Indices for the Assessment of Glycoalkaloids in Potato Tubers Based on Surface Color and Chlorophyll Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Color Measurement and Analysis
2.3. Extraction and Quantification of Chls and GAs
- Chl a (mg g−1) peel fresh weight) = [(12.7*A663) − (2.69*A645)]*(V/1000*W)
- Chl b (mg g−1) peel fresh weight) = [(22.9*A645) − (4.68*A663)]*(V/1000*W)
- Total Chls = Chl a + Chl b
2.4. Procedure to Develop Indices for the Estimation of Total GAs
2.5. Statistical Analysis
3. Results and Discussion
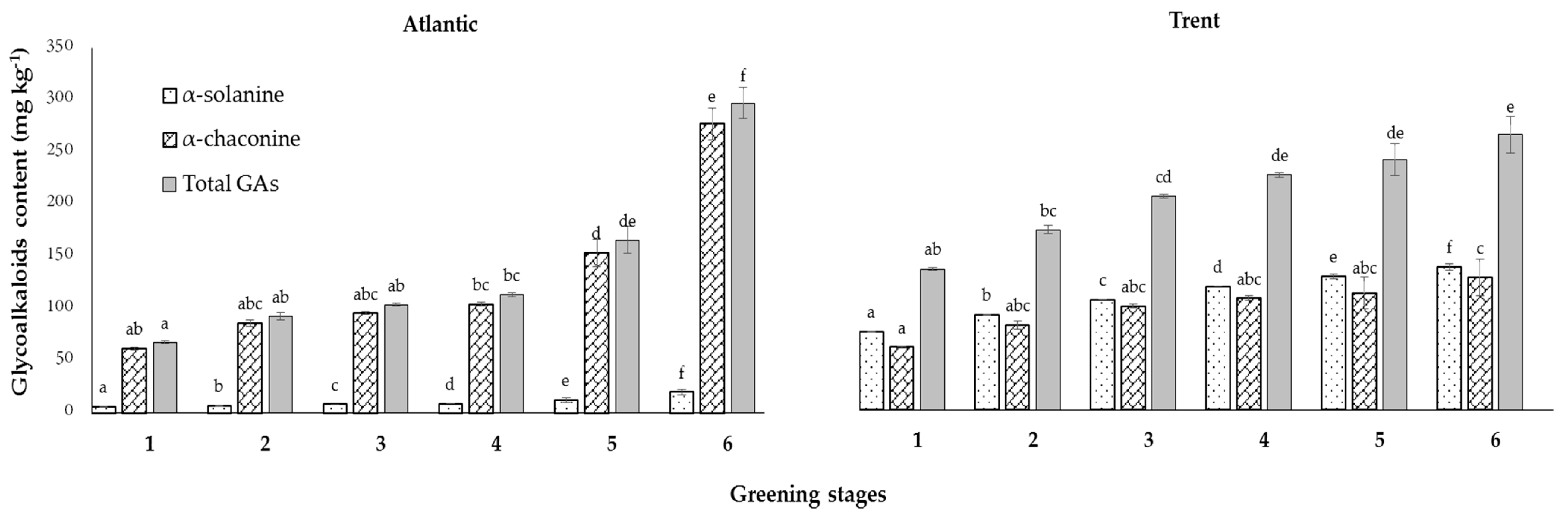
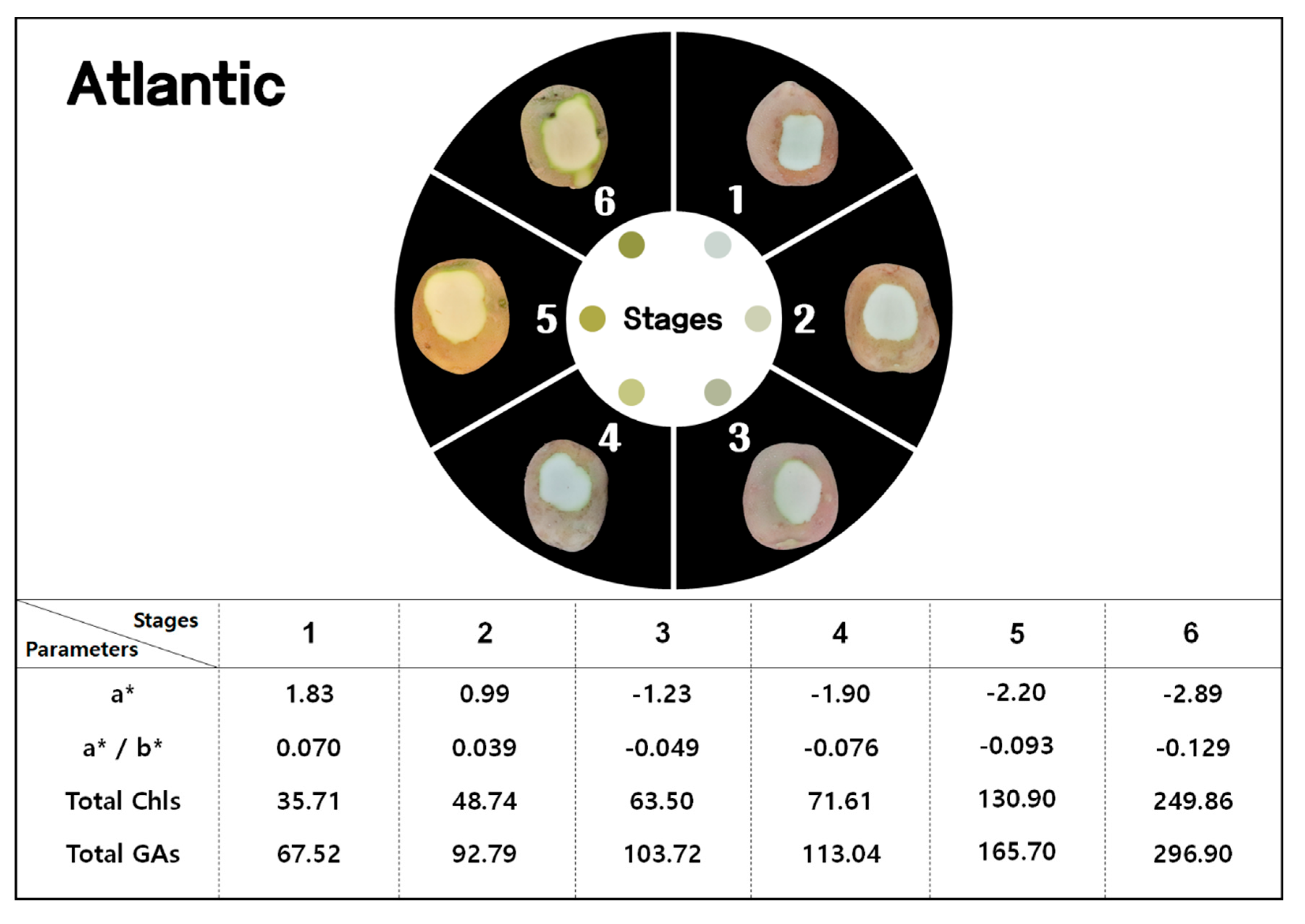
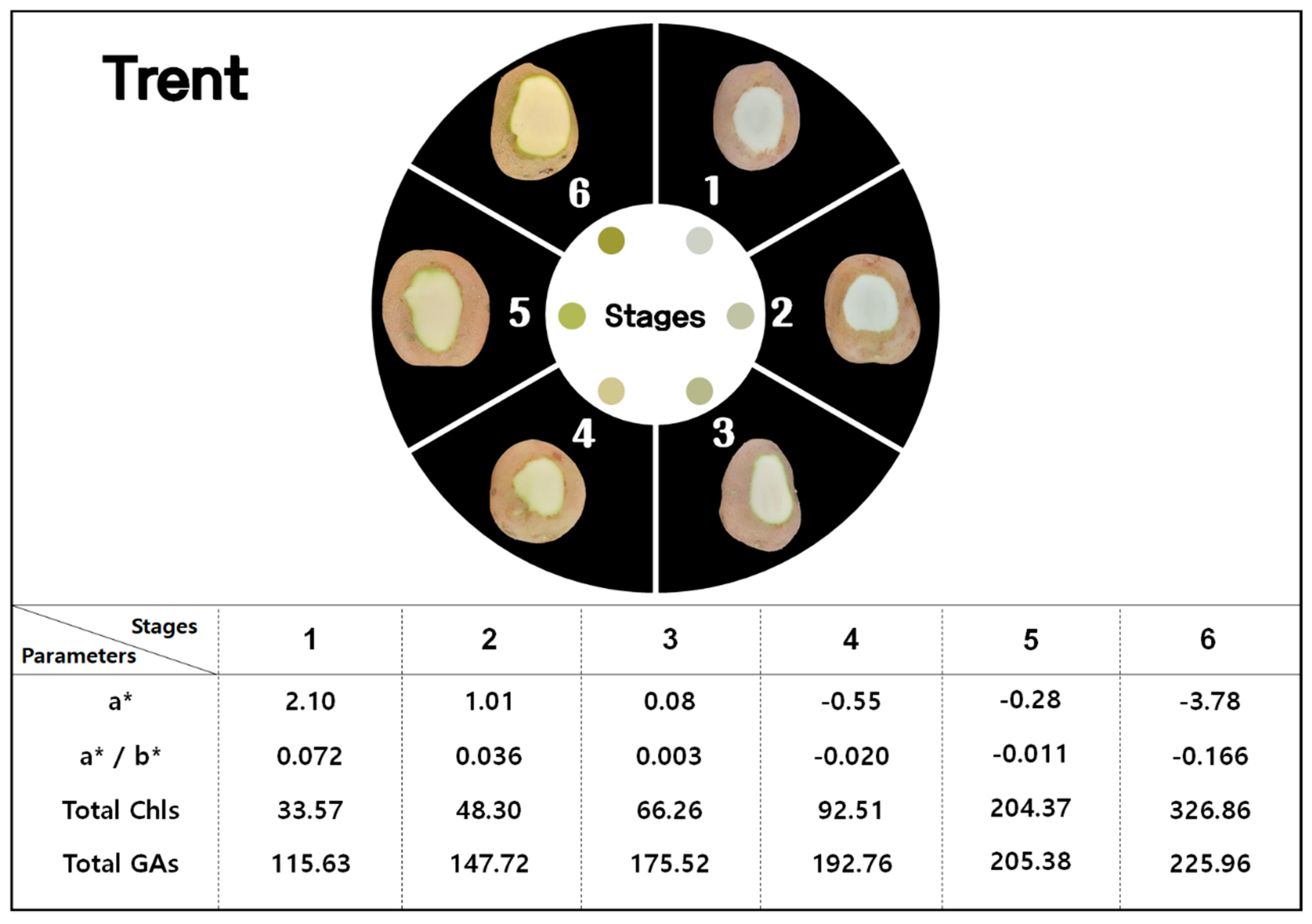
3.1. Color Variables and GAs
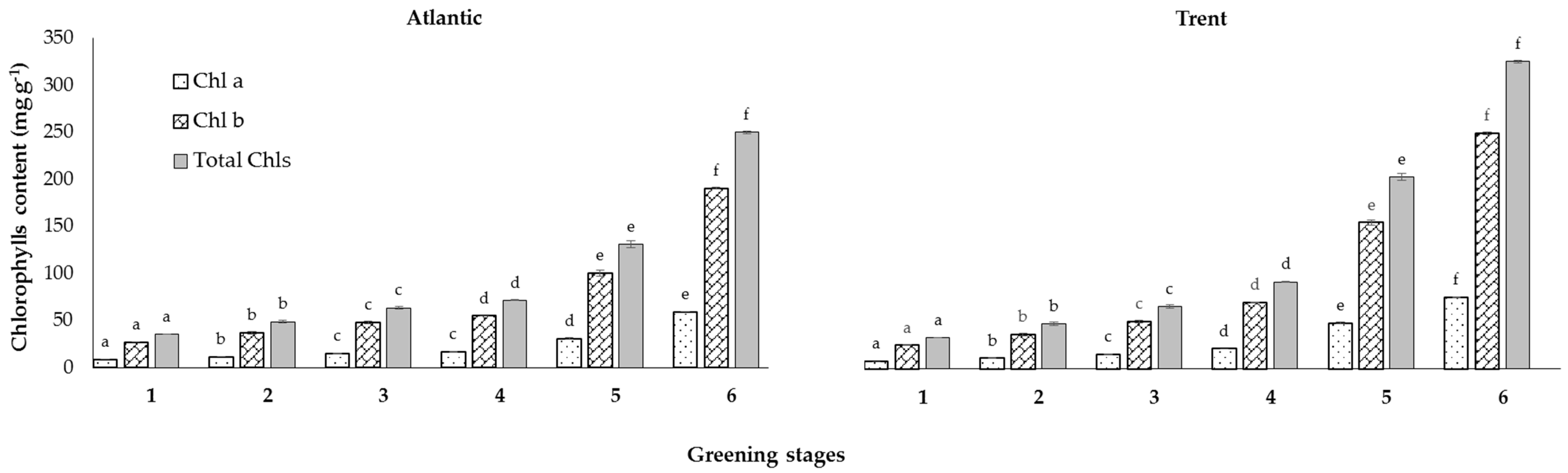
3.2. Chls and GAs
3.3. Correlation of the Evaluated Parameters and Indices for the Estimation of Total GAs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAOSTAT Food & Agriculture Organization of the United Nations Statistics Division. Available online: http://faostat3.fao.org/home/index.html (accessed on 20 October 2020).
- Prokop, S.; Albert, J. Potatoes, Nutrition and Diet-International Year of the Potato; Food and Agriculture Organization of the United Nations Factsheet: Roma, Italy, 2008; pp. 1–2. [Google Scholar]
- Hussain, T. Potatoes: Ensuring Food for the Future. Adv. Plants Agric. Res. 2016, 3, 178–182. [Google Scholar] [CrossRef]
- Beals, K.A. Potatoes, Nutrition and Health. Am. J. Potato Res. 2019, 96, 102–110. [Google Scholar] [CrossRef]
