Incident Light and Leaf Age Influence Leaflet Element Concentrations of Cycas micronesica Trees
Abstract
1. Introduction
2. Materials and Methods
2.1. Incident Light
2.2. Leaf Age
2.3. Soils
2.4. Statistics
3. Results and Discussion
3.1. Incident Light
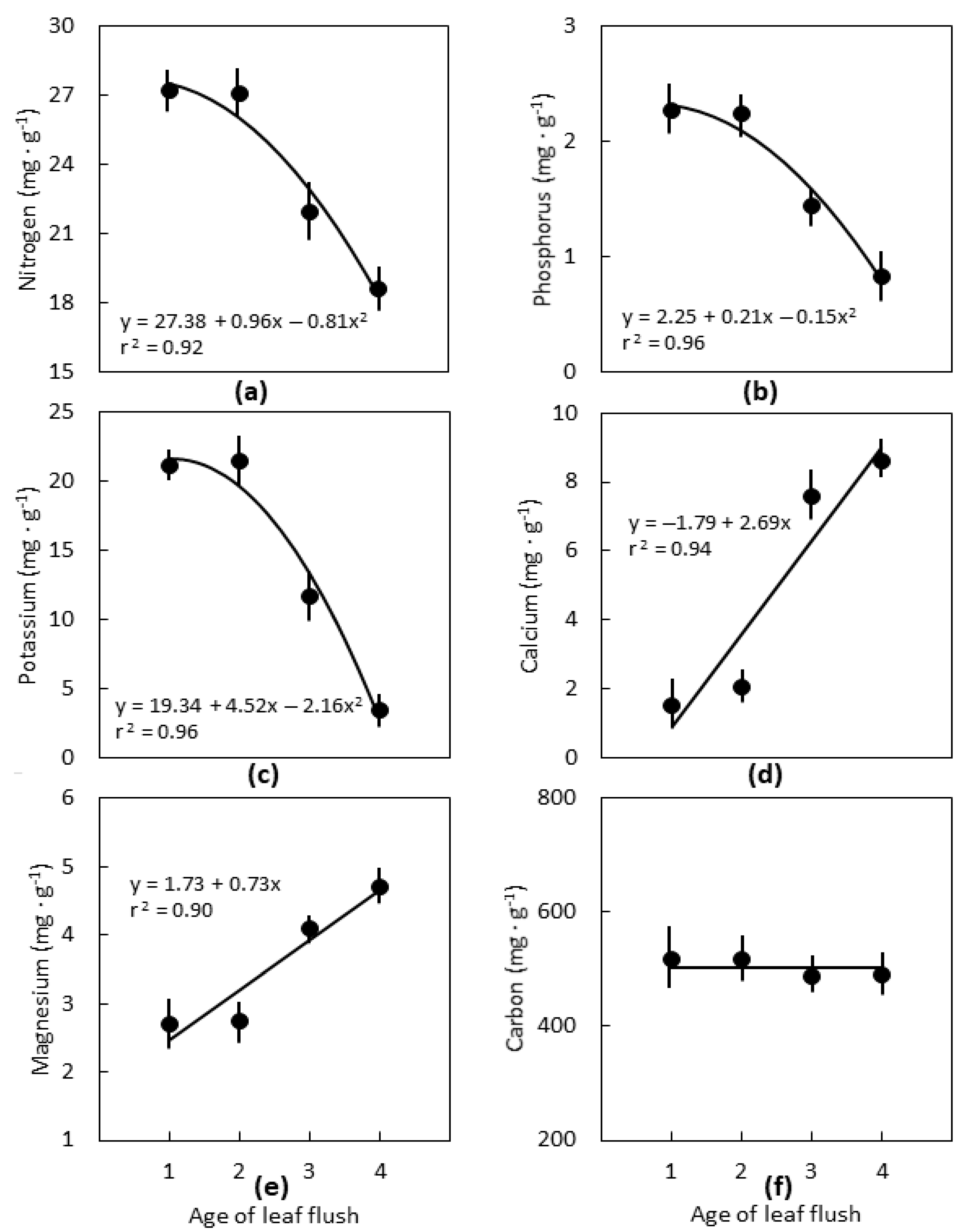
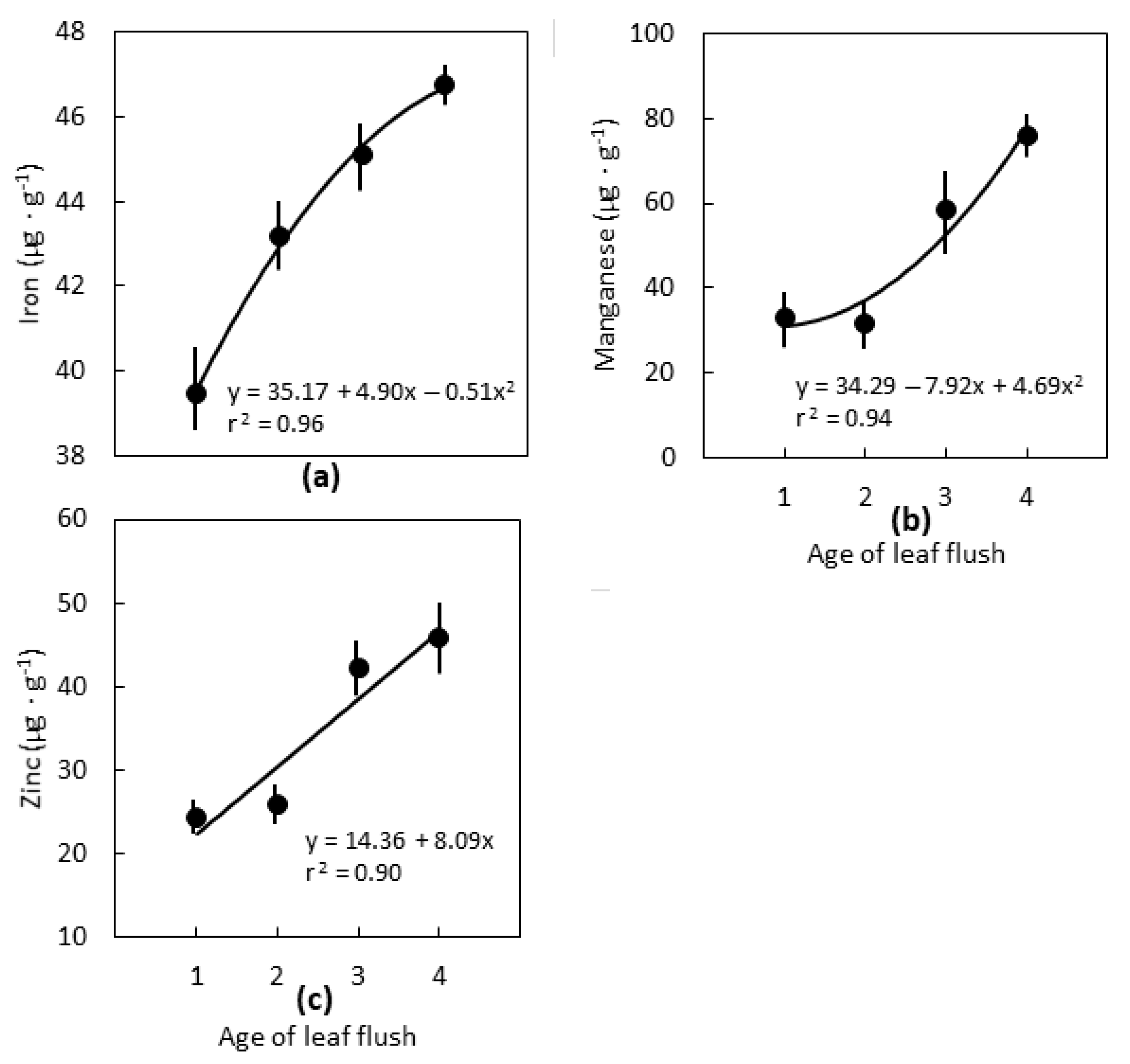
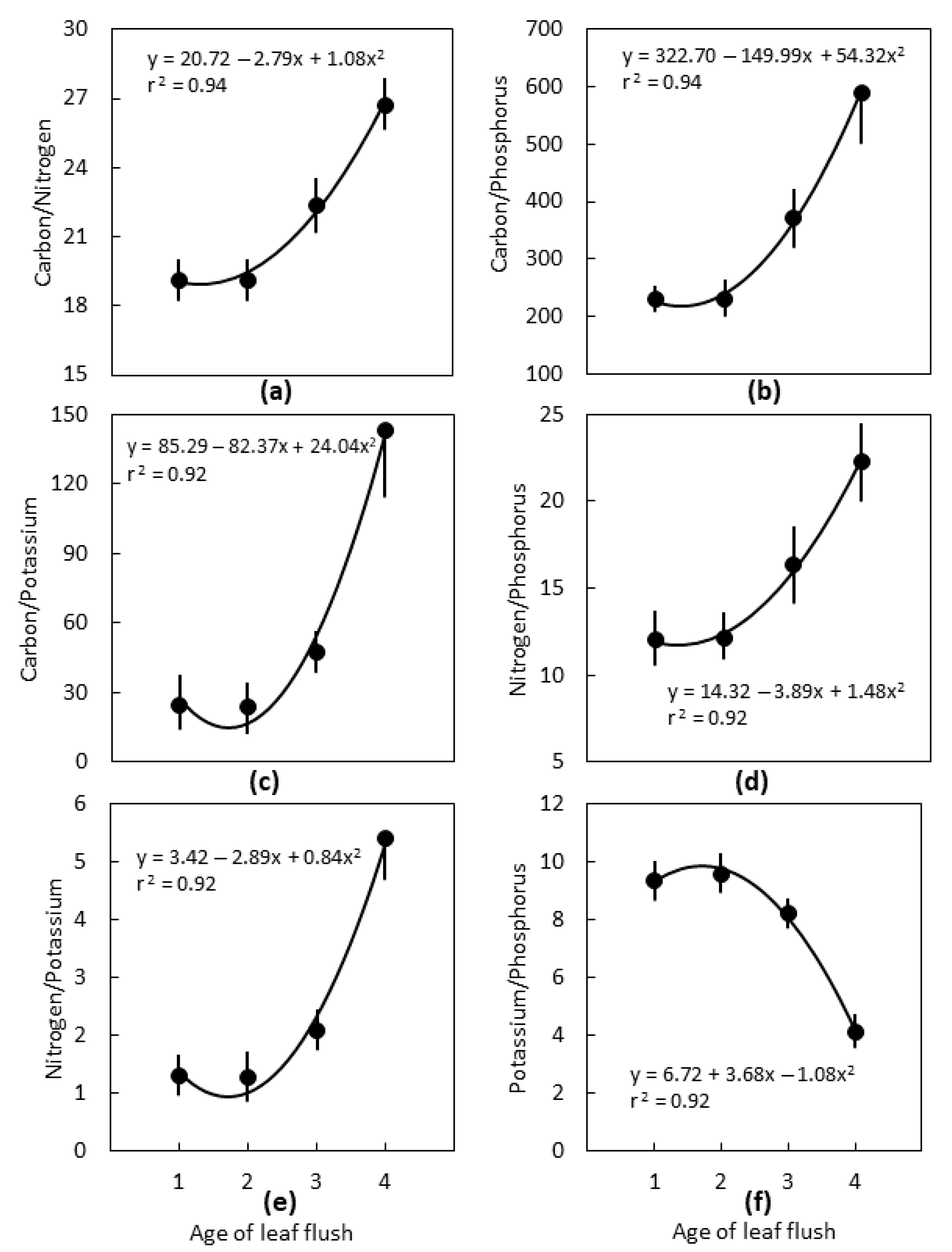
3.2. Leaf Age
3.3. Interpretations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Norstog, K.J.; Nicholls, T.J. The Biology of the Cycads; Cornell University Press: Ithaca, NY, USA, 1997; ISBN 978-0-8014-3033-6. [Google Scholar]
- Brenner, E.D.; Stevenson, D.W.; Twigg, R.W. Cycads: Evolutionary innovations and the role of plant-derived neurotoxins. Trends Plant. Sci. 2003, 8, 446–452. [Google Scholar] [CrossRef]
- Marler, T.E.; Lindström, A.J. Inserting cycads into global nutrient relations data sets. Plant. Signal. Behav. 2018, 13, e1547578. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E.; Krishnapillai, M.V. Does plant size influence leaf elements in an arborescent cycad. Biology 2018, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E.; Krishnapillai, M.V. Distribution of elements along the rachis of Cycas micronesica leaves: A cautionary note for sampling design. Horticulturae 2019, 5, 33. [Google Scholar] [CrossRef]
