The Effect of Leaching Fraction-Based Irrigation on Fertilizer Longevity and Leachate Nutrient Content in a Greenhouse Environment
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. LF and Leachate Nutrient Content
3.2. LF and Fertilizer Longevity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Biernbaum, J.A. Root-zone management of greenhouse container-grown crops to control water and fertilizer. Hortic. Tech. 1992, 2, 127–132. [Google Scholar] [CrossRef]
- Howarth, R.W. Nutrient limitation of net primary production in marine ecosystems. Ann. Rev. Ecol. 1988, 19, 89–110. [Google Scholar] [CrossRef]
- Kabashima, J.N. Innovative irrigation techniques in nursery production to reduce water usage. Hortic. Sci. 1993, 28, 291–293. [Google Scholar] [CrossRef]
- Adams, C.; Frantz, J.; Bugbee, B. Macro-and micronutrient-release characteristics of three polymer-coated fertilizers: Theory and measurements. J. Plant Nutr. Soil Sci. 2013, 176, 76–88. [Google Scholar] [CrossRef]
- Kochba, M.; Gambash, S.; Avnimelech, Y. Studies on slow release fertilizers: 1. Effects of temperature, soil moisture, and water vapor pressure. Soil Sci. 1990, 149, 339–343. [Google Scholar] [CrossRef]
- Rathier, T.M.; Frink, C.R. Nitrate in runoff water from container grown juniper and Alberta spruce under different irrigation and N fertilization. J. Environ. Hortic. 1989, 7, 32–35. [Google Scholar]
- Bayer, A.; Ruter, J.; van Iersel, M.W. Optimizing irrigation and fertilization of Gardenia jasminoides for good growth and minimal leaching. Hortic. Sci. 2015, 50, 994–1001. [Google Scholar] [CrossRef]
- Million, J.B.; Yeager, T.H. Periodic versus real-time adjustment of a leaching fraction-based container-grown plants. Hortic. Sci. 2020, 55, 83–88. [Google Scholar] [CrossRef]
- Chappell, M.; Dove, S.K.; van Iersel, M.W.; Thomas, P.A.; Ruter, J. Implementation of wireless sensor networks for irrigation control in three container nurseries. Hortic. Tech. 2013, 23, 747–753. [Google Scholar] [CrossRef]
- Stanley, J. Using leaching fractions to maximize irrigation efficiency©. Proc. Int. Plant Propag. Soc. 2012, 62, 331–334. [Google Scholar] [CrossRef]
- Owen, J.S., Jr.; Warren, S.L.; Bilderback, T.E.; Albano, J.P. Phosphorus rate, leaching fraction, and substrate influence on influent quantity, effluent nutrient content, and response of a containerized woody ornamental crop. Hortic. Sci. 2008, 43, 906–912. [Google Scholar] [CrossRef]
- Tyler, H.H.; Warren, S.L.; Bilderback, T.E. Reduced leaching fractions improve irrigation use efficiency and nutrient efficacy. J. Environ. Hortic. 1996, 14, 199–204. [Google Scholar]
- Prehn, A.E.; Owen, J.S., Jr.; Warren, S.L.; Bilderback, T.E.; Albano, J.P. Comparison of water management in container-grown nursery crops using leaching fraction or weight-based on demand irrigation control. J. Environ. Hortic. 2010, 28, 117–123. [Google Scholar]
- Du, C.W.; Zhou, J.M.; Shaviv, A. Release characteristics of nutrients from polymer-coated compound controlled release fertilizers. J. Polym. Environ. 2006, 14, 223–230. [Google Scholar] [CrossRef]
- Owens, J.S., Jr.; LeBude, A.V.; Chappell, M.; Hoskins, T. Advanced Irrigation Management for Container-Grown Ornamental Crop Production. Available online: http://digitalpubs.ext.vt.edu/vcedigitalpubs/3312473486515986/MobilePagedReplica.action?pm=2&folio=1#pg1 (accessed on 10 August 2018).
