Valorization of Spent Coffee Grounds, Biochar and other residues to Produce Lightweight Clay Ceramic Aggregates Suitable for Nursery Grapevine Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Lightweight Aggregates Production and Characterization
2.2. Nursery Greenhouse Experiments
2.3. Recorded Parameters
2.4. Data Analysis
3. Results and Discussion
3.1. Lightweight Aggregates Characteristics
3.2. Bare-Rooted Vines Production
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reynolds, A.G. Managing Wine Quality: Viticulture and Wine Quality; Woodhead Publishing: Cambridge, UK, 2010. [Google Scholar]
- Ronga, D.; Francia, E.; Allesina, G.; Pedrazzi, S.; Zaccardelli, M.; Pane, C.; Tava, A.; Bignami, C. Valorization of vineyard by-products to obtain composted digestate and biochar suitable for nursery grapevine (Vitis vinifera L.) production. Agronomy 2019, 9, 420. [Google Scholar] [CrossRef]
- Tesic, D.; Keller, M.; Hutton, R.J. Influence of vineyard floor management practices on grapevine vegetative growth, yield, and fruit composition. Am. J. Enol. Vitic. 2007, 58, 1–11. [Google Scholar]
- Arrobas, M.; Ferreira, I.Q.; Freitas, S.; Verdial, J.; Rodrigues, M.Â. Guidelines for fertilizer use in vineyards based on nutrient content of grapevine parts. Sci. Hortic. 2014, 172, 191–198. [Google Scholar] [CrossRef]
- Palliotti, A.; Tombesi, S.; Silvestroni, O.; Lanari, V.; Gatti, M.; Poni, S. Changes in vineyard establishment and canopy management urged by earlier climate-related grape ripening: A review. Sci. Hortic. 2014, 178, 43–54. [Google Scholar] [CrossRef]
- Eldon, J.; Gershenson, A. Effects of Cultivation and Alternative Vineyard Management Practices on Soil Carbon Storage in Diverse Mediterranean Landscapes: A Review of the Literature. Agroecol. Sustain. Food Syst. 2015, 39, 516–550. [Google Scholar] [CrossRef]
- Medrano, H.; Tomás, M.; Martorell, S.; Bota, J. Improving water use efficiency of vineyards in semi-arid regions. A review. Agron. Sustain. Dev. 2015, 35, 499–517. [Google Scholar] [CrossRef]
- Borsellino, V.; Galati, A.; Schimmenti, E. Survey on the innovation in the Sicilian grapevine nurseries. J. Wine Res. 2012, 23, 1–13. [Google Scholar] [CrossRef]
- Waite, H.; May, P.; Bossinger, G. Variations in phytosanitary and other management practices in Australian grapevine nurseries. Phytopathol. Mediterr. 2013, 52, 369–379. [Google Scholar]
- Whitelaw-Weckert, M.A.; Rahman, L.; Appleby, L.M.; Hall, A.; Clark, A.C.; Waite, H.; Hardie, W.J. Co-infection by B otryosphaeriaceae and I lyonectria spp. fungi during propagation causes decline of young grafted grapevines. Plant Pathol. 2013, 62, 1226–1237. [Google Scholar] [CrossRef]
- Salomé, C.; Coll, P.; Lardo, E.; Metay, A.; Villenave, C.; Marsden, C.; Blanchart, E.; Hinsinger, P.; Le Cadre, E. The soil quality concept as a framework to assess management practices in vulnerable agroecosystems: A case study in Mediterranean vineyards. Ecol. Indic. 2016, 61, 456–465. [Google Scholar] [CrossRef]
- Bozzolo, A.; Pizzeghello, D.; Cardinali, A.; Francioso, O.; Nardi, S. Effects of moderate and high rates of biochar and compost on grapevine growth in a greenhouse experiment. AIMS Agric. Food 2017, 2, 113–128. [Google Scholar] [CrossRef]
- Ciesielczuk, T.; Rosik-Dulewska, C.; Wiśniewska, E. Possibilities of coffee spent ground use as a slow action organo-mineral fertilizer. Rocz. Ochr. Srodowiska 2015, 17, 422–437. [Google Scholar]