- Marler, T.E.; Krishnapillai, M.V. Cycas micronesica trees alter local soil traits. Forests 2018, 9, 565. [Google Scholar] [CrossRef]
- Álvarez-Yépiz, J.C.; Cueva, A.; Dovčiak, M.; Teece, M.; Yepez, E.A. Ontogenetic resource-use strategies in a rare long-lived cycad along environmental gradients. Conserv. Physiol. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Grove, T.S.; O’Connell, A.M.; Malajczuk, N. Effects of fire on the growth, nutrient content and rate of nitrogen fixation of the cycad Macrozamia riedlei. Austral. J. Bot. 1980, 28, 271–281. [Google Scholar] [CrossRef]
- Krieg, C.; Watkins, J.E.; Chambers, S.; Husby, C.E. Sex-specific differences in functional traits and resource acquisition in five cycad species. AoB PLANTS 2017, 9, plx013. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E.; Ferreras, U.F. Disruption of leaf nutrient remobilization in coastal Cycas trees by tropical cyclone damage. J. Geogr. Nat. Disast. 2015, 5, 142. [Google Scholar] [CrossRef]
- Marler, T.E.; Ferreras, U.F. Current status, threats and conservation needs of the endemic Cycas wadei Merrill. J. Biodivers. Endanger. Species 2017, 5, 193. [Google Scholar] [CrossRef]
