Growth Responses and Root Characteristics of Lettuce Grown in Aeroponics, Hydroponics, and Substrate Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation Systems
2.2. Planting and Experimental Arrangement
2.3. Harvesting and Measurement
2.3.1. Biomass and Root/Shoot Ratio
2.3.2. Root Characteristics
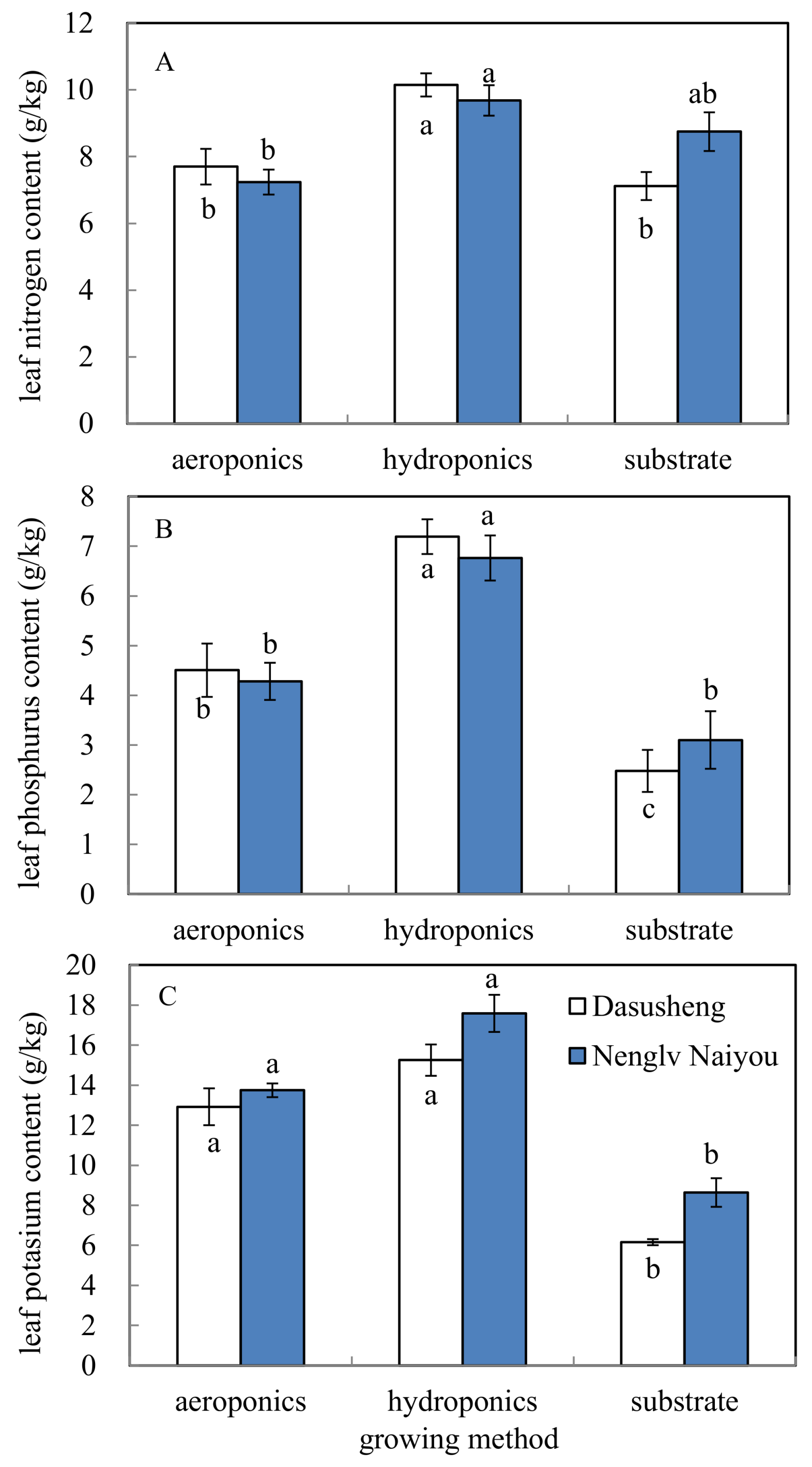
2.3.3. Plant Leaf Nitrogen, Phosphorus, and Potassium Content
2.4. Statistical Analysis
3. Results
3.1. Plant Growth and Biomass
3.2. Root Characteristics
3.3. Leaf N, P, and K Contents
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lal, R. Feeding 11 billion on 0.5 billion hectare of area under cereal crops. Food Energy Secur. 2016, 5, 239–251. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.B., Jr. Complete Guide for Growing Plants Hydroponically; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Kratsch, H.A.; Graves, W.R.; Gladon, R.J. Aeroponic system for control of root-zone atmosphere. Environ. Exp. Bot. 2006, 55, 70–76. [Google Scholar] [CrossRef]
- He, J. Farming of Vegetables in Space-Limited Environments. Cosmos 2015, 11, 21–36. [Google Scholar] [CrossRef]
- Clawson, J.; Hoehn, A.; Stodieck, L.; Todd, P.; Stoner, R. Re-Examining Aeroponics for Spaceflight Plant Growth; SAE Technical Paper 0148-7191; SAE International: Warrendale, PA, USA, 2000. [Google Scholar]
- Resh, H.M. Hydroponic Food Production: A Definitive Guidebook for the Advanced Home Gardener and the Commercial Hydroponic Grower; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Buckseth, T.; Sharma, A.K.; Pandey, K.K.; Singh, B.P.; Muthuraj, R. Methods of pre-basic seed potato production with special reference to aeroponics—A review. Sci. Hortic. 2016, 204, 79–87. [Google Scholar] [CrossRef]
- Tierno, R.; Carrasco, A.; Ritter, E.; de Galarreta, J.I.R. Differential Growth Response and Minituber Production of Three Potato Cultivars Under Aeroponics and Greenhouse Bed Culture. Am. J. Potato Res. 2013, 91, 346–353. [Google Scholar] [CrossRef]