- Stanley, G.; Matthews, M.A. The influence of Phosphorus availability, scion, and rootstock on grapevine shoot growth, leaf area, and petiole Phosphorus concentration. Am. J. Enol. Vitic. 1996, 47, 217–224. [Google Scholar]
- Baldi, E.; Miotto, A.; Ceretta, C.A.; Brunetto, G.; Muzzi, E.; Sorrenti, G.; Quartieri, M. Soil application of P can mitigate the copper toxicity in grapevine: Physiological implications. Sci. Hortic. 2018, 238, 400–407. [Google Scholar] [CrossRef]
- Gautier, A.; Cookson, S.J.; Hevin, C.; Vivin, P.; Lauvergeat, V.; Mollier, A. Phosphorus acquisition efficiency and phosphorus remobilization mediate genotype-specific differences in shoot phosphorus content in grapevine. Tree Physiol. 2018, 38, 1742–1751. [Google Scholar] [CrossRef]
- Ronga, D.; Mantovi, P.; Pacchioli, M.T.; Pulvirenti, A.; Bigi, F.; Allesina, G.; Pedrazzi, S.; Dal Prà, A. Combined Effects of Dewatering, Composting and Pelleting to Valorize and Delocalize Livestock Manure, Improving Agricultural Sustainability. Agronomy 2020, 10, 661. [Google Scholar] [CrossRef]
- Bortolini, S.; Macavei, L.I.; Saadoun, J.H.; Foca, G.; Ulrici, A.; Bernini, F.; Malferrari, D.; Setti, L.; Ronga, D.; Maistrello, L. Hermetia illucens (L.) larvae as chicken manure management tool for circular economy. J. Clean. Prod. 2020, 262, 121289. [Google Scholar] [CrossRef]
- Ronga, D.; Pellati, F.; Brighenti, V.; Laudicella, K.; Laviano, L.; Fedailaine, M.; Benvenuti, S.; Pecchioni, N.; Francia, E. Testing the influence of digestate from biogas on growth and volatile compounds of basil (Ocimum basilicum L.) and peppermint (Mentha x piperita L.) in hydroponics. J. Appl. Res. Med. Aromat. Plants 2018, 11, 18–26. [Google Scholar] [CrossRef]
- Ronga, D.; Caradonia, F.; Parisi, M.; Bezzi, G.; Parisi, B.; Allesina, G.; Pedrazzi, S.; Francia, E. Using digestate and biochar as fertilizers to improve processing tomato production sustainability. Agronomy 2020, 10, 138. [Google Scholar] [CrossRef]
- Andreola, F.; Borghi, A.; Pedrazzi, S.; Allesina, G.; Tartarini, P.; Lancellotti, I.; Barbieri, L. Spent Coffee Grounds in the Production of Lightweight Clay Ceramic Aggregates in View of Urban and Agricultural Sustainable Development. Materials 2019, 12, 3581. [Google Scholar] [CrossRef]
- Andreola, F.; Lancellotti, I.; Manfredini, T.; Barbieri, L. The circular economy of agro and post-consumer residues as raw materials for sustainable ceramics. Int. J. Appl. Ceram. Technol. 2020, 17, 22–31. [Google Scholar] [CrossRef]
- Andreola, F.; Barbieri, L.; Lancellotti, I.; Pozzi, P.; Tartarini, P.; Vezzali, V.; Allesina, G.; Pedrazzi, S. Patent Pending 102018000009844, Procedimento per Utilizzare Char da Gassificazione e/o Pirolisi con Altri Scarti Industriali per la Formulazione di Materiali Alleggeriti con Effetto Fertilizzante e di Materiali Polimerici per Isolamento Termico; University of Modena and Reggio Emilia: Emilia, Italy, 2018. [Google Scholar]
- European Coffee Federation. European Coffee Report 2017–2018. 2019. Available online: https://www.ecf-coffee.org/publications/european-coffee-report (accessed on 1 September 2020).
- Comitato Italiano del Caffè. Esportazione Caffè 2017–2019. Available online: http://comitcaf.it/index.php/esportazione-caffe/ (accessed on 30 July 2020).