- Watanabe, T.; Broadley, M.R.; Jansen, S.; White, P.J.; Takada, J.; Satake, K.; Takamatsu, T.; Tuah, S.J.; Osaki, M. Evolutionary control of leaf element composition in plants. New Phytol. 2007, 174, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, K.; Sack, L.; Li, N.; Wei, X.; Goldstein, G. Extending the generality of leaf economic design principles in the cycads, an ancient lineage. New Phytol. 2015, 206, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Sack, L.; Cao, K.-F.; Wei, X.-W.; Li., N. Speed versus endurance tradeoff in plants: Leaves with higher photosynthetic rates show stronger seasonal declines. Sci. Rep. 2017, 7, 42085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Sack, L.; Goldstein, G.; Cao, K.-F. Hydraulic determination of leaf nutrient concentrations in cycads. Mem. NY Bot. Gard. 2018, 117, 179–192. [Google Scholar]
- Hill, K.D. The Cycas rumphii complex (Cycadaceae) in New Guinea and the Western Pacific. Aust. Syst. Bot. 1994, 7, 543–567. [Google Scholar] [CrossRef]
- Smith, C.W. Soil Survey of Islands of Yap Federated States of Micronesia; U.S. Dept. of Agric. Soil Conservation Service: Washington, DC, USA, 1983. [Google Scholar]
- Dumas, J.B.A. Procedes de L’analyse Organique. Ann. Chim. Phys. 1831, 47, 198–205. [Google Scholar]
- Hou, X.; Jones, B.T. Inductively coupled plasma/optical emission spectrometry. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons: Chichester, UK, 2000; pp. 9468–9485. [Google Scholar]