- Omayio, D.G.; Abong, G.O.; Okoth, M.W. A review of occurrence of glycoalkaloids in potato and potato products. Curr. Res. Nutr. Food Sci. 2016, 4, 195–202. [Google Scholar] [CrossRef]
- Shen, K.H.; Liao, A.C.H.; Hung, J.H.; Lee, W.J.; Hu, K.C.; Lin, P.T.; Liao, R.F.; Chen, P.S. α-Solanine inhibits invasion of human prostate cancer cell by suppressing epithelial-mesenchymal transition and MMPs expression. Molecules 2014, 19, 11896–11914. [Google Scholar] [CrossRef] [PubMed]
- Tilahun, S.; An, H.S.; Hwang, I.G.; Choi, J.H.; Baek, M.W.; Choi, H.R.; Park, D.S.; Jeong, C.S. Prediction of α-Solanine and α-Chaconine in Potato Tubers from Hunter Color Values and VIS/NIR Spectra. J. Food Qual. 2020. [Google Scholar] [CrossRef]
- Kirui, G.K.; Misra, A.K.; Olanya, O.M.; Friedman, M.; El-Bedewy, R.; Ewell, P.T. Glycoalkaloid content of some superior potato (Solanum tuberosum L.) clones and commercial cultivars. Arch. Phytopathol. Plant Prot. 2009, 42, 453–463. [Google Scholar] [CrossRef]
- Tilahun, S.; An, H.S.; Choi, H.R.; Park, D.S.; Lee, J.S.; Jeong, C.S. Effects of Storage Temperature on Glycoalkaloid Content, Acrylamide Formation, and Processing-related Variables of Potato Cultivars. Agric. Life Environ. Sci. 2019, 31, 128–142. [Google Scholar]
- Jakuczun, H.; Zimnoch-guzowska, E. Inheritance of tuber greening under light exposure in diploid potatoes. Am. J. Potato Res. 2006, 83, 211–221. [Google Scholar] [CrossRef]
- Grunenfelder, L.A.; Knowles, L.O.; Hiller, L.K.; Knowles, N.R. Glycoalkaloid development during greening of fresh market potatoes (Solanum tuberosum L.). J. Agric. Food Chem. 2006, 54, 5847–5854. [Google Scholar] [CrossRef]
