Effects of the Biofertilizer OYK (Bacillus sp.) Inoculation on Endophytic Microbial Community in Sweet Potato
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth Condition, Inoculation, and Cultivation of Sweet Potato
2.2. Sample Collection and Isolation of Endophytic Bacteria
2.3. Genetic Analysis of Endophytes
2.4. Analysis of the Community Structure of Endophytes
2.5. Nucleotide Sequence Accession Numbers
2.6. Statistical Analysis
3. Results
3.1. Effects of OYK Inoculation
3.2. Isolation of Endophytic Bacterial Strains
3.3. Genetic Analysis of Endophytes
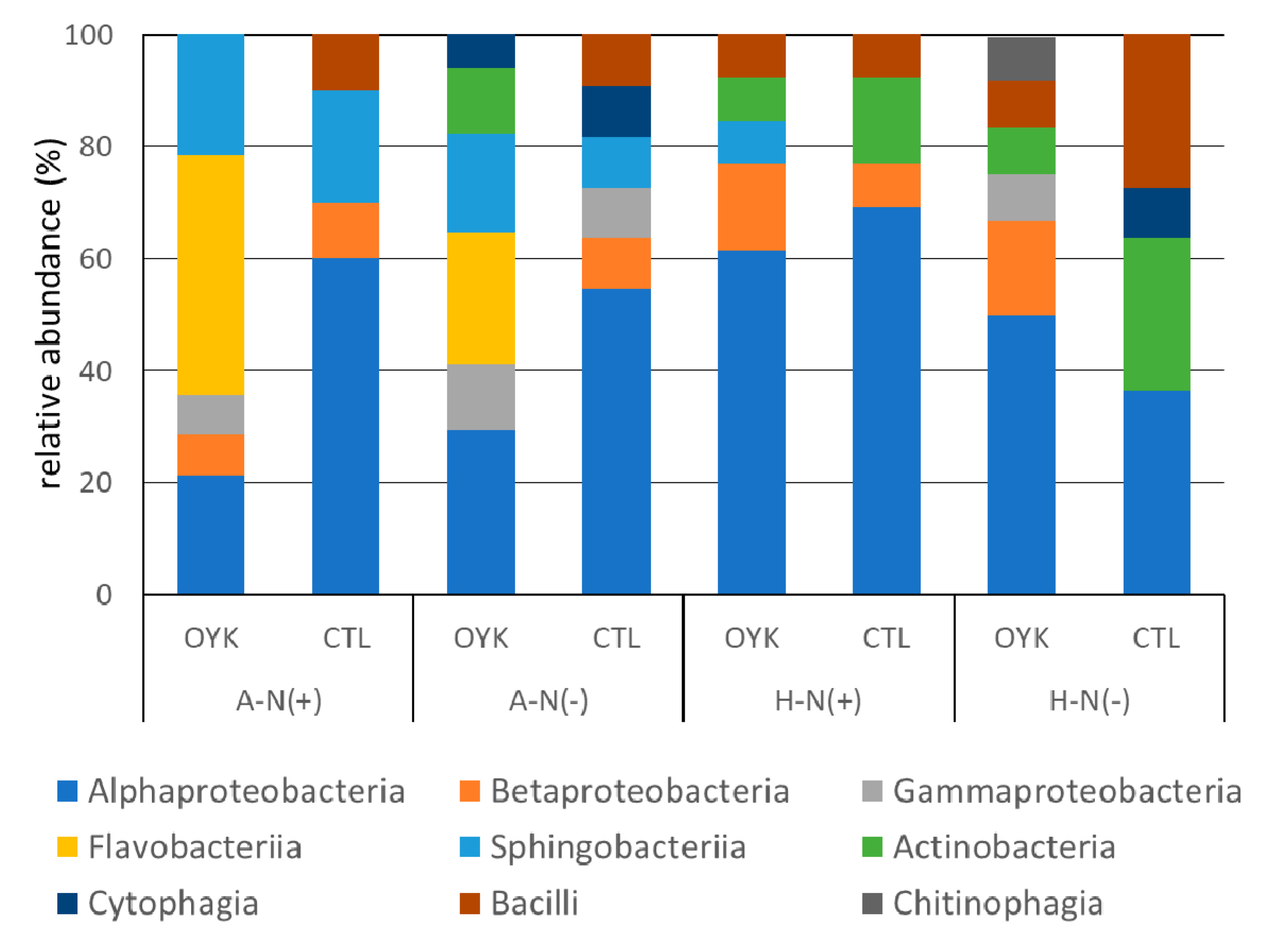
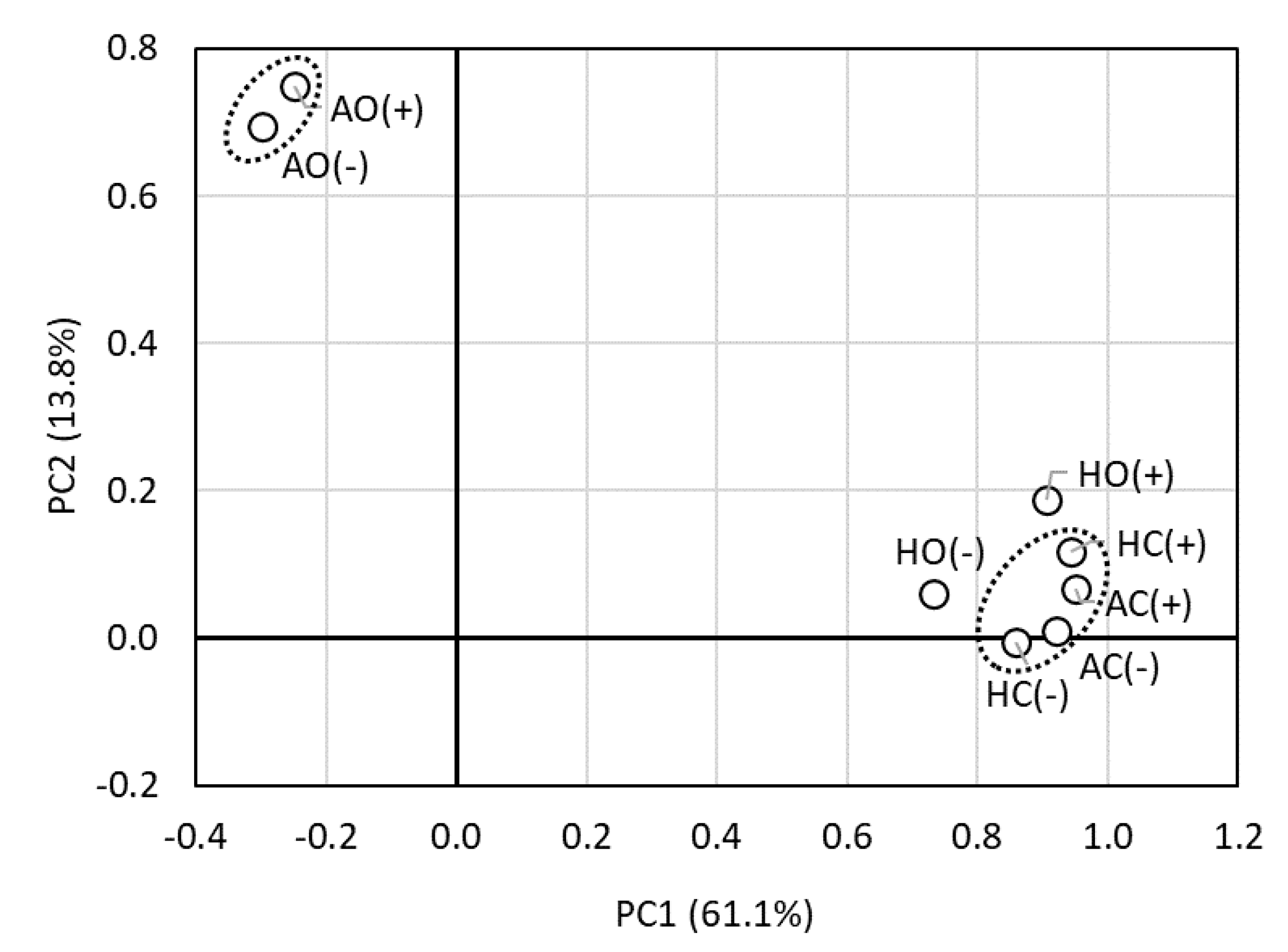
3.4. Community Structure of Endophytes
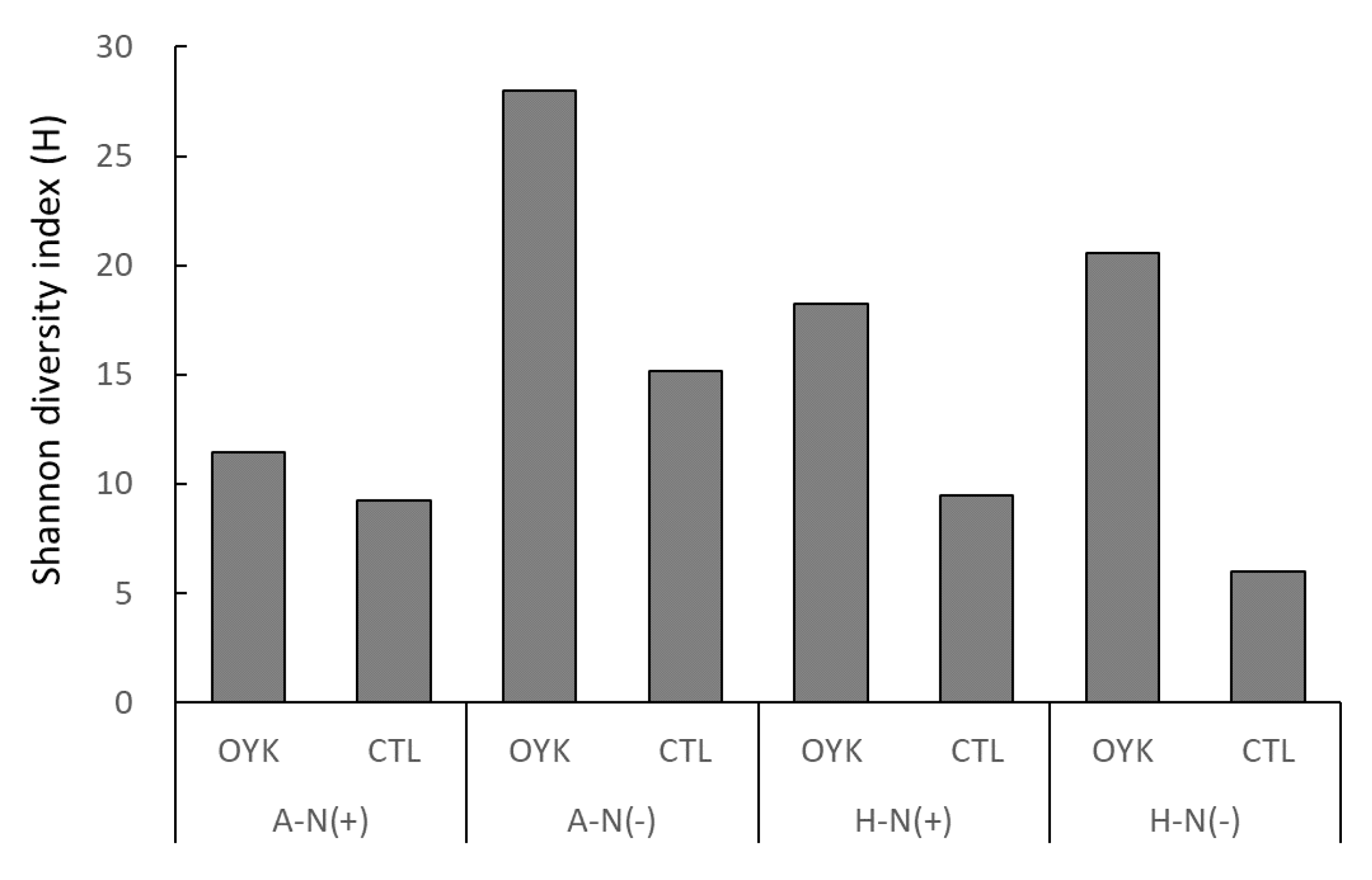
3.5. Diversity of Endophytes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- Adesemoye, A.O.; Torbert, H.A.; Kloepper, J.W. Plant growth-promoting rhizobacteria allow reduced application rates of chemical fertilizers. Microb. Ecol. 2009, 58, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.F.O.; Hower, J.C.; Izquierdo, M.; Querol, X. Complex nanominerals and ultrafine particles assemblages in phosphogypsum of the fertilizer industry and implications on human exposure. Sci. Total Environ. 2010, 408, 5117–5122. [Google Scholar] [CrossRef] [PubMed]
- Ashrafuzzaman, M.; Hossen, F.A.; Ismail, M.R.; Hoque, M.A.; Islam, M.Z.; Shahidullah, S.M.; Meon, S. Efficiency of plant growth-promoting rhizobacteria (PGPR) for the enhancement of rice growth Afr. J. Biotechnol. 2009, 8, 1247–1252. [Google Scholar]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Z.; Li, W.; Yan, R.; Li, L.; Li, Y.; Li, M. Growth promoting effect of a transgenic Bacillus mucilaginosus on tobacco planting. Appl. Microbiol. Biotechnol. 2007, 74, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Nosratabad, A.R.F.; Etesami, H.; Shariati, S. Integrated use of organic fertilizer and bacterial inoculant improves phosphorus use efficiency in wheat (Triticum aestivum L.) fertilized with triple superphosphate. Rhizosphere 2017, 3, 109–111. [Google Scholar] [CrossRef]
- Dawwam, G.E.; Elbeltagy, A.; Emara, H.M.; Abbas, I.H.; Hassan, M.M. Beneficial effect of plant growth promoting bacteria isolated from the roots of potato plant. Annal. Agric. Sci. 2013, 58, 195–201. [Google Scholar] [CrossRef]