- Mateus-Rodriguez, J.R.; de Haan, S.; Andrade-Piedra, J.L.; Maldonado, L.; Hareau, G.; Barker, I.; Chuquillanqui, C.; Otazú, V.; Frisancho, R.; Bastos, C.; et al. Technical and Economic Analysis of Aeroponics and other Systems for Potato Mini-Tuber Production in Latin America. Am. J. Potato Res. 2013, 90, 357–368. [Google Scholar] [CrossRef]
- Margaret, C. Potential of aeroponics system in the production of quality potato (Solanum tuberosum L.) seed in developing countries. Afr. J. Biotechnol. 2012, 11, 3993–3999. [Google Scholar]
- Oteng-Darko, P.; Kyei-Baffour, N.; Otoo, E.; Agyare, W.A. Growing Seed Yams in the Air: The Agronomic Performance of Two Aeroponics Systems Developed in Ghana. Sustain. Agric. Res. 2017, 6, 106–116. [Google Scholar] [CrossRef]
- Maroya, N.; Balogun, M.; Asiedu, R.; Aighewi, B.; Kumar, P.L.; Augusto, J. Yam propagation using aeroponics technology. Annu. Res. Rev. Biol. 2014, 4, 3894–3903. [Google Scholar] [CrossRef]
- Hayden, A.; Yokelsen, T.; Giacomelli, G.; Hoffmann, J. Aeroponics: An alternative production system for high-value root crops. Acta Hortic. 2004, 629, 207–213. [Google Scholar] [CrossRef]
- Hayden, A.L.; Brigham, L.A.; Giacomelli, G.A. Aeroponic cultivation of ginger (Zingiber officinale) rhizomes. Acta Hortic. 2004, 659, 397–402. [Google Scholar] [CrossRef]
- Hayden, A.L. Aeroponic and hydroponic systems for medicinal herb, rhizome, and root crops. Hortscience 2006, 41, 536–538. [Google Scholar]
- Souret, F.F.; Weathers, P.J. The Growth of Saffron (Crocus sativus L.) in Aeroponics and Hydroponics. J. Herbs Spices Med. Plants 2000, 7, 25–35. [Google Scholar] [CrossRef]
- Tabatabaei, S.J. Effects of Cultivation Systems on the Growth, and Essential Oil Content and Composition of Valerian. J. Herbs Spices Med. Plants 2008, 14, 54–67. [Google Scholar] [CrossRef]
