Scholar] [CrossRef]
- Lee, J.M.; Kubota, C.; Tsao, S.J.; Bie, Z.; Hoyos Echevarria, P.; Morra, L.; Oda, M. Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hortic. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Massa, D.; Salerno, A.; Rea, E. Yield, fruit quality and mineral composition of grafted melon plants grown under saline conditions. J. Hortic. Sci. Biotechnol. 2006, 81, 146–152. [Google Scholar] [CrossRef]
- Johnson, G. Grafted Watermelons Revisited. Weekly Crop Update. University of Delaware Cooperative Extension. 2017. Available online: https://sites.udel.edu/weeklycropupdate/?p=10563 (accessed on 10 September 2018).
- Barrett, C.E.; Zhao, X.; Sims, C.A.; Brecht, J.K.; Dreyer, E.Q.; Gao, Z. Fruit composition and sensory attributes of organic heirloom tomatoes as affected by grafting. HortTechnology 2012, 22, 804–809. [Google Scholar] [CrossRef]
- Galinato, S.P.; Gallardo, R.K. Cost analysis for vegetable grafting. In Grafting Manual: How to Produce Grafted Vegetable Plants; Kubota, C., Miles, C., Zhao, X., Eds.; National Institute of Food and Agriculture: Washington, DC, USA, 2017; Chapter 6.1. [Google Scholar]
- Lewis, M.; Kubota, C.; Tronstad, R.; Son, Y.-J. Scenario-based cost analysis for vegetable grafting nurseries of different technologies and sizes. HortScience 2014, 49, 917–930. [Google Scholar] [CrossRef]
- Taylor, M.; Bruton, B.; Fish, W.; Roberts, W. Cost benefit analysis of using grafted watermelon transplants for fusarium wilt disease control. Acta Hortic. 2008, 782, 343–350. [Google Scholar] [CrossRef]
- Galinato, S.P.; Miles, C.A.; Wimer, J.A. Non-grafted and grafted seedless watermelon transplants: Comparative economic feasibility analysis. Acta Hortic. 2016, 311–312. [Google Scholar] [CrossRef]
- Tateishi, K. Grafting watermelon onto pumpkin. J. Jpn. Hortic. 1927, 39, 5–8. Available online: http://portainnestiortive.it/wp-content/uploads/sites/3/2016/06/Translation-of-Tateishi-1927.pdf (accessed on 22 April 2018). (In Japanese).
- Kubota, C.; McClure, M.A.; Kokalis-Burelle, N.; Bausher, M.G.; Rosskopf, E.N. Vegetable grafting: History, use and current technology status in North America. HortScience 2008, 43, 1664–1669. [Google Scholar] [CrossRef]
- Kubota, C. Environmental control for growth suppression and quality preservation of transplants. Environ. Control. Biol. 2003, 41, 97–105. [Google Scholar] [CrossRef][Green Version]
- Carpenter, J.; Gianessi, L.; Lynch, L. The Economic Impact of the Scheduled U.S. Phaseout of Methyl Bromide. National Center for Food and Agricultural Policy. 2000. Available online: https://www.ncfap.org/documents/mb.pdf (accessed on 22 October 2019).
- U.S. Environmental Protection Agency. The Phase out of Methyl Bromide. Ozone Layer Protection: Regulatory Program. Available online: https://www.epa.gov/ods-phaseout/methyl-bromide (accessed on 17 November 2017).
- Traka-Mavrona, E.; Koutsika-Sotiriou, M.; Pritsa, T. Response of squash (Cucurbita spp.) as rootstock for melon (Cucumis melo L.). Scientia. Hortic. 2000, 83, 353–362. [Google Scholar] [CrossRef]
- Ertle, J.; Kubota, C. North American Grafting Survey. Ohio State University. 2019. Available online: https://cpb-us-w2.wpmucdn.com/u.osu.edu/dist/e/50423/files/2020/03/North-American-Grafting-Survey.pdf (accessed on 11 March 2020).
- U.S. Department of Agriculture. National Agricultural Statistics Service. Vegetables 2019 Summary. 2020. Available online: https://www.nass.usda.gov/Publications/Todays_Reports/reports/vegean20.pdf (accessed on 26 October 2020).
- Gunar, N.; (Sakata Seed America, Morgan Hill, CA, USA). Personal communication, 2020.