- Carter, M.R.; Gregorich, E.G. (Eds.) Soil Sampling and Methods of Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; ISBN 978-0-8493-3586-0. [Google Scholar]
- Berghage, R.D.; Krauskopf, D.M.; Warncke, D.D.; Widders, I. Micronutrient testing of plant growth media extractant, identification and evaluation. Commun. Soil Sci. Plant. Anal. 1987, 18, 1089–1109. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Warman, P.R. Comparison of three digestion methods for the recovery of 17 plant essential nutrients and trace elements from six composts. Compost Sci. Utiliz. 2002, 10, 197–203. [Google Scholar] [CrossRef]
- Hue, N.V.; Ikawa, H.; Huang, X. Predicting soil phosphorus requirements. In Plant Nutrient Management in Hawaii’s Soils, Approaches for Tropical and Subtropical Agriculture; Silva, J.A., Uchida, R., Eds.; College of Tropical Agriculture and Human Resources, University of Hawaii: Manoa, HI, USA, 2000; pp. 95–99. [Google Scholar]
- Marler, T.E. Elemental profiles in Cycas micronesica stems. Plants 2018, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Calonje, M.; Stevenson, D.W.; Osborne, R. The World List of Cycads, online edition. 2013–2019. Available online: http://www.cycadlist.org (accessed on 26 July 2019).