- Reiter, B.; Burgmann, H.; Burg, K.; Sessitsch, A. Endophytic gene diversity in African sweet potato. Can. J. Microbiol. 2003, 49, 549–555. [Google Scholar] [CrossRef][Green Version]
- Souza, V.C.; Lorenzi, H. Botânica sistemática. In Guia Ilustrado Para Identificação das Famílias de Angiospermas da Flora Brasileira; Souza, V.C., Lorenzi, H., Eds.; Instituto Plantarum: Nova Odessa, Brazil, 2008; p. 640. [Google Scholar]
- Hartemink, A.E.; Poloma, S.; Maino, M.; Powell, K.S.; Egenae, J.; O’Sullivan, J.N. Yield decline of sweet potato in the humid lowlands of Papua New Guinea. Agric. Ecosyst. Environ. 2000, 79, 259–269. [Google Scholar] [CrossRef]
- Hill, W.A.; Hortense, D.; Hahn, S.K.; Mulongoy, K.; Adeyeye, S.O. Sweet potato root and biomass production with and without nitrogen fertilization. Agron. J. 1990, 82, 1120. [Google Scholar] [CrossRef]
- Yonebayashi, K.; Katsumi, N.; Nishi, T.; Okazaki, M. Activation of nitrogen-fixing endophytes is associated with the tuber growth of sweet potato. Mass Spectrom. 2014, 3, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, C.B.; Pasternak, J.J.; Glick, B.R. Partial purification and characterization of 1-aminocyclopropane-1-carboxylate deaminase from the plant growth promoting rhizobacterium Pseudomonas putida GR 12–2. Can. J. Microbiol. 1994, 40, 1019–1025. [Google Scholar] [CrossRef]
- Dhungana, S.A.; Adachi, F.; Hayashi, S.; Puri, R.R.; Itoh, K. Plant growth promoting effects of Nepalese sweet potato endophytes. Horticulturae 2018, 4, 53. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Bacterial modulation of plant ethylene levels. Plant Physiol. 2015, 169, 13–22. [Google Scholar] [CrossRef]
- O’Sullivan, D.J.; O’Gara, F. Traits of fluorescent Pseudomonas spp. involved in suppression of plant root pathogens. Microbiol. Rev. 1992, 56, 662–676. [Google Scholar] [CrossRef]
- Khan, M.S.; Gao, J.; Chen, X.; Zhang, M.; Yang, F.; Du, Y.; Moe, T.S.; Munir, I.; Xue, J.; Zhang, X. Isolation and characterization of plant growth-promoting endophytic bacteria Paenibacillus polymyxa SK1 from Lilium lancifolium. Biomed. Res. Int. 2020, 8650957. [Google Scholar] [CrossRef]
- Terakado-Tonooka, J.; Fujihara, S.; Ohwaki, Y. Possible contribution of Bradyrhizobium on nitrogen fixation in sweet potatoes. Plant Soil 2013, 367, 639–650. [Google Scholar] [CrossRef]