- Guan, W.; Zhao, X. Techniques for Melon Grafting. Acta Hortic. 2016, 1140, 335–336. [Google Scholar] [CrossRef]
- Sakata, Y.; Ohara, T.; Sugiyama, M. The history and present state of the grafting of cucurbitaceous vegetables in Japan. Acta Hortic. 2007, 731, 159–170. [Google Scholar] [CrossRef]
- Miles, C.; Devi, P.; Zhao, X.; Guan, W. Watermelon and melon grafting. In Grafting Manual: How to Produce Grafted Vegetable Plants; Kubota, C., Miles, C., Zhao, X., Eds.; National Institute of Food and Agriculture: Washington, DC, USA, 2017; Chapter 3.2.1. [Google Scholar]
- Kubota, C. History of grafting. In Grafting Manual: How to Produce Grafted Vegetable Plants; Kubota, C., Miles, C., Zhao, X., Eds.; National Institute of Food and Agriculture: Washington, DC, USA, 2016; Chapter 1.1. [Google Scholar]
- Saito, M. New Grafting Methods for Vegetables; Nobunkyo—Rural Culture Association: Japan, Tokyo, 1981; 162p. [Google Scholar]
- Miles, C.; Hesnault, L.; Johnson, S.; Kreider, P.; Dabirian, S. Vegetable Grafting: Watermelon. Washington State University Extension Fact Sheet FS100E. 2016. Available online: https://s3.wp.wsu.edu/uploads/sites/2071/2014/04/Grafting-Watermelon-FS100E.pdf (accessed on 20 March 2018).
- Hassell, R.L.; Memmott, F.; Liere, D.G. Grafting methods for watermelon production. HortScience 2008, 43, 1677–1679. [Google Scholar] [CrossRef]
- Memmott, F.D.; Hassell, R.L. Watermelon (Citrullus lanatus) grafting method to reduce labor cost by eliminating rootstock side shoots. Acta Hortic. 2010, 871, 389–394. [Google Scholar] [CrossRef]
- Kurata, K. Cultivation of grafted vegetables II. Development of grafting robots in Japan. HortScience 1994, 29, 240–244. [Google Scholar] [CrossRef]
- Daley, S.L.; Hassell, R.L. Fatty alcohol application to control meristematic regrowth in bottle gourd and interspecific hybrid squash rootstocks used for grafting watermelon. HortScience 2014, 49, 60–264. [Google Scholar] [CrossRef]
- Oda, M. Vegetable seedling grafting in Japan. Acta Hortic. 2007, 759, 175–180. [Google Scholar] [CrossRef]
- Rivard, C.L.; Sydorovych, O.; O’Connell, S.; Peet, M.M.; Louws, F.J. An economic analysis of two grafted tomato transplant production systems in the United States. HortTechnology 2010, 20, 794–803. [Google Scholar] [CrossRef]
- Dabirian, S.; Miles, C.A. Increasing survival of splice-grafted watermelon seedlings using a sucrose application. HortScience 2017, 52, 579–583. [Google Scholar] [CrossRef]
- Devi, P.; Lukas, S.; Miles, C. Fruit maturity and quality of splice-grafted and one-cotyledon grafted watermelon. HortScience 2020, 55, 1090–1098. [Google Scholar] [CrossRef]
- Crafts, A.S. Phloem anatomy, exudation, and transport of organic nutrients in cucurbits. Plant Physiol. 1932, 7, 183–225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, X.; Ayre, B.; Turgeon, R. The origin and composition of cucurbit “phloem” exudate. Plant Physiol. 2012, 158, 1873–1882. [Google Scholar] [CrossRef]
- Aloni, B.; Karni, L.; Deventurero, G.; Levin, Z.; Cohen, R.; Katzir, N.; Lotan-Pompan, M.; Edelstein, M.; Aktas, H.; Turhan, E.; et al. Physiological and biochemical changes at the rootstock−scion interface in graft combinations between Cucurbita rootstocks and a melon scion. J. Hortic. Sci. Biotech. 2008, 83, 777–783. [Google Scholar] [CrossRef]
- Cushman, K. Grafting Techniques for Watermelon; University of Florida Cooperative Extension Service: Gainesville, FL, USA, 2006; Available online: https://ufdcimages.uflib.ufl.edu/IR/00/00/33/82/00001/HS33000.pdf (accessed on 10 June 2018).
- Guan, W.; Zhao, X. Effects of grafting methods and root excision on growth characteristics of grafted muskmelon plants. HortTechnology 2015, 25, 706–713. [Google Scholar] [CrossRef]
- Hunter, J.J.; Volschenk, C.G.; Le Roux, D.J.; Adams, L. Plant Material Quality: A Compilation of Research; ARC Infruitec-Nietvoorbij: Stellenbosch, South Africa, 2004; Available online: http://www.winetech.co.za/documents/plantmaterial/plantmaterialquality.pdf (accessed on 15 December 2018).