| Nitrogen | LF | Leached from Pot (mg) | Left in Pot (mg) | ||
|---|---|---|---|---|---|
| 0.05 | 102 | c z | 583 | a | |
| 0.15 | 135 | bc | 547 | ab | |
| 0.25 | 153 | bc | 534 | ab | |
| 0.35 | 213 | ab | 472 | bc | |
| 0.45 | 205 | ab | 476 | bc | |
| 0.55 | 270 | a | 422 | c | |
| Significance y | L *** | Q *** | |||
| Phosphorus | |||||
| 0.05 | 12 | b | 89 | a | |
| 0.15 | 16 | b | 84 | ab | |
| 0.25 | 18 | b | 82 | ab | |
| 0.35 | 27 | ab | 73 | bc | |
| 0.45 | 23 | b | 76 | ab | |
| 0.55 | 39 | a | 61 | c | |
| Significance | L *** | Q *** | |||
| Potassium | |||||
| 0.05 | 193 | d | 386 | a | |
| 0.15 | 215 | d | 375 | a | |
| 0.25 | 241 | cd | 353 | ab | |
| 0.35 | 305 | bc | 284 | bc | |
| 0.45 | 347 | b | 240 | c | |
| 0.55 | 460 | a | 136 | d | |
| Significance | Q *** | Q *** | |||
| Leached from Pot (mg) | ||||||||||||
| LF | 0.05 | 0.15 | 0.25 | 0.35 | 0.45 | 0.55 | ||||||
| Calcium | 92.3 | d z | 120.7 | d | 135.3 | cd | 179.0 | cd | 189.8 | b | 260.5 | a |
| Magnesium | 43.8 | d | 55.1 | d | 61.8 | cd | 79.8 | bc | 85.6 | b | 117.4 | a |
| SO4-Sulfur | 66.0 | c | 79.2 | c | 88.9 | cd | 119.5 | b | 127.4 | b | 188.1 | a |
| Iron | 0.5 | bc | 0.4 | c | 0.7 | bc | 1.0 | b | 0.9 | b | 1.5 | a |
| Manganese | 0.1 | b | 0.1 | b | 0.2 | b | 0.2 | b | 0.2 | b | 0.4 | a |
| Boron | 0.0 | ab | 0.0 | b | 0.0 | b | 0.0 | a | 0.0 | ab | 0.0 | a |
| Copper | 0.1 | bc | 0.1 | c | 0.2 | bc | 0.3 | ab | 0.2 | bc | 0.4 | a |
| Zinc | 1.1 | bc | 0.9 | c | 1.2 | bc | 1.8 | ab | 1.8 | ab | 2.3 | ab |
| Molybdenum | 0.0 | ab | 0.0 | a | 0.0 | a | 0.0 | a | 0.0 | a | 0.0 | a |
| Sodium | 18.2 | e | 22.3 | de | 25.8 | d | 35.6 | c | 44.8 | b | 66.7 | a |
| Aluminum | 0.1 | d | 0.1 | d | 0.2 | cd | 0.2 | cd | 0.2 | ab | 0.2 | ab |
| Silicon | 5.7 | c | 6.7 | c | 7.6 | bc | 9.7 | b | 12.9 | a | 14.7 | a |
| Left in Pot (mg) | ||||||||||||
| LF | 0.05 | 0.15 | 0.25 | 0.35 | 0.45 | 0.55 | ||||||
| Calcium | −92.3 y | a | −120.7 | a | −136.3 | ab | −179.9 | bc | −190.4 | c | −260.1 | d |
| Magnesium | −58.9 | a | −70.5 | a | −80.0 | ab | −98.1 | bc | −104.6 | b | −134.6 | a |
| SO4-Sulfur | 109.8 | a | 95.7 | a | 78.7 | ab | 47.9 | bc | 37.2 | c | −16.2 | d |