- Mehandru, P.; Shekhawat, N.S.; Rai, M.K.; Kataria, V.; Gehlot, H.S. Evaluation of aeroponics for clonal propagation of Caralluma edulis, Leptadenia reticulata and Tylophora indica—Three threatened medicinal Asclepiads. Physiol. Mol. Biol. Plants 2014, 20, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Martin-Laurent, F.; Lee, S.-K.; Tham, F.-Y.; Jie, H.; Diem, H.G. Aeroponic production of Acacia mangium saplings inoculated with AM fungi for reforestation in the tropics. For. Ecol. Manag. 1999, 122, 199–207. [Google Scholar] [CrossRef]
- Soffer, H.; Burger, D.W. Effects of dissolved oxygen concentrations in aero-hydroponics on the formation and growth of adventitious roots. J. Am. Soc. Hortic. Sci. 1988, 113, 218–221. [Google Scholar]
- Weathers, P.; Liu, C.; Towler, M.; Wyslouzil, B. Mist reactors: Principles, comparison of various systems, and case studies. Electron. J. Integr. Biosci. 2008, 3, 29–37. [Google Scholar]
- Tisserat, B.; Jones, D.; Galletta, P.D. Construction and use of an inexpensive in vitro ultrasonic misting system. HortTechnology 1993, 3, 75–78. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil, 2nd ed.; Circular 347; California Agricultural Experiment Station: Berkeley, CA, USA, 1950. [Google Scholar]
- Galkovskyi, T.; Mileyko, Y.; Bucksch, A.; Moore, B.; Symonova, O.; Price, C.A.; Topp, C.N.; Iyer-Pascuzzi, A.S.; Zurek, P.R.; Fang, S. GiA Roots: Software for the high throughput analysis of plant root system architecture. BMC Plant Biol. 2012, 12, 116. [Google Scholar] [CrossRef] [PubMed]
- Horneck, D.A.; Miller, R.O. Determination of total nitrogen in plant tissue. In Handbook of Reference Methods for Plant Analysis; Kalra, Y.P., Ed.; CRC Press LLC: Boca Raton, FL, USA, 1998; pp. 75–83. [Google Scholar]
- Jones, J.B., Jr. Laboratory Guide for Conducting Soil Tests and Plant Analysis; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Horneck, D.; Hanson, D. Determination of potassium and sodium by flame emission spectrophotometry. In Handbook of Reference Methods for Plant Analysis; Kalra, Y.P., Ed.; CRC Press LLC: Boca Raton, FL, USA, 1998; pp. 153–155. [Google Scholar]