| Leaf Trait | Sun | Shade | Significance 1 |
|---|---|---|---|
| Carbon (mg·g−1) | 492.91 ± 1.57 | 484 ± 1.99 | 0.0441 |
| Nitrogen (mg·g−1) | 23.42 ± 0.87 | 26.79 ± 0.82 | 0.0153 |
| Phosphorus (mg·g−1) | 1.76 ± 0.08 | 2.47 ± 0.11 | 0.0002 |
| Potassium (mg·g−1) | 14.10 ± 1.01 | 19.08 ± 0.93 | 0.0035 |
| Calcium (mg·g−1) | 1.83 ± 0.30 | 1.17 ± 0.34 | 0.1687 |
| Magnesium (mg·g−1) | 2.52 ± 0.24 | 2.57 ± 0.19 | 0.8596 |
| Iron (µg·g−1) | 42.86 ± 1.79 | 43.57 ± 2.34 | 0.8110 |
| Manganese (µg·g−1) | 26.14 ± 2.35 | 31.0 ± 2.91 | 0.2233 |
| Zinc (µg·g−1) | 20.43 ± 2.53 | 23.71 ± 3.21 | 0.4432 |
| Copper (µg·g−1) | 3.14 ± 0.27 | 3.14 ± 0.26 | 1.0000 |
| Boron (µg·g−1) | 11.57 ± 1.29 | 14.29 ± 0.75 | 0.0934 |
| Leaf Trait | Sun | Shade | Significance 1 |
|---|---|---|---|
| Carbon/nitrogen | 21.21 ± 0.75 | 18.17 ± 0.53 | 0.0034 |
| Carbon/potassium | 282.39 ± 12.85 | 197.90 ± 8.45 | <0.0001 |
| Carbon/phosphorus | 36.51 ± 0.66 | 25.75 ± 1.34 | 0.0033 |
| Nitrogen/potassium | 13.38 ± 0.66 | 10.95 ± 0.59 | 0.0142 |
| Nitrogen/phosphorus | 1.73 ± 0.16 | 1.43 ± 0.09 | 0.1327 |
| Potassium/phosphorus | 8.00 ± 0.58 | 7.75 ± 0.36 | 0.8032 |
| Leaf Trait | Sun | Shade | Significance 1 |
|---|---|---|---|
| Carbon (g·m−2) | 72.11 ± 0.18 | 40.41 ± 0.08 | <0.0001 |
| Nitrogen (g·m−2) | 3.43 ± 0.13 | 2.24 ± 0.07 | <0.0001 |
| Phosphorus (g·m−2) | 0.26 ± 0.01 | 0.21 ± 0.01 | 0.0035 |
| Potassium (g·m−2) | 2.06 ± 0.15 | 1.59 ± 0.07 | 0.0172 |
| Calcium (g·m−2) | 0.27 ± 0.04 | 0.10 ± 0.03 | 0.0067 |
| Magnesium (g·m−2) | 0.37 ± 0.04 | 0.21 ± 0.02 | 0.0020 |
| Iron (mg·m−2) | 6.25 ± 0.26 | 3.65 ± 0.19 | <0.0001 |
| Manganese (mg·m−2) | 3.84 ± 0.35 | 2.59 ± 0.24 | 0.0132 |
| Zinc (mg·m−2) | 3.00 ± 0.38 | 1.97 ± 0.27 | 0.0471 |
| Copper (mg·m−2) | 0.44 ± 0.04 | 0.27 ± 0.02 | 0.0016 |
| Boron (mg·m−2) | 1.69 ± 0.16 | 1.19 ± 0.09 | 0.0239 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marler, T.E.; Krishnapillai, M.V. Incident Light and Leaf Age Influence Leaflet Element Concentrations of Cycas micronesica Trees. Horticulturae 2019, 5, 58. https://doi.org/10.3390/horticulturae5030058
Marler TE, Krishnapillai MV. Incident Light and Leaf Age Influence Leaflet Element Concentrations of Cycas micronesica Trees. Horticulturae. 2019; 5(3):58. https://doi.org/10.3390/horticulturae5030058
Chicago/Turabian StyleMarler, Thomas E., and Murukesan V. Krishnapillai. 2019. "Incident Light and Leaf Age Influence Leaflet Element Concentrations of Cycas micronesica Trees" Horticulturae 5, no. 3: 58. https://doi.org/10.3390/horticulturae5030058
APA StyleMarler, T. E., & Krishnapillai, M. V. (2019). Incident Light and Leaf Age Influence Leaflet Element Concentrations of Cycas micronesica Trees. Horticulturae, 5(3), 58. https://doi.org/10.3390/horticulturae5030058