- Bangera, M.G.; Thomashow, L.S. Characterization of a genomic locus required for synthesis of the antibiotic 2, 4-diacetylphloroglucinol by the biological control agent Pseudomonas fluorescens Q2–87. Mol. Plant Microbe. Interact. 1996, 9, 83–90. [Google Scholar] [CrossRef]
- Benhamou, N.; Kloepper, J.W.; Quadt-Hallman, A.; Tuzun, S. Induction of defence related ultrastructural modifications in pea root tissues inoculated with endophytic bacteria. Plant Physiol. 1996, 112, 919–929. [Google Scholar] [CrossRef]
- Hallmann, J.; Quadt-Hallmann, A.; Mahaffee, W.F.; Kloepper, J.W. Bacterial endophytes in agricultural crops. Can. J. Microbiol. 1997, 43, 895–914. [Google Scholar] [CrossRef]
- Sturz, A.V.; Christie, B.R.; Nowak, J. Bacterial endophytes: Potential role in developing sustainable systems of crop production. Critic. Rev. Plant Sci. 2000, 19, 1–30. [Google Scholar] [CrossRef]
- Adachi, K.; Nakatani, M.; Mochida, H. Isolation of an endophytic diazotroph, Klebsiella oxytoca, from sweet potato stems in Japan. Soil Sci. Plant Nutr. 2002, 48, 889–895. [Google Scholar] [CrossRef]
- Asis, C.A.; Adachi, K. Isolation of endophytic diazotroph Pantoea agglomerans and nondiazotroph Enterobacter asburiae from sweet potato stem in Japan. Lett. Appl. Microbiol. 2004, 38, 19–23. [Google Scholar] [CrossRef]
- Khan, Z.; Doty, S.L. Characterization of bacterial endophytes of sweet potato plants. Plant Soil 2009, 322, 197–207. [Google Scholar] [CrossRef]
- Marques, J.M.; da Silva, T.F.; Vollú, R.E.; de Lacerda, J.R.M.; Blank, A.F.; Smalla, K.; Seldin, L. Bacterial endophytes of sweet potato tuberous roots affected by the plant genotype and growth stage. Appl. Soil Ecol. 2015, 96, 273–281. [Google Scholar] [CrossRef]
- Puri, R.R.; Dangi, S.; Dhungana, S.A.; Itoh, K. Diversity and plant growth promoting ability of culturable endophytic bacteria in Nepalese sweet potato. Ad. Microbiol. 2018, 8, 734–761. [Google Scholar] [CrossRef]
- Trabelsi, D.; Mhamdi, R. Microbial inoculants and their impact on soil microbial communities: A review. BioMed Res. Int. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Conn, V.M.; Franco, C.M. Effect of microbial inoculants on the indigenous actinobacterial endophyte population in the roots of wheat as determined by terminal restriction fragment length polymorphism. Appl. Environ. Microbiol. 2004, 70, 6407–6413. [Google Scholar] [CrossRef]