- Prabhat, K.N.; Ramayya, N.; Duncan, E.; Niranjan, K. Potato glycoalkaloids: Formation and strategies for mitigation. J. Sci. Food Agric. 2008, 88, 1869–1881. [Google Scholar] [CrossRef]
- Şengül, M.; Keleş, F.; Keleş, M.S. The effect of storage conditions (temperature, light, time) and variety on the glycoalkaloid content of potato tubers and sprouts. Food Control. 2004, 15, 281–286. [Google Scholar] [CrossRef]
- Friedman, M. Potato glycoalkaloids and metabolites: Roles in the plant and in the diet. J. Agric. Food Chem. 2006, 54, 8655–8681. [Google Scholar] [CrossRef] [PubMed]
- Cantwell, M. A Review of Important Facts about Potato Glycoalkaloids. Perishables Handl. Newsl. 1996, 2, 27. [Google Scholar]
- Kodamatani, H.; Saito, K.; Niina, N.; Yamazaki, S.; Tanaka, Y. Simple and sensitive method for determination of glycoalkaloids in potato tubers by high-performance liquid chromatography with chemiluminescence detection. J. Chromatogr. A 2005, 1100, 26–31. [Google Scholar] [CrossRef]
- Sotelo, A.; Serrano, B. High-performance liquid chromatographic determination of the glycoalkaloids α-solanine and α-chaconine in 12 commercial varieties of Mexican potato. J. Agric. Food Chem. 2000, 48, 2472–2475. [Google Scholar] [CrossRef] [PubMed]
- Coxon, D.T.; Price, K.R.; Jones, P.G. A simplified method for the determination of total glycoalkaloids in potato tubers. J. Sci. Food Agric. 1979, 30, 1043–1049. [Google Scholar] [CrossRef]
- Abu Bakar Siddique, M.; Brunton, N. Food Glycoalkaloids: Distribution, Structure, Cytotoxicity, Extraction, and Biological Activity. Alkaloids Their Importance Nat. Hum. Life 2019. [Google Scholar] [CrossRef]