- Ogata, T.; Kabashima, Y.; Shiozaki, S.; Horiuchi, S. Regeneration of the vascular bundle at the graft interface in auto- and heterografts with juvenile nucellar seedlings of satsuma mandarin, yuzu and trifoliate orange. J. Jpn. Soc. Hortic. Sci. 2005, 74, 214–220. [Google Scholar] [CrossRef][Green Version]
- Melnyk, C.W. Monitoring vascular regeneration and xylem connectivity in Arabidopsis thaliana. Methods Mol. Biol. 2017, 1544, 91–102. [Google Scholar] [CrossRef]
- Bhalerao, R.P.; Eklöf, J.; Ljung, K.; Marchant, A.; Bennett, M.; Sandberg, G. Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J. 2002, 29, 325–332. [Google Scholar] [CrossRef]
- Nanda, A.K.; Melnyk, C.W. The role of plant hormones during grafting. J. Plant Res. 2018, 131, 49–58. [Google Scholar] [CrossRef]
- Procko, C.; Crenshaw, C.M.; Ljung, K.; Noel, J.P.; Chory, J. Cotyledon-generated auxin is required for shade-induced hypocotyl growth in Brassica rapa. Plant Physiol. 2014, 165, 1285–1301. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.C.; Kwon, S.W.; Ko, B.R.; Choi, J.S. Using chemical controls to inhibit axillary buds of Lagenaria as rootstock for grafted watermelon (Citrullus lanatus). Acta Hortic. 2002, 588, 43–48. [Google Scholar] [CrossRef]
- Bruton, B.D.; Fletcher, J.; Pair, S.D.; Shaw, M.; Sittertz-Bhatkar, H. Association of a phloem-limited bacterium with yellow vine disease in cucurbits. Plant Dis. 1998, 82, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Hagimori, M. Studies in establishment of transplant production methods of sweet pepper (Capsicum annuum L.) by grafting shoots harvested from mother plants: Effect of healing conditions of grafts on the rate and quality of successful union. J. Jpn. Soc. Hortic. Sci. 2004, 73, 380–385. [Google Scholar] [CrossRef]
- Fernandez-Garcia, N.; Carvajal, M.; Olmos, E. Graft union formation in tomato plants: Peroxidase and catalase involvement. Ann. Bot. 2004, 93, 53–60. [Google Scholar] [CrossRef]
- Melnyk, C.W.; Gabel, A.; Hardcastle, T.J.; Robinson, S.; Miyashima, S.; Grosse, I.; Meyerowitz, E.M. Transcriptome dynamics at Arabidopsis graft junctions reveal an intertissue recognition mechanism that activates vascular regeneration. Proc. Natl. Acad. Sci. USA 2018, 115, E2447–E2456. [Google Scholar] [CrossRef]
- Dabirian, S.; Miles, C.A. Antitranspirant application increases grafting success of watermelon. HortTechnology 2017, 52, 579–583. [Google Scholar] [CrossRef]
- Marguerit, E.; Brendel, O.; Lebon, E.; Van Leeuwen, C.; Ollat, N. Rootstock control of scion transpiration and its acclimation to water deficit are controlled by different genes. New Phytol. 2012, 194, 416–429. [Google Scholar] [CrossRef]
- Daszkowska-Golec, A.; Szarejko, I. Open or close the gate–stomata action under the control of phytohormones in drought stress conditions. Front. Plant Sci. 2013, 4, 138. [Google Scholar] [CrossRef]
- Leung, J.; Giraudat, J. Abscisic acid signal transduction. Annu. Rev. Plant Biol. 1998, 49, 199–222. [Google Scholar] [CrossRef]
- Moftah, A.E.; Al-Redhaiman, K.N. Effects of preharvest foliar spray of ’Limewash’ on water relations, quantity, quality, and shelf life of bell pepper under water deficit conditions. Eur. J. Hortic. Sci. 2006, 71, 78–83. [Google Scholar] [CrossRef]
- Kramer, P. Water Relations of Plants; Academic Pres Inc.: New York, NY, USA, 1983. [Google Scholar]
- Dunn, B.L.; Cole, J.C.; Payton, M.E. Use of antitranspirants to reduce water stress on herbaceous and woody ornamentals. J. Environ. Hortic. 2012, 30, 137–145. [Google Scholar] [CrossRef]
- Gomez-Cadenas, A.; Arbona, V.; Jacas, J.; Primo-Millo, E.; Talon, M. Abscisic acid reduces leaf abscission and increases salt tolerance in citrus plants. J. Plant Growth Regul. 2003, 21, 234–240. [Google Scholar] [CrossRef]
- Tambussi, E.A.; Bort, J.; Araus, J.L. Water use efficiency in C3 cereals under Mediterranean conditions: A review of physiological aspects. Ann. Appl. Biol. 2007, 150, 307–321. [Google Scholar] [CrossRef]
- Johnson, G. Can Antitranspirants and Antidesiccants Improve Vegetable Transplant Survival? Weekly Crop Update. University of Delaware Cooperative Extension. 2012. Available online: https://agdev.anr.udel.edu/weeklycropupdate/?p=3942 (accessed on 20 September 2018).