- Gadhave, K.R.; Devlin, P.F.; Ebertz, A.; Ross, A.; Gange, A.C. Soil inoculation with Bacillus spp. modifies root endophytic bacterial diversity, evenness, and community composition in a context-specific manner. Microbial. Ecol. 2018, 76, 741–750. [Google Scholar] [CrossRef]
- Ono, K.; Yamanaka, D.; Watanabe, N.U.S. Microorganism. International Patent No. WO99/11756; GB Application No. 09/485,939; U.S. Patent 6,399,056, 4 June 2002. [Google Scholar]
- Aizaki, M.; Sumita, A. Effect of hydrophytes on the control of water temperature in model wetland type green roof garden. Environ. Sci. 2005, 18, 535–540. [Google Scholar]
- Itoh, K.; Ohashi, K.; Yakai, N.; Adachi, F.; Hayashi, H. Changes in acetylene reduction activities and nifH genes associated with field-grown sweet potatoes with different nursery farmers and cultivars. Horticulturae 2019, 5, 1–9. [Google Scholar]
- Elbeltagy, A.; Nishioka, K.; Sato, T.; Suzuki, H.; Ye, B.; Hamada, T.; Isawa, T.; Mitsui, H.; Minamisawa, K. Endophytic colonization and in planta nitrogen fixation by a Herbaspirillum sp. isolated from wild rice species. Appl. Environ. Microbiol. 2001, 67, 5285–5293. [Google Scholar] [PubMed]
- Saeki, Y.; Kaneko, A.; Hara, T.; Suzuki, K.; Yamakawa, T.; Nguyen, M.T.; Nagatomo, Y.; Akao, S. Phylogenetic analysis of soybean-nodulating rhizobia isolated from alkaline soils in Vietnam. Soil Sci. Plant Nutr. 2005, 51, 1043–1052. [Google Scholar]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S Ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [PubMed]
- Poly, F.; Monrozier, L.J.; Bally, R. Improvement in the RFLP procedure for studying the diversity of nifH genes in communities of nitrogen fixers in soil. Res. Microbiol. 2001, 152, 95–103. [Google Scholar]
- Adhikari, D.; Kaneto, M.; Itoh, K.; Suyama, K.; Pokharel, B.B.; Gaihre, Y.K. Genetic diversity of soybean-nodulating rhizobia in Nepal in relation to climate and soil properties. Plant Soil 2012, 357, 131–145. [Google Scholar]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2. Bioinformatics 2007, 23, 2947–2948. [Google Scholar]
- Felici, C.; Vettori, L.; Giraldi, E.; Forino, L.M.C.; Toffanin, A.; Tagliasacchi, A.M.; Nuti, M. Single and co-inoculation of Bacillus subtilis and Azospirillum brasilense on Lycopersicon esculentum: Effects on plant growth and rhizosphere microbial community. Appl. Soil. Ecol. 2008, 40, 260–270. [Google Scholar]