- Uluwaduge, D.I.; Lecturer, S.; Lanka, S. Glycoalkaloids, bitter tasting toxicants in potatoes: A review. Int. J. Food Sci. Nutr. 2018, 3, 188–193. [Google Scholar]
- Tilahun, S.; Seo, M.H.; Park, D.S.; Jeong, C.S. Effect of cultivar and growing medium on the fruit quality attributes and antioxidant properties of tomato (Solanum lycopersicum L.). Hortic. Environ. Biotechnol. 2018, 59. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of Objective Color Measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Jadhav, S.J.; Salunkhe, D.K. Formation and Control of Chlorophyll and Glycoalkaloids in Tubers of Solanum tuberosum L. and Evaluation of Glycoalkaloid Toxicity*. Adv. Food Res. 1975, 21, 307–354. [Google Scholar] [CrossRef]
- Hiscox, J.D.; Israelstam, G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. In Proceedings of the 2010 International Conference on E-Business and E-Government, Guangzhou, China, 7–9 May 2010; pp. 5474–5478. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Zywicki, B.; Catchpole, G.; Draper, J.; Fiehn, O. Comparison of rapid liquid chromatography-electrospray ionization-tandem mass spectrometry methods for determination of glycoalkaloids in transgenic field-grown potatoes. Anal. Biochem. 2005, 336, 178–186. [Google Scholar] [CrossRef]
- Nie, X.; Zhang, G.; Lv, S.; Guo, H. Steroidal glycoalkaloids in potato foods as affected by cooking methods. Int. J. Food Prop. 2018, 21, 1875–1887. [Google Scholar] [CrossRef]