- Allesina, G.; Pedrazzi, S.; Lovato, F.; Allegretti, F.; Tartarini, P.; Siligardi, C. Discussion of possible coffee grounds disposal chains for energy production. In Proceedings of the 23rd EUBCE (European Biomass Conference and Exhibition), Wien, Austria, 1–4 June 2015. [Google Scholar]
- Van Zwieten, L.; Kimber, S.; Sinclair, K.; Chan, K.Y.; Downie, A. Biochar: Potential for climate change mitigation, improved yield and soil health. In Pastures at the Cutting Edge: Proceedings of the 23rd Annual Conference of the Grassland Society of NSW, Tamworth, Australia, 21–23 July 2008; Boschma, S.P., Serafin, L.M., Ayres, J.F., Eds.; Grassland Society of NSW Inc: Orange, Australia, 2008; pp. 30–33152. 152p. [Google Scholar]
- Yamato, M.; Okimori, Y.; Wibowo, I.F.; Anshori, S.; Ogawa, M. Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. Soil Sci. Plant Nutr. 2006, 52, 489–495. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Windeatt, J.H.; Ross, A.A.; Williams, P.T.; Forster, P.M.; Nahil, M.A.; Singh, S. Characteristics of biochars from crop residues: Potential for carbon sequestration and soil amendment. J. Environ. Manag. 2014, 146, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Decree, L. Decreto Legislativo n. 75, 29 Aprile 2010: Riordino e Revisione Della Disciplina in Materia di Fertilizzanti, a Norma Dell’articolo 13 Della Legge 7 Luglio 2009 n. 88. Gazzetta Ufficiale della Repubblica Italiana 2010, 106; European Parliament and European Council. Regulation (EC) No 2003/2003 of the European Parliament and of the Council Related to Fertilizers; European Union: Brussels, Belgium, 2003. [Google Scholar]
- Lorenz, D.H.; Eichhorn, K.W.; Blei-Holder, H.; Klose, R.; Meier, U.; Weber, E. Phenological growth stages and BBCH—Identification keys of grapevine (Vitis vinifera L. ssp. vinifera). In Growth Stages of Mono and Dicotyledonous Plants—BBCH Monograph, 2nd ed.; Meier, U., Ed.; Federal Biological Research Centre for Agriculture and Forestry: Berlin, Germany, 2001; p. 158. [Google Scholar]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant 2012, 146, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Ronga, D.; Pentangelo, A.; Parisi, M. Optimizing N Fertilization to Improve Yield, Technological and Nutritional Quality of Tomato Grown in High Fertility Soil Conditions. Plants 2020, 9, 575. [Google Scholar] [CrossRef]