- Valenzuela-Soto, J.H.; Estrada-Hernandez, M.G.; Ibarra-Laclette, E.; Delano-Frier, J.P. Inoculation of tomato plants (Solanum lycopersicum) with growth-promoting Bacillus subtilis retards whitefly Bemisia tabaci development. Planta 2010, 231, 397–410. [Google Scholar] [PubMed]
- Qiao, J.; Yu, X.; Liang, X.; Liu, Y.; Borriss, R.; Liu, Y. Addition of plant-growth-promoting Bacillus subtilis PTS-394 on tomato rhizo- sphere has no durable impact on composition of root microbiome. BMC Microbiol. 2017, 17, 131. [Google Scholar]
- Nascimento, F.X.; Hernández, A.G.; Glick, B.R.; Rossi, M.J. Plant growth-promoting activities and genomic analysis of the stress-resistant Bacillus megaterium STB1 a bacterium of agricultural and biotechnological interest. Biotechnol. Rep. 2019, 25, e00406. [Google Scholar] [CrossRef] [PubMed]
- Weifang, X.; Wang, F.; Zhang, M.; Ou, T.; Wang, R.; Strobel, G.; Xiang, Z.; Zhou, Z.; Xie, J. Diversity of cultivable endophytic bacteria in mulberry and their potential for antimicrobial and plant growth-promoting activities. Microbiol. Res. 2019, 229, 126328. [Google Scholar]
- Chowdhury, S.P.; Dietel, K.; Ranndler, M.; Schmid, M.; Junge, H.; Borriss, R.; Hartmann, A.; Grosch, R. Effects of Bacillus amyloliquefaciens FZB42 on lettuce growth and health under pathogen pressure and its impact on the rhizosphere bacterial community. PLoS ONE 2013, 8, e68818. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, Y.; Lai, X.H.; Shan, C.; Deng, Z.; Ji, Y. Screening and characterization of endophytic Bacillus and Paenibacillus strains from medicinal plant Lonicera japonica for use as potential plant growth promoters. Braz. J. Microbiol. 2015, 46, 977–989. [Google Scholar]
- Yu, X.; Ai, C.; Xin, L.; Zhou, G. The siderophore-producing bacterium, Bacillus subtilis CAS15, has a biocontrol effect on Fusarium wilt and promotes the growth of pepper. Eur. J. Soil Biol. 2011, 47, 138–145. [Google Scholar]
- Khedher, S.B.; Kilani-Feki, O.; Dammak, M.; Jabnoun-Khiareddine, H.; Daami-Remadi, M.; Tounsi, S. Efficacy of Bacillus subtilis V26 as a biological control agent against Rhizoctonia solani on potato. Comptes Rendus Biol. 2015, 338, 784–792. [Google Scholar] [CrossRef]