- Nitzsche, P.; Berkowitz, G.A.; Rabin, J. Development of a seedling applied antitranspirant formulation to enhance water status, growth, and yield of transplanted bell pepper. J. Am. Soc. Hortic. Sci. 1991, 116, 405–411. [Google Scholar] [CrossRef]
- Bonner, J.; Galston, A.W. Principles of plant physiology. Agron. J. 1952, 44, 277–278. [Google Scholar] [CrossRef]
- Rost, T.L.; Barbour, M.G.; Thornton, R.M.; Wiever, T.E.; Stocking, C.R. Botany; Wiley & Sons: New York, NY, USA, 1984; 342p. [Google Scholar]
- Raven, P.H.; Evert, R.F.; Eichhom, S.E. Biology of Plants; Worth Publishers: New York, NY, USA, 1992; 791p. [Google Scholar]
- Salisbury, F.B.; Ross, C.W. Plant Physiology; Wadsworth Publishing Company: Belmont, CA, USA, 1992; 682p. [Google Scholar]
- Preece, J.; Read, P. The Biology of Horticulture; Wiley & Sons: New York, NY, USA, 1993; 480p. [Google Scholar]
- Friml, J.; Palme, K. Polar auxin transport–old questions and new concepts? Plant Mol. Biol. 2002, 49, 273–284. [Google Scholar] [CrossRef]
- Donner, T.J.; Sherr, I.; Scarpella, E. Regulation of preprocambial cell state acquisition by auxin signaling in Arabidopsis leaves. Development 2009, 136, 3235–3246. [Google Scholar] [CrossRef]
- Wenzel, C.L.; Schuetz, M.; Yu, Q.; Mattsson, J. Dynamics of MONOPTEROS and PIN-FORMED1 expression during leaf vein pattern formation in Arabidopsis thaliana. Plant J. 2007, 49, 387–398. [Google Scholar] [CrossRef]
- Aloni, R. Role of auxin and sucrose in the differentiation of sieve and tracheary elements in plant tissue cultures. Planta 1980, 150, 255–263. [Google Scholar] [CrossRef]
- Mazur, E.; Benková, E.; Friml, J. Vascular cambium regeneration and vessel formation in wounded inflorescence stems of Arabidopsis. Sci. Rep. 2016, 6, 33754. [Google Scholar] [CrossRef] [PubMed]
- Sauer, M.; Balla, J.; Luschnig, C.; Wiśniewska, J.; Reinöhl, V.; Friml, J.; Benková, E. Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Genes Dev. 2006, 20, 2902–2911. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M. Metabolic regulation of photosynthesis. In Photosynthesis and the Environment; Baker, N.R., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; pp. 151–190. [Google Scholar]
- Sachs, T. On the determination of the pattern of vascular tissue in peas. Ann. Bot. 1968, 32, 781–790. [Google Scholar] [CrossRef]
- Katsumi, M.; Chiba, Y.; Fukuvama, M. The roles of the cotyledons and auxin in the adventitious root formation of hypocotyl cuttings of light-grown cucumber seedlings. Physiol. Plant. 1969, 22, 993–1000. [Google Scholar] [CrossRef]
- El-Eslamboly, A.S.A. Seedless watermelon propagation by cuttings: A. Effect of planting containers, cutting types and IBA on transplants production from cuttings. Bul. Fac. Agric. Cairo Univ. 2014, 65, 183–192. [Google Scholar]
- Buajaila, F.A.; Devi, P.; Miles, C.A. Effect of environment on survival of eggplant, pepper, and tomato in a small-scale healing chamber. HortTechnology 2018, 28, 668–675. [Google Scholar] [CrossRef]
- Johnson, S.J.; Miles, C.A. Effect of healing chamber design on the survival of grafted eggplant, tomato, and watermelon. HortTechnology 2011, 21, 752–758. [Google Scholar] [CrossRef]
- Kubota, C.; Miles, C.A. Healing and acclimatization methods and design principles. In Grafting Manual: How to Produce Grafted Vegetable Plants; Kubota, C., Miles, C., Zhao, X., Eds.; National Institute of Food and Agriculture: Washington, DC, USA, 2016; Chapter 2.4. [Google Scholar]