- Grunenfelder, L.; Hiller, L.K.; Knowles, N.R. Color indices for the assessment of chlorophyll development and greening of fresh market potatoes. Postharvest Biol. Technol. 2006, 40, 73–81. [Google Scholar] [CrossRef]
- Haase, N.U. Glycoalkaloid Concentration in Potato Tubers Related to Storage and Consumer Offering. Potato Res. 2010, 53, 297–307. [Google Scholar] [CrossRef]
- Tilahun, S.; Park, D.S.; Taye, A.M.; Jeong, C.S. Effect of ripening conditions on the physicochemical and antioxidant properties of tomato (Lycopersicon esculentum Mill.). Food Sci. Biotechnol. 2017, 26. [Google Scholar] [CrossRef]
- Tajner-Czopek, A.; Jarych-Szyszka, M.; Lisińska, G. Changes in glycoalkaloids content of potatoes destined for consumption. Food Chem. 2008, 106, 706–711. [Google Scholar] [CrossRef]
- Percival, G.C.; Dixon, G.R. Glycoalkaloids. In Handbook of Plant and Fungal Toxins; Felix D’Mello, J.P., Ed.; CRC Press: Boca Raton, FL, USA, 1997; pp. 19–35. [Google Scholar]
- Okamoto, H.; Ducreux, L.J.M.; Allwood, J.W.; Hedley, P.E.; Wright, A.; Gururajan, V.; Terry, M.J.; Taylor, M.A. Light Regulation of Chlorophyll and Glycoalkaloid Biosynthesis During Tuber Greening of Potato S. tuberosum. Front. Plant Sci. 2020, 11, 1–19. [Google Scholar] [CrossRef]
- Arias, R.; Lee, T.C.; Logendra, L.; Janes, H. Correlation of lycopene measured by HPLC with the L*, a*, b* color readings of a hydroponic tomato and the relationship of maturity with color and lycopene content. J. Agric. Food Chem. 2000, 48, 1697–1702. [Google Scholar] [CrossRef]