- Dutta, S.; Rani, T.S.; Podile, A.R. Root exudate-induced alterations in Bacillus cereus cell wall contribute to root colonization and plant growth promotion. PLoS ONE 2013, 8, e78369. [Google Scholar]
- Sharaf-Eldin, M.; Elkholy, S.; Fernandez, J.A.; Junge, H.; Cheetham, R.; Guardiola, J.; Weathers, P. Bacillus subtilis FZB24 (R) affects flower quantity and quality of saffron (Crocus sativus). Planta Med. 2008, 74, 1316. [Google Scholar]
- Lacey, L.A.; Frutos, R.; Kaya, H.K.; Vail, P. Insect pathogens as biological control agents: Do they have a future? Biol. Control 2001, 21, 230–248. [Google Scholar] [CrossRef]
- Paulitz, T.C.; Belanger, R.R. Biological control in greenhouse systems. Ann. Rev. Phytopathol. 2001, 39, 103–133. [Google Scholar]
- Singh, B.K.; Dawson, L.A.; Macdonald, C.A.; Buckland, S.M. Impact of biotic and abiotic interaction on soil microbial communities and functions: A field study. Appl. Soil Ecol 2009, 41, 239–248. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Overbeek, V.L.S.; Elsas, J.D.V. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 2008, 16, 463–471. [Google Scholar] [CrossRef]
- Puri, R.R.; Adachi, F.; Omichi, M.; Saeki, Y.; Yamamoto, A.; Hayashi, S.; Itoh, K. Culture-dependent analysis of endophytic bacterial community of sweet potato (Ipomoea batatas) in different soils and climates. J. Adv. Microbiol. 2018, 13, 1–12. [Google Scholar] [CrossRef]
- Marques, J.M.; Da Silva, T.F.; Vollu, R.E.; Blank, A.F.; Ding, G.-C.; Seldin, L.; Smalla, K. Plant age and genotype affect the bacterial community composition in the tuber rhizosphere of field-grown sweet potato plants. FEMS Microbiol. Ecol. 2014, 88, 424–435. [Google Scholar] [CrossRef]
- Dalmastri, C.; Chiarini, L.; Cantale, C.; Bevivino, A.; Tabacchiono, S. Soil type and maize cultivar affect the genetic diversity of maize root-associated Burkholderia cepacia populations. Microb. Ecol. 1999, 38, 273–284. [Google Scholar] [CrossRef]
- Miller, H.J.; Henken, G.; Van Veen, J.A. Variation and composition of bacterial populations in the rhizosphere of maize, wheat and grass cultivars. Can. J. Microbiol. 1989, 35, 656–660. [Google Scholar] [CrossRef]
- Fromin, N.; Achouak, W.; Thiery, J.M.; Heulin, T. The genotypic diversity of Pseudomonas brassicacearum populations isolated from roots of Arabidopsis thaliana: Influence of plant genotype. FEMS Microbiol. Ecol. 2001, 37, 21–29. [Google Scholar] [CrossRef]