- Salachas, G.; Savvas, D.; Argyropoulou, K.; Tarantillis, P.; Kapotis, G. Yield and nutritional quality of aeroponically cultivated basil as affected by the available root-zone volume. Emir. J. Food Agric. 2015, 27, 911–918. [Google Scholar] [CrossRef]
- Chang, D.C.; Park, C.S.; Kim, S.Y.; Lee, Y.B. Growth and Tuberization of Hydroponically Grown Potatoes. Potato Res. 2012, 55, 69–81. [Google Scholar] [CrossRef]
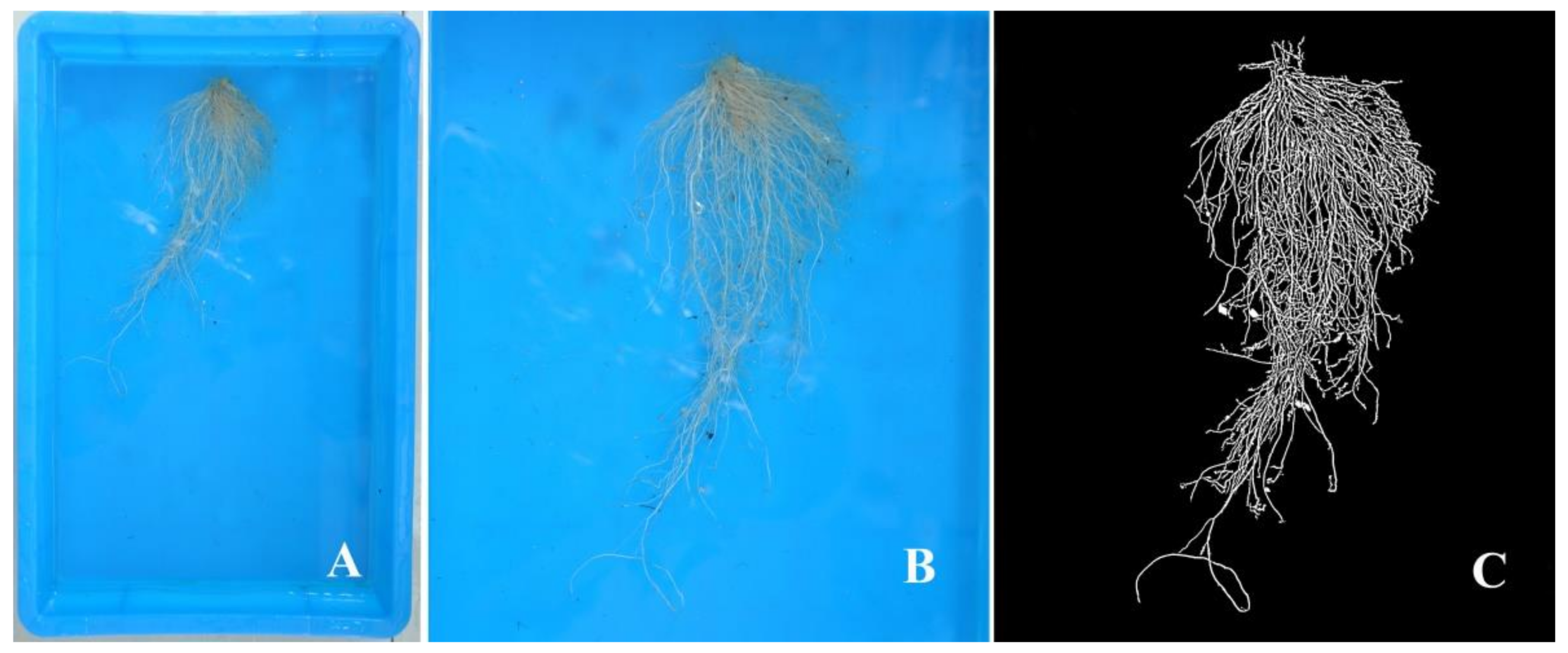

| Shoot FW (g) | Root FW (g) | Shoot DW (g) | Root DW (g) | Root/Shoot Ratio | |
|---|---|---|---|---|---|
| Lactuca sativa ‘Dasusheng’ | |||||
| aeroponics | 37.8 ± 2.67 bz | 8.67 ± 1.20 a | 2.40 ± 0.17 b | 0.80 ± 0.15 a | 0.32 ± 0.04 a |
| hydroponics | 88.8 ± 9.47 a | 8.78 ± 1.24 a | 4.86 ± 0.54 a | 0.59 ± 0.06 b | 0.12 ± 0.01 c |
| substrate | 49.2 ± 2.34 b | 6.92 ± 0.43 b | 3.23 ± 0.13 b | 0.69 ± 0.03 b | 0.22 ± 0.02 b |
| Lactuca sativa ‘Nenglv Naiyou’ | |||||
| aeroponics | 50.9 ± 2.60 b | 10.3 ± 0.46 a | 2.58 ± 0.11 b | 0.77 ± 0.07 a | 0.30 ± 0.03 a |
| hydroponics | 96.1 ± 4.23 a | 11.5 ± 1.07 a | 4.80 ± 0.16 a | 0.54 ± 0.03 b | 0.11 ± 0.01 b |
| substrate | 39.4 ± 1.72 c | 3.9 ± 0.35 b | 2.03 ± 0.06 c | 0.26 ± 0.03 c | 0.13 ± 0.01 b |
| Significance | |||||
| Cultivation system (CS) | *** y | *** | *** | ** | *** |
| Genotype (G) | Ns | Ns | * | * | ns |
| CS × G | * | ** | * | * | ns |
| Average Root Diameter (mm) | Root Length (cm) | Root Area (cm2) | Root Volume (cm3) | Maximum No. of Roots | Median No. of Roots | Network Perimeter (cm) | |
|---|---|---|---|---|---|---|---|
| Lactuca sativa ‘Dasusheng’ | |||||||
| aeroponics | 0.501 ± 0.017 az | 3043 ± 231 a | 479 ± 42 a | 7.24 ± 0.77 a | 75.0 ± 5.8 a | 39.0 ± 4.1 a | 6019 ± 473 a |
| hydroponics | 0.551 ± 0.025 a | 581 ± 113 b | 100 ± 20 b | 1.64 ± 0.35 b | 24.6 ± 2.9 b | 13.8 ± 1.6 b | 1164 ± 221 b |