- Helyes, L.; Pék, Z.; Lugasi, A. Tomato fruit quality and content depend on stage of maturity. HortScience 2006, 41, 1400–1401. [Google Scholar] [CrossRef]
- Spoladore, D.S.; Zullo, M.A.T.; Teixeira, J.P.F.; Coelho, S.M.B.M.; de Silva Miranda Filho, H. Synthesis of chlorophylls and glycoalkaloids in mature potato tubers stored under natural light. Bragantia 1985, 44, 197–208. [Google Scholar] [CrossRef]
| Parameters | Cultivars | Greening Stages | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| L* | Atlantic | 58.30 aA | 56.74 bcB | 55.66 bcdA | 55.37 bcdA | 55.29 cdA | 54.30 dA |
| Trent | 56.88 bB | 58.93 aA | 55.95 bcA | 55.84 bcA | 56.28 bcA | 52.64 dB | |
| a* | Atlantic | 1.83 aA | 0.99 bcA | −1.23 cdA | −1.90 deA | −2.20 deA | −2.89 fA |
| Trent | 2.10 aA | 1.01 bcA | 0.08 cdB | −0.55 deB | −0.28 deB | −3.78 fA | |
| b* | Atlantic | 26.10 abB | 25.21 bcB | 24.86 cB | 25.82 abcB | 23.72 dB | 22.46 eA |
| Trent | 29.22 aA | 28.32 abA | 27.16 cA | 27.81 bcA | 26.41 cdA | 22.76 eA | |
| a*/b* | Atlantic | 0.070 aA | 0.039 bcA | −0.049 cdB | −0.076 deB | −0.093 deB | −0.129 fA |
| Trent | 0.072 aA | 0.036 bcA | 0.003 cdA | −0.020 deA | −0.011 deA | −0.166 fB | |
| Hunter L* | Hunter a* | Hunter b* | a*/b* | Chl a | Chl b | Total Chl | α-solanine | α-chaconine | Total GA | |
|---|---|---|---|---|---|---|---|---|---|---|
| Hunter L* | 1 | 0.89 *** | 0.73 ** | 0.90 *** | −0.78 ** | −0.79 ** | −0.80 ** | −0.16 ns | −0.55 ns | −0.62 * |
| Hunter a* | 1 | 0.82 *** | 0.99 *** | −0.78 ** | −0.78 ** | −0.82 *** | −0.05 ns | −0.67 * | −0.65 ** | |
| Hunter b* | 1 | 0.83 *** | −0.67 * | −0.67 * | −0.69 * | 0.35 ns | −0.71 ** | −0.40 ns | ||