- Germida, J.J.; Siciliano, S.D.; Renato, D.F.J.; Seib, A.M. Diversity of root-associated bacteria associated with field-grown canola (Brassica napus L.) and wheat (Triticum aestivum L.). FEMS Microbiol. Ecol. 1998, 26, 43–50. [Google Scholar] [CrossRef]
- Tian, B.; Zhang, C.; Ye, Y.; Wen, J.; Wu, Y.; Wang, H.; Li, H.; Cai, S.; Cai, W.; Cheng, Z.; et al. Beneficial traits of bacterial endophytes belonging to the core communities of the tomato root microbiome. Agric. Ecosyst. Environ. 2017, 247, 149–156. [Google Scholar] [CrossRef]
- Souza, S.A.; Xavier, A.A.; Costa, M.R.; Cardoso, A.M.S.; Pereira, M.C.T.; Nietsche, S. Endophytic bacterial diversity in banana “Prata Anã” (Musa spp.) roots. Gene Mol. Biol. 2013, 36, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Greissworth, E.; Mucci, C.; Williams, M.A.; Bolt, S.D. Characterization of culturable bacterial endophytes of switchgrass (Panicum virgatum L.) and their capacity to influence plant growth. GCB Bioenergy 2013, 5, 674–682. [Google Scholar] [CrossRef]
- Liu, B.; Qiao, H.; Huang, L.; Buchenauer, H.; Han, Q.; Kang, Z.; Gong, Y. Biological control of take-all in wheat by endophytic Bacillus subtilis E1R-j and potential mode of action. Biol. Control 2009, 49, 277–285. [Google Scholar] [CrossRef]

| Sample a | CFU b | Isolated c | Morphology d | Selected e | Identified f |
|---|---|---|---|---|---|
| AO-N(+) | 32 | 32 | 11 | 14 | 14 |
| AO-N(−) | 42 | 40 | 17 | 17 | 17 |
| AC-N(+) | 22 | 13 | 6 | 10 | 10 |
| AC-N(−) | 24 | 18 | 9 | 12 | 11 |
| HO-N(+) | 50 | 50 | 12 | 15 | 13 |
| HO-N(−) | 46 | 42 | 14 | 15 | 12 |
| HC-N(+) | 31 | 21 | 11 | 13 | 13 |
| HC-N(−) | 22 | 16 | 11 | 13 | 11 |
| Total | 269 | 232 | - | 109 | 101 |
| Class/Genus | Beniazuma (A) | Beniharuka (H) | ||||||
|---|---|---|---|---|---|---|---|---|
| N (+) | N (−) | N (+) | N (−) | |||||
| OYK | CTL | OYK | CTL | OYK | CTL | OYK | CTL | |
| α-Proteobacteria | 21 | 60 | 29 | 55 | 62 | 69 | 50 | 36 |
| Novosphingobium | - | 50 | - | 45 | 38 | 54 | 25 | 36 |
| Rhizobium | - | 10 | 6 | - | - | 15 | - | - |
| Sphingomonas | 21 | - | 6 | - | 8 | - | 8 | - |
| Sphingobium | - | - | 12 | - | 15 | - | 8 | - |
| Caulobacter | - | - | 6 | 9 | - | - | 8 | - |
| β-Proteobacteria | 7 | 10 | - | 9 | 15 | 8 | 17 | - |
| Methylibium | - | 10 | - | - | - | - | - | - |
| Burkholderia | - | - | - | 9 | - | - | - | - |