| substrate | 0.501 ± 0.007 a | 724 ± 126 b | 114 ± 21 b | 1.67 ± 0.35 b | 32.6 ± 5.3 b | 17.4 ± 2.2 b | 1437 ± 245 b |
| Lactuca sativa ‘Nenglv Naiyou’ | |||||||
| aeroponics | 0.511 ± 0.0023 ab | 2634 ± 260 a | 424 ± 46 a | 6.63 ± 0.84 a | 63.0 ± 7.4 a | 34.4 ± 3.1 a | 5197 ± 557 a |
| hydroponics | 0.554 ± 0.0009 a | 1688 ± 239 b | 296 ± 46 b | 4.91 ± 0.85 ab | 54.4 ± 3.0 a | 19.0 ± 4.8 b | 3379 ± 473 b |
| substrate | 0.487 ± 0.0006 b | 1378 ± 58 b | 211 ± 10 b | 2.99 ± 0.15 b | 51.2 ± 1.6 a | 37.0 ± 2.6 a | 2755 ± 113 b |
| Significance | |||||||
| Cultivation system (CS) | ns y | *** | ** | * | *** | *** | * |
| Genotype (G) | Ns | *** | *** | *** | *** | ** | *** |
| CS × G | * | * | ns | Ns | ns | * | * |
| N | P | K | |
|---|---|---|---|
| Cultivation system (CS) | 11.6 *** z | 50.6 *** | 84.6 ** |
| Genotype (G) | 0.28 ns | 0.01 ns | 10.7 ** |
| CS × G | 2.51 ns | 0.88 ns | 0.84 ns |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Li, X.; Tang, B.; Gu, M. Growth Responses and Root Characteristics of Lettuce Grown in Aeroponics, Hydroponics, and Substrate Culture. Horticulturae 2018, 4, 35. https://doi.org/10.3390/horticulturae4040035
Li Q, Li X, Tang B, Gu M. Growth Responses and Root Characteristics of Lettuce Grown in Aeroponics, Hydroponics, and Substrate Culture. Horticulturae. 2018; 4(4):35. https://doi.org/10.3390/horticulturae4040035
Chicago/Turabian StyleLi, Qiansheng, Xiaoqiang Li, Bin Tang, and Mengmeng Gu. 2018. "Growth Responses and Root Characteristics of Lettuce Grown in Aeroponics, Hydroponics, and Substrate Culture" Horticulturae 4, no. 4: 35. https://doi.org/10.3390/horticulturae4040035
APA StyleLi, Q., Li, X., Tang, B., & Gu, M. (2018). Growth Responses and Root Characteristics of Lettuce Grown in Aeroponics, Hydroponics, and Substrate Culture. Horticulturae, 4(4), 35. https://doi.org/10.3390/horticulturae4040035