- Waite, H.; Whitelaw-Weckert, M.; Torley, P. Grapevine propagation: Principles and methods for the production of high-quality grapevine planting material. N. Z. J. Crop Hortic. Sci. 2015, 43, 144–161. [Google Scholar] [CrossRef]
- Valdés-Gómez, H.; Celette, F.; García de Cortázar-Atauri, I.; Jara-Rojas, F.; Ortega-Farías, S.; Gary, C. Modelling soil water content and grapevine growth and development with STICS crop-soil model under two different water management strategies. J. Int. Sci. Vigne Vin 2009, 43, 13–28. [Google Scholar] [CrossRef]
- Carey, D.E.; McNamara, P.J.; Zitomer, D.H. Biochar from pyrolysis of biosolids for nutrient adsorption and turfgrass cultivation. Water Environ. Res. 2015, 87, 2098–2106. [Google Scholar] [CrossRef]
- Caradonia, F.; Ronga, D.; Catellani, M.; Giaretta Azevedo, C.V.; Terrazas, R.A.; Robertson-Albertyn, S.; Francia, E.; Bulgarelli, D. Nitrogen fertilizers shape the composition and predicted functions of the microbiota of field-grown tomato plants. Phytobiomes J. 2019, 3, 315–325. [Google Scholar] [CrossRef]
- Prichard, T. Vineyard Irrigation Systems. In Raisin Production Manual University of California Agricultural and Natural Resources; Apex: Oakland, CA, USA, 2000; Volume 3393, pp. 57–63. [Google Scholar]
- Richards, D. The grape root system. Hortic. Rev. 1983, 5, 127–168. [Google Scholar]
- Clarke, S.J.; Lamont, K.J.; Pan, H.Y.; Barry, L.A.; Hall, A.; Rogiers, S.Y. Spring root-zone temperature regulates root growth, nutrient uptake and shoot growth dynamics in grapevines. Aust. J. Grape Wine Res. 2015, 21, 479–489. [Google Scholar] [CrossRef]
- Rogiers, S.Y.; Clarke, S.J.; Schmidtke, L.M. Elevated root-zone temperature hastens vegetative and reproductive development in Shiraz grapevines. Aust. J. Grape Wine Res. 2014, 20, 123–133. [Google Scholar] [CrossRef]
- Jacobs, D.F.; Timmer, V.R. Fertilizer-induced changes in rhizosphere electrical conductivity: Relation to forest tree seedling root system growth and function. New For. 2005, 30, 147–166. [Google Scholar] [CrossRef]