| a*/b* | 1 | −0.80 ** | −0.80 ** | −0.84 *** | −0.06 ns | −0.69 * | −0.67 ** | |||
| Chl a | 1 | 0.99 *** | 0.99 *** | 0.39 ns | 0.60 * | 0.82 ** | ||||
| Chl b | 1 | 0.99 *** | 0.39 ns | 0.59 * | 0.81 ** | |||||
| Total Chl | 1 | 0.38 ns | 0.63 * | 0.84 *** | ||||||
| α−solanine | 1 | −0.25 ns | 0.47 ns | |||||||
| α−chaconine | 1 | 0.74** | ||||||||
| Total GA | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tilahun, S.; An, H.S.; Solomon, T.; Baek, M.W.; Choi, H.R.; Lee, H.C.; Jeong, C.S. Indices for the Assessment of Glycoalkaloids in Potato Tubers Based on Surface Color and Chlorophyll Content. Horticulturae 2020, 6, 107. https://doi.org/10.3390/horticulturae6040107
Tilahun S, An HS, Solomon T, Baek MW, Choi HR, Lee HC, Jeong CS. Indices for the Assessment of Glycoalkaloids in Potato Tubers Based on Surface Color and Chlorophyll Content. Horticulturae. 2020; 6(4):107. https://doi.org/10.3390/horticulturae6040107
Chicago/Turabian StyleTilahun, Shimeles, Hee Sung An, Tifsehit Solomon, Min Woo Baek, Han Ryul Choi, Hee Cheol Lee, and Cheon Soon Jeong. 2020. "Indices for the Assessment of Glycoalkaloids in Potato Tubers Based on Surface Color and Chlorophyll Content" Horticulturae 6, no. 4: 107. https://doi.org/10.3390/horticulturae6040107
APA StyleTilahun, S., An, H. S., Solomon, T., Baek, M. W., Choi, H. R., Lee, H. C., & Jeong, C. S. (2020). Indices for the Assessment of Glycoalkaloids in Potato Tubers Based on Surface Color and Chlorophyll Content. Horticulturae, 6(4), 107. https://doi.org/10.3390/horticulturae6040107