| Variovorax | 7 | - | - | - | 8 | 8 | - | - |
| Mitsuaria | - | - | - | 8 | - | 17 | ||
| γ-Proteobacteria | 7 | - | 12 | 9 | - | - | 8 | - |
| Pseudoxanthomonas | 7 | - | - | - | - | - | 8 | - |
| Stenotrophomonas | - | - | 6 | - | - | - | - | - |
| Pseudomonas | - | - | 6 | - | - | - | - | - |
| Dyella | - | - | - | 9 | - | - | - | - |
| Flavobacteria | 43 | - | 24 | - | - | - | - | - |
| Chryseobacterium | 21 | - | 24 | - | - | - | - | - |
| Acinetobacter | 21 | - | - | - | - | - | - | - |
| Sphingobacteria | 21 | 20 | 18 | 9 | 8 | - | - | - |
| Mucilaginibacter | - | 20 | - | 9 | 8 | - | - | - |
| Sphingobacterium | 21 | - | - | - | - | - | - | - |
| Pedobacter | - | - | 18 | - | - | - | - | - |
| Actinobacteria | - | - | 12 | - | 8 | 15 | 8 | 27 |
| Microbacterium | - | - | - | - | 8 | 15 | 8 | 27 |
| Streptomyces | - | - | 6 | - | - | - | - | - |
| Lysinimonas | - | - | 6 | - | - | - | - | - |
| Cytophagia | - | - | 6 | 9 | - | - | - | 9 |
| Dyadobacter | - | - | 6 | - | - | - | - | 9 |
| Chryseolinea | - | - | - | 9 | - | - | - | - |
| Bacilli | - | 10 | - | 9 | 8 | 8 | 8 | 27 |
| Bacillus | - | 10 | - | 9 | 8 | 8 | 8 | 27 |
| Chitinophagia | - | - | - | - | - | - | 8 | - |
| Filimonas | - | - | - | - | - | - | 8 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salehin, A.; Hafiz, M.H.R.; Hayashi, S.; Adachi, F.; Itoh, K. Effects of the Biofertilizer OYK (Bacillus sp.) Inoculation on Endophytic Microbial Community in Sweet Potato. Horticulturae 2020, 6, 81. https://doi.org/10.3390/horticulturae6040081
Salehin A, Hafiz MHR, Hayashi S, Adachi F, Itoh K. Effects of the Biofertilizer OYK (Bacillus sp.) Inoculation on Endophytic Microbial Community in Sweet Potato. Horticulturae. 2020; 6(4):81. https://doi.org/10.3390/horticulturae6040081
Chicago/Turabian StyleSalehin, Ahsanul, Md Hafizur Rahman Hafiz, Shohei Hayashi, Fumihiko Adachi, and Kazuhito Itoh. 2020. "Effects of the Biofertilizer OYK (Bacillus sp.) Inoculation on Endophytic Microbial Community in Sweet Potato" Horticulturae 6, no. 4: 81. https://doi.org/10.3390/horticulturae6040081
APA StyleSalehin, A., Hafiz, M. H. R., Hayashi, S., Adachi, F., & Itoh, K. (2020). Effects of the Biofertilizer OYK (Bacillus sp.) Inoculation on Endophytic Microbial Community in Sweet Potato. Horticulturae, 6(4), 81. https://doi.org/10.3390/horticulturae6040081