- Abad, M.; Noguera, P.; Bures, S. National inventory of organic wastes for use as growing media for ornamental potted plant production: Case study in Spain. Bioresour. Technol. 2001, 77, 197–200. [Google Scholar] [CrossRef]
- Ronga, D.; Pane, C.; Zaccardelli, M.; Pecchioni, N. Use of spent coffee ground compost in peat-based growing media for the production of basil and tomato potting plants. Commun. Soil Sci. Plant 2016, 47, 356–368. [Google Scholar] [CrossRef]
- Schmidt, H.P.; Kammann, C.; Niggli, C.; Evangelou, M.W.; Mackie, K.A.; Abiven, S. Biochar and biochar-compost as soil amendments to a vineyard soil: Influences on plant growth, nutrient uptake, plant health and grape quality. Agric. Ecosyst. Environ. 2014, 191, 117–123. [Google Scholar] [CrossRef]
- Vaccari, F.P.; Maienza, A.; Miglietta, F.; Baronti, S.; Di Lonardo, S.; Guagnoni, L.; Lagomarsino, A.; Pozzi, A.; Pusceddu, E.; Ranieri, R.; et al. Biochar stimulates plant growth but not fruit yield of processing tomato in a fertile soil. Agric. Ecosyst. Environ. 2015, 207, 163–170. [Google Scholar] [CrossRef]
- Sigua, G.C.; Novak, J.M.; Watts, D.W.; Johnson, M.G.; Spokas, K. Efficacies of designer biochars in improving biomass and nutrient uptake of winter wheat grown in a hard setting subsoil layer. Chemosphere 2016, 142, 176–183. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, X.; Zheng, W.; Scott, J.W.; Sharma, B.K.; Chen, X. Copyrolysis of biomass with phosphate fertilizers to improve biochar carbon retention, slow nutrient release, and stabilize heavy metals in soil. ACS Sustain. Chem. Eng. 2016, 4, 1630–1636. [Google Scholar] [CrossRef]
- Gwenzi, W.; Nyambishi, T.J.; Chaukura, N.; Mapope, N. Synthesis and nutrient release patterns of a biochar-based N–P–K slow-release fertilizer. Int. J. Environ. Sci. Technol. 2018, 15, 405–414. [Google Scholar] [CrossRef]
- Setti, L.; Francia, E.; Pulvirenti, A.; Gigliano, S.; Zaccardelli, M.; Pane, C.; Caradonia, F.; Bortolini, S.; Maistrello, L.; Ronga, D. Use of black soldier fly (Hermetia illucens (L.), Diptera: Stratiomyidae) larvae processing residue in peat-based growing media. Waste Manag. 