- Aloni, B.; Cohen, R.; Karni, L.; Aktas, H.; Edelstein, M. Hormonal signaling in rootstock-scion interactions. Sci Hortic. 2010, 127, 119–126. [Google Scholar] [CrossRef]
- Pina, A.; Errea, P. A review of new advances in mechanism of graft compatibility–incompatibility. Sci. Hortic. 2005, 106, 1–11. [Google Scholar] [CrossRef]
- Mullendore, D.L.; Windt, C.W.; Van As, H.; Knoblauch, M. Sieve tube geometry in relation to phloem flow. Plant Cell 2010, 22, 579–593. [Google Scholar] [CrossRef]
- Turgeon, R.; Oparka, K. The secret phloem of pumpkins. Proc. Natl. Acad. Sci. USA 2010, 107, 13201–13202. [Google Scholar] [CrossRef] [PubMed]
- Van Bel, A.J.; Gaupels, F. Pathogen-induced resistance and alarm signals in the phloem. Mol. Plant Path. 2004, 5, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tolstikov, V.; Turnbull, C.; Hicks, L.M.; Fiehn, O. Divergent metabolome and proteome suggest functional independence of dual phloem transport systems in cucurbits. Proc. Natl. Acad. Sci. USA 2010, 107, 13532–13537. [Google Scholar] [CrossRef] [PubMed]
- Asahina, M.; Iwai, H.; Kikuchi, A.; Yamaguchi, S.; Kamiya, Y.; Kamada, H.; Satoh, S. Gibberellin produced in the cotyledon is required for cell division during tissue-reunion in the cortex of cut cucumber and tomato hypocotyls. Plant Physiol. 2002, 129, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Kubota, C.; Meng, C.; Son, Y.J.; Lewis, M.; Spalholz, H.; Tronstad, R. Horticultural, systems-engineering and economic evaluations of short-term plant storage techniques as a labor management tool for vegetable grafting nurseries. PLoS ONE 2017, 12, 1–17. [Google Scholar] [CrossRef]
- Ito, T. Present state of transplant production practices in Japanese horticultural industry. In Transplant Production Systems; Kurata, K., Kozai, T., Eds.; Springer: Berlin, Germany, 1992. [Google Scholar]
- Kobayashi, K. Grafting Robot for Fruit Vegetables. Research Journal of Food and Agriculture (Japan). 2005. Available online: https://agris.fao.org/agris-search/search.do?recordID=JP2006005207 (accessed on 12 February 2019).
- Bie, Z.; Nawaz, M.A.; Huang, Y.; Lee, J.M.; Colla, G. Introduction of vegetable grafting. In Vegetable Grafting: Principles and Practices; CABI Publishing: Wallingford, UK, 2017; pp. 1–21. [Google Scholar]
- Lee, J.M.; Oda, M. Grafting of herbaceous vegetable and ornamental crops. Hortic. Rev. 2003, 28, 61–123. [Google Scholar] [CrossRef]
- Suzuki, M.; Sasaya, S.; Kobayashi, K. Present status of vegetable grafting systems. Jpn. Agric. Res. Q. 1998, 32, 105–112. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devi, P.; Lukas, S.; Miles, C. Advances in Watermelon Grafting to Increase Efficiency and Automation. Horticulturae 2020, 6, 88. https://doi.org/10.3390/horticulturae6040088
Devi P, Lukas S, Miles C. Advances in Watermelon Grafting to Increase Efficiency and Automation. Horticulturae. 2020; 6(4):88. https://doi.org/10.3390/horticulturae6040088
Chicago/Turabian StyleDevi, Pinki, Scott Lukas, and Carol Miles. 2020. "Advances in Watermelon Grafting to Increase Efficiency and Automation" Horticulturae 6, no. 4: 88. https://doi.org/10.3390/horticulturae6040088
APA StyleDevi, P., Lukas, S., & Miles, C. (2020). Advances in Watermelon Grafting to Increase Efficiency and Automation. Horticulturae, 6(4), 88. https://doi.org/10.3390/horticulturae6040088