| Iron | 6.9 | a | 6.9 | a | 6.6 | ab | 6.3 | b | 6.4 | b | 5.8 | c |
| Manganese | 3.5 | a | 3.4 | ab | 3.2 | bc | 3.2 | cd | 3.2 | c | 2.9 | d |
| Boron | 0.0 | ab | 0.0 | a | 0.0 | ab | 0.0 | b | 0.0 | ab | 0.0 | b |
| Copper | 3.5 | a | 3.5 | a | 3.4 | a | 3.3 | ab | 3.4 | ab | 3.2 | b |
| Zinc | 2.4 | ab | 2.7 | a | 2.3 | ab | 1.8 | bc | 1.7 | bc | 1.3 | c |
| Molybdenum | 0.1 | a | 0.1 | a | 0.1 | a | 0.0 | ab | 0.1 | a | 0.1 | a |
| Sodium | −6.1 | a | −10.0 | ab | −13.6 | bc | −22.9 | cd | −31.8 | d | −53.5 | e |
| Aluminum | 0.0 | ab | −0.1 | ab | −0.1 | bc | −0.2 | cd | −0.2 | d | −0.3 | e |
| Silicon | −5.7 | a | −6.7 | a | −7.6 | ab | −9.7 | b | −12.9 | c | −14.7 | c |
| Target LF | |||||||
|---|---|---|---|---|---|---|---|
| Weeks | 0.05 | 0.15 | 0.25 | 0.35 | 0.45 | 0.55 | Significancex |
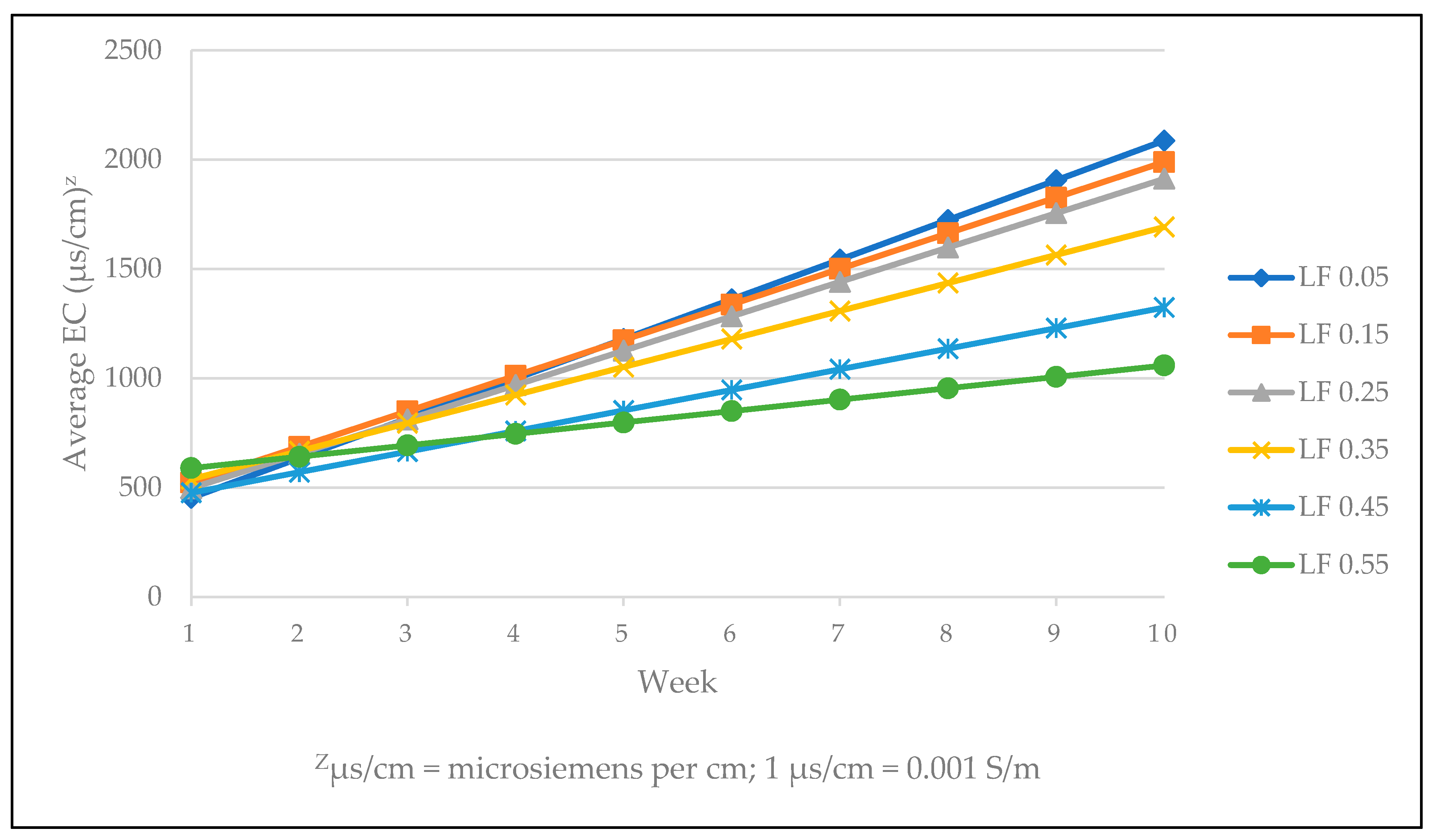
| 1 | 582.6 | 370.1 | 437.1 | 507.9 | 337.1 | 480.3 | ns |
| 2 | 619.0 | 667.0 | 634.1 | 583.0 | 608.5 | 516.0 | ns |
| 3 | 669.3 | 780.8 | 668.3 | 736.0 | 618.1 | 669.8 | ns |
| 4 | 757.2 | 991.9 | 936.7 | 1000.8 | 906.0 | 1053.6 | ns |
| 5 | 1505.6 | 1438.3 | 1331.4 | 1269.0 | 926.7 | 939.6 | L ** |
| 6 | 1481.0 | 1519.0 | 1562.3 | 1048.0 | 905.0 | 792.3 | L *** |
| 7 | 1333.3 | 1602.3 | 1415.5 | 1334.8 | 938.5 | 739.3 | Q * |
| 8 | 1648.5 | 1562.0 | 1541.0 | 1544.8 | 1195.5 | 1093.8 | L *** |
| 9 | 2023.0 | 1806.8 | 1698.3 | 1460.0 | 1247.3 | 1037.0 | L *** |
| 10 | 2084.0 | 1823.3 | 1822.8 | 1642.5 | 1277.5 | 917.5 | Q * |
| Significance | L *** | Q * | L *** | L *** | L *** | Q * | |
| Target LF | |||||||
|---|---|---|---|---|---|---|---|
| Weeks | 0.05 | 0.15 | 0.25 | 0.35 | 0.45 | 0.55 | Significance z |
| 1 | 6.55 | 6.82 | 6.66 | 6.62 | 6.70 | 6.47 | Q * |
| 2 | 6.87 | 6.97 | 6.94 | 6.90 | 6.90 | 6.94 | ns |
| 3 | 6.70 | 6.82 | 6.80 | 6.69 | 6.80 | 6.77 | ns |
| 4 | 6.72 | 6.77 | 6.76 | 6.66 | 6.71 | 6.55 | ns |
| 5 | 6.39 | 6.41 | 6.42 | 6.36 | 6.55 | 6.47 | ns |
| 6 | 6.21 | 5.85 | 5.80 | 5.97 | 6.08 | 6.17 | Q *** |
| 7 | 5.71 | 5.67 | 5.68 | 5.73 | 5.88 | 6.06 | Q * |
| 8 | 6.31 | 5.90 | 5.83 | 5.81 | 5.98 | 6.06 | Q *** |
| 9 | 5.68 | 5.40 | 5.39 | 5.35 | 5.67 | 5.82 | Q *** |
| 10 | 5.70 | 5.41 | 5.30 | 5.33 | 5.51 | 5.73 | Q *** |