2019, 95, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Raviv, M.; Oka, Y.; Katan, J.; Hadar, Y.; Yogev, A.; Medina, S.; Krasnovskya, A.; Ziadna, H. High-nitrogen compost as a medium for organic container-grown crops. Bioresour. Technol. 2005, 96, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Amendola, C.; Montagnoli, A.; Terzaghi, M.; Trupiano, D.; Oliva, F.; Baronti, S.; Miglietta, F.; Chiatante, D.; Scippa, G.S. Short-term effects of biochar on grapevine fine root dynamics and arbuscular mychorrhizae production. Agric. Ecosyst. Environ. 2017, 239, 236–245. [Google Scholar] [CrossRef]
- Gaiotti, F.; Marcuzzo, P.; Belfiore, N.; Lovat, L.; Fornasier, F.; Tomasi, D. Influence of compost addition on soil properties, root growth and vine performances of Vitis vinifera cv Cabernet sauvignon. Sci. Hortic. 2017, 225, 88–95. [Google Scholar] [CrossRef]
- Bernal-Vicente, A.; Ros, M.; Tittarelli, F.; Intrigliolo, F.; Pascual, J.A. Citrus compost and its water extract for cultivation of melon plants in greenhouse nurseries. Evaluation of nutriactive and biocontrol effects. Bioresour. Technol. 2008, 99, 8722–8728. [Google Scholar] [CrossRef]
- Ronga, D.; Caradonia, F.; Setti, L.; Hagassou, D.; Giaretta Azevedo, C.V.; Milc, J.; Pedrazzi, S.; Allesina, G.; Arru, L.; Francia, E. Effects of innovative biofertilizers on yield of processing tomato cultivated in organic cropping systems in northern Italy. Acta Hortic. 2019, 1233, 129–136. [Google Scholar] [CrossRef]
- Herrera, F.; Castillo, J.E.; Chica, A.F.; López Bellido, L. Use of municipal solid waste compost (MSWC) as a growing medium in the nursery production of tomato plants. Bioresour. Technol. 2008, 99, 287–296. [Google Scholar] [CrossRef]
- Ericsson, T.; Rytter, L.; Vapaavuori, E. Physiology of carbon allocation in trees. Biomass Bioenergy 1996, 11, 115–127. [Google Scholar] [CrossRef]
- Ronga, D.; Setti, L.; Salvarani, C.; De Leo, R.; Bedin, E.; Pulvirenti, A.; Mil, J.; Francia, E. Effects of solid and liquid digestate for hydroponic baby leaf lettuce (Lactuca sativa L.) cultivation. Sci. Hortic. 2019, 244, 172–181. [Google Scholar] [CrossRef]
- Leghari, S.J.; Wahocho, N.A.; Laghari, G.M.; HafeezLaghari, A.; MustafaBhabhan, G.; HussainTalpur, K.; Lashari, A.A. Role of nitrogen for plant growth and development: A review. Adv. Environ. Biol. 2016, 10, 209–219. [Google Scholar]
- Araujo, F.; Williams, L.E.; Matthews, M.A. A comparative study of young ‘Thompson Seedless’ grapevines (Vitis vinifera L) under drip and furrow irrigation. II. Growth, water use efficiency and nitrogen partitioning. Sci. Hortic. 1995, 60, 251–265. [Google Scholar] [CrossRef]
- Graber, E.R.; Tsechansky, L.; Mayzlish-Gati, E.; Shema, R.; Koltai, H. A humic substances product extracted from biochar reduces Arabidopsis root hair density and length under P-sufficient and P-starvation conditions. Plant Soil 2015, 395, 21–30. [Google Scholar] [CrossRef]
- Jindo, K.; Martim, S.A.; Navarro, E.C.; Pérez-Alfocea, F.; Hernandez, T.; Garcia, C.; Aguiar, N.O.; Canellas, L.P. Root growth promotion by humic acids from composted and non-composted urban organic wastes. Plant Soil 2012, 353, 209–220. [Google Scholar] [CrossRef]
| Physical Properties | CSCG15FG30 | CBSCG15FG30 |
| Weight Loss (%) | 16.73 | 17.82 |
| Water Absorption (%) | 14.29 | 13.48 |
| App. Density (kg/m3) | 1490 | 1400 |
| Calculated Total Porosity (%) | 42.21 | 45.35 |
| Chemical Properties | CSCG15FG30 | CBSCG15FG30 |
| pH | 7.24 | 8.31 |
| Electrical Conductivity (dS m−1) | 0.24 | 0.36 |
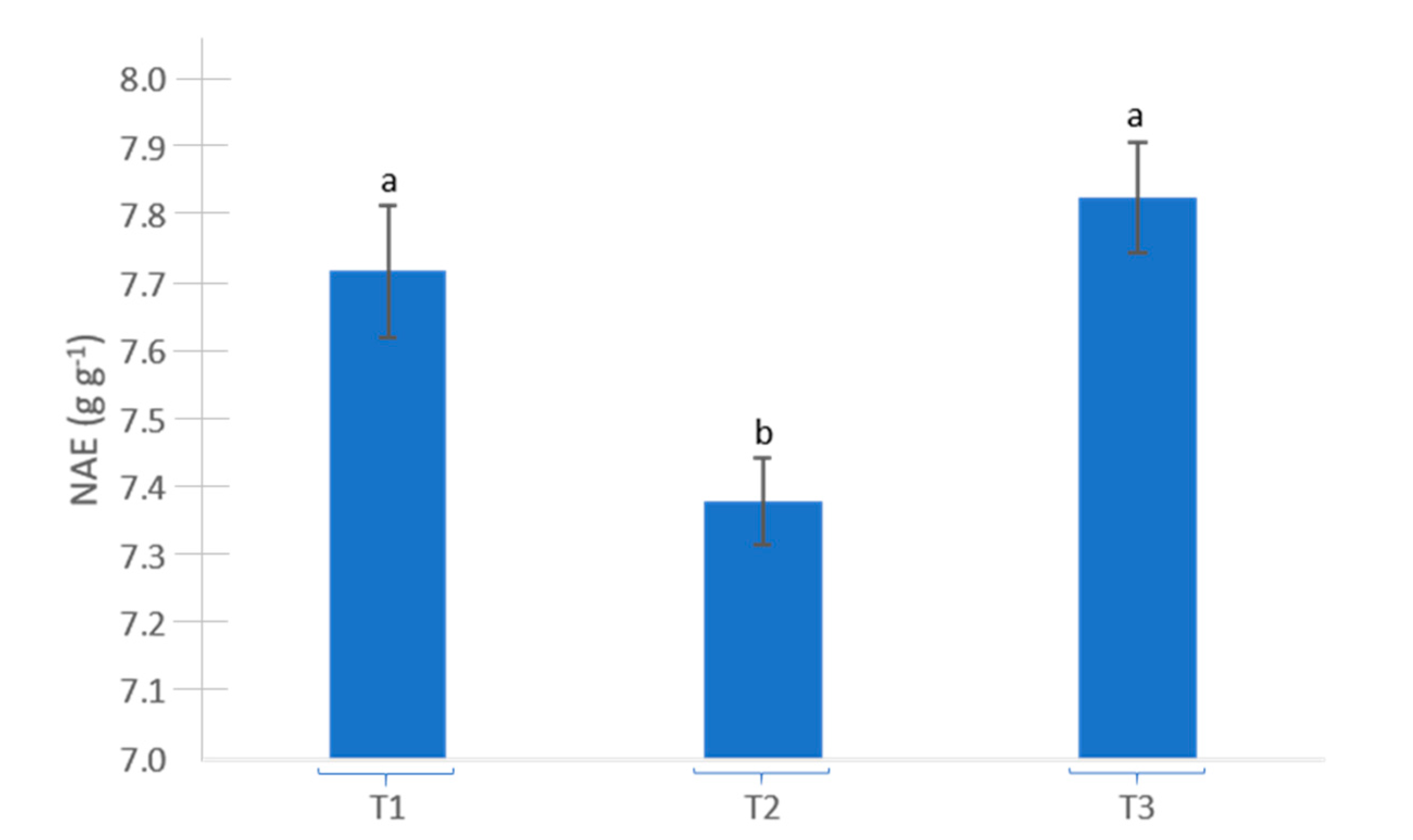
| Treatment | Growing Media Water Content (m3 m−3) | Growing Media Temperature (°C) | Growing Media EC (dS m−1) | |||
|---|---|---|---|---|---|---|
| T0 | 0.18 | b | 23.48 | b | 0.24 | a |
| T1 | 0.15 | c | 23.46 | b | 0.15 | c |
| T2 | 0.18 | b | 24.08 | a | 0.22 | b |
| T3 | 0.21 | a | 24.12 | a | 0.24 | a |
| (A) Treatment | Plant Height (cm) | Leaf Number (No.) | Node Number (No.) | Shoot Diameter (mm) | ||||||
| T0 | 80.00 | d | 3.00 | d | 13.00 | c | 3.71 | c | ||
| T1 | 83.50 | c | 10.00 | c | 14.00 | b | 3.85 | b | ||
| T2 | 86.00 | b | 14.00 | a | 16.00 | a | 4.38 | a | ||
| T3 | 89.00 | a | 11.67 | b | 5.33 | a | 4.65 | a | ||
| (B)Treatment | Chl (μg cm−2) | Flav (μg cm−2) | Anth (μg cm−2) | NBI (-) | Leaf Temperature (°C) | |||||
| T0 | 35.80 | c | 0.89 | b | 0.24 | b | 40.18 | b | 24.18 | a |
| T1 | 38.95 | b | 0.88 | bc | 0.24 | b | 44.06 | a | 23.77 | b |
| T2 | 22.10 | d | 0.84 | c | 0.34 | a | 26.25 | c | 24.78 | a |