| Significance | L *** | L *** | L *** | L *** | L *** | L *** | |
| LF | pH | EC (µS/cm) z | ||
|---|---|---|---|---|
| 0.05 | 6.0 | b z | 805.0 | a |
| 0.15 | 6.3 | ab | 715.0 | ab |
| 0.25 | 6.4 | a | 620.0 | b |
| 0.35 | 6.4 | a | 645.0 | b |
| 0.45 | 6.4 | a | 652.5 | b |
| 0.55 | 6.4 | ab | 622.5 | b |
| Sign. x | Q ** | Q ** | ||
| Nitrogen | LF | Fertilizer Loss (mg) z | Remaining in Fertilizer (mg) | ||
| 0.05 | 685.2 | ns y | 119.2 | ns | |
| 0.15 | 682.1 | 122.3 | |||
| 0.25 | 687.5 | 116.9 | |||
| 0.35 | 684.5 | 119.9 | |||
| 0.45 | 681.7 | 122.7 | |||
| 0.55 | 691.6 | 112.8 | |||
| Phosphorus | |||||
| 0.05 | 101.8 | ns | 31.2 | ns | |
| 0.15 | 99.9 | 33.1 | |||
| 0.25 | 100.5 | 32.5 | |||
| 0.35 | 99.9 | 33.1 | |||
| 0.45 | 99.7 | 33.3 | |||
| 0.55 | 100.8 | 32.2 | |||
| Potassium | |||||
| 0.05 | 578.6 | ns | 170.4 | ns | |
| 0.15 | 590.8 | 158.2 | |||
| 0.25 | 593.8 | 155.2 | |||
| 0.35 | 589.1 | 159.9 | |||
| 0.45 | 586.3 | 162.7 | |||
| 0.55 | 595.3 | 153.7 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krofft, C.E.; Pickens, J.M.; Newby, A.F.; Sibley, J.L.; Fain, G.B. The Effect of Leaching Fraction-Based Irrigation on Fertilizer Longevity and Leachate Nutrient Content in a Greenhouse Environment. Horticulturae 2020, 6, 43. https://doi.org/10.3390/horticulturae6030043
Krofft CE, Pickens JM, Newby AF, Sibley JL, Fain GB. The Effect of Leaching Fraction-Based Irrigation on Fertilizer Longevity and Leachate Nutrient Content in a Greenhouse Environment. Horticulturae. 2020; 6(3):43. https://doi.org/10.3390/horticulturae6030043
Chicago/Turabian StyleKrofft, Claire E., Jeremy M. Pickens, Adam F. Newby, Jeff L. Sibley, and Glenn B. Fain. 2020. "The Effect of Leaching Fraction-Based Irrigation on Fertilizer Longevity and Leachate Nutrient Content in a Greenhouse Environment" Horticulturae 6, no. 3: 43. https://doi.org/10.3390/horticulturae6030043
APA StyleKrofft, C. E., Pickens, J. M., Newby, A. F., Sibley, J. L., & Fain, G. B. (2020). The Effect of Leaching Fraction-Based Irrigation on Fertilizer Longevity and Leachate Nutrient Content in a Greenhouse Environment. Horticulturae, 6(3), 43. https://doi.org/10.3390/horticulturae6030043