| T3 | 41.20 | a | 0.91 | a | 0.19 | c | 45.22 | a | 24.37 | a |
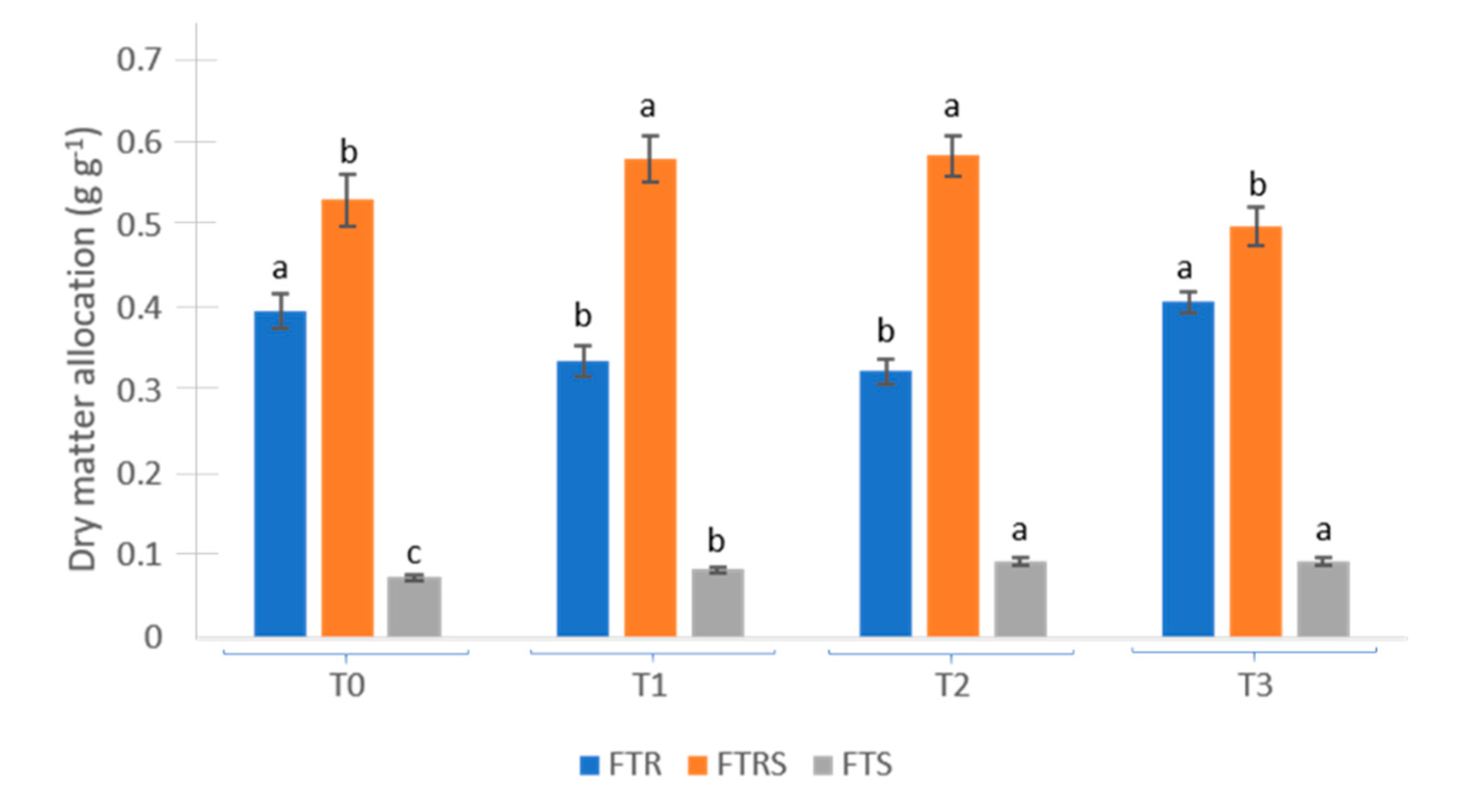
| (A) Treatment | Root Fresh Weight (g plant−1) | Rootstock Fresh Weight (g plant−1) | Shoot Fresh Weight (g plant−1) | Total Fresh Weight (g plant−1) | ||||
| T0 | 38.42 | c | 29.90 | c | 7.17 | c | 75.48 | c |
| T1 | 31.67 | d | 43.17 | a | 10.00 | b | 84.83 | b |
| T2 | 39.83 | b | 37.00 | b | 11.50 | b | 88.33 | b |
| T3 | 44.17 | a | 38.50 | b | 12.83 | a | 95.50 | a |
| (B)Treatment | Root Dry Weight (g plant−1) | Rootstock Dry Weight (g plant−1) | Shoot Dry Weight (g plant−1) | Total Dry Weight (g plant−1) | ||||
| T0 | 13.28 | c | 17.81 | c | 2.46 | d | 33.55 | c |
| T1 | 15.57 | b | 26.90 | a | 3.85 | c | 46.32 | a |
| T2 | 14.33 | b | 25.88 | a | 4.08 | b | 44.29 | b |
| T3 | 19.13 | a | 23.45 | b | 4.40 | a | 46.97 | a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ronga, D.; Parisi, M.; Barbieri, L.; Lancellotti, I.; Andreola, F.; Bignami, C. Valorization of Spent Coffee Grounds, Biochar and other residues to Produce Lightweight Clay Ceramic Aggregates Suitable for Nursery Grapevine Production. Horticulturae 2020, 6, 58. https://doi.org/10.3390/horticulturae6040058
Ronga D, Parisi M, Barbieri L, Lancellotti I, Andreola F, Bignami C. Valorization of Spent Coffee Grounds, Biochar and other residues to Produce Lightweight Clay Ceramic Aggregates Suitable for Nursery Grapevine Production. Horticulturae. 2020; 6(4):58. https://doi.org/10.3390/horticulturae6040058
Chicago/Turabian StyleRonga, Domenico, Mario Parisi, Luisa Barbieri, Isabella Lancellotti, Fernanda Andreola, and Cristina Bignami. 2020. "Valorization of Spent Coffee Grounds, Biochar and other residues to Produce Lightweight Clay Ceramic Aggregates Suitable for Nursery Grapevine Production" Horticulturae 6, no. 4: 58. https://doi.org/10.3390/horticulturae6040058
APA StyleRonga, D., Parisi, M., Barbieri, L., Lancellotti, I., Andreola, F., & Bignami, C. (2020). Valorization of Spent Coffee Grounds, Biochar and other residues to Produce Lightweight Clay Ceramic Aggregates Suitable for Nursery Grapevine Production. Horticulturae, 6(4), 58. https://doi.org/10.3390/horticulturae6040058









