Ripening Characteristics and Pigment Changes in Russeted Pear Fruit in Response to Ethylene and 1-MCP
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Measurement of Ethylene Production and Flesh Firmness
2.3. Pigment Analysis
2.4. RNA Isolation and Quantitative Polymerase Chain Reaction (qRT-PCR)
2.5. Statistical Analyses
3. Results
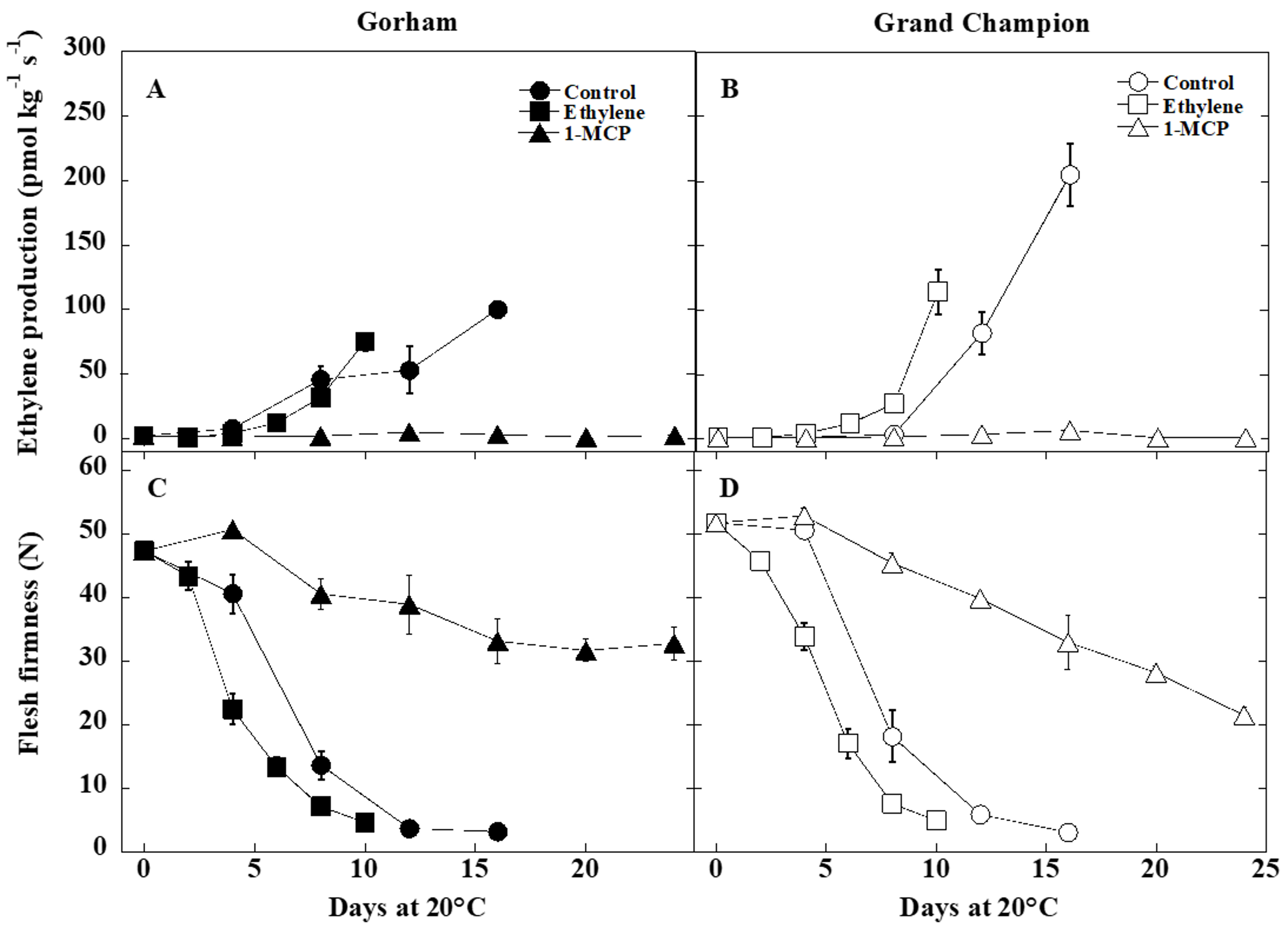
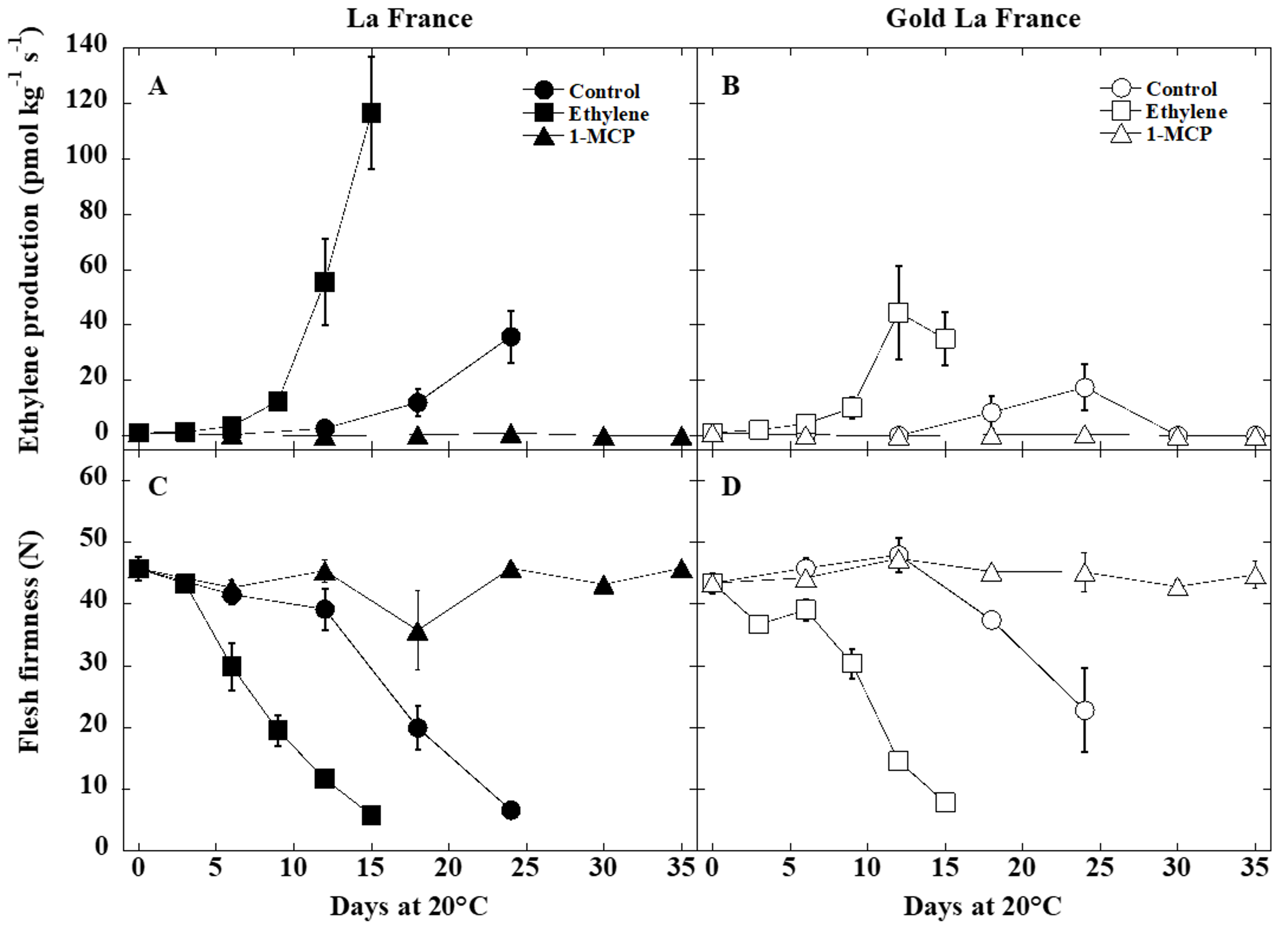
3.1. Ethylene Production and Fruit Softening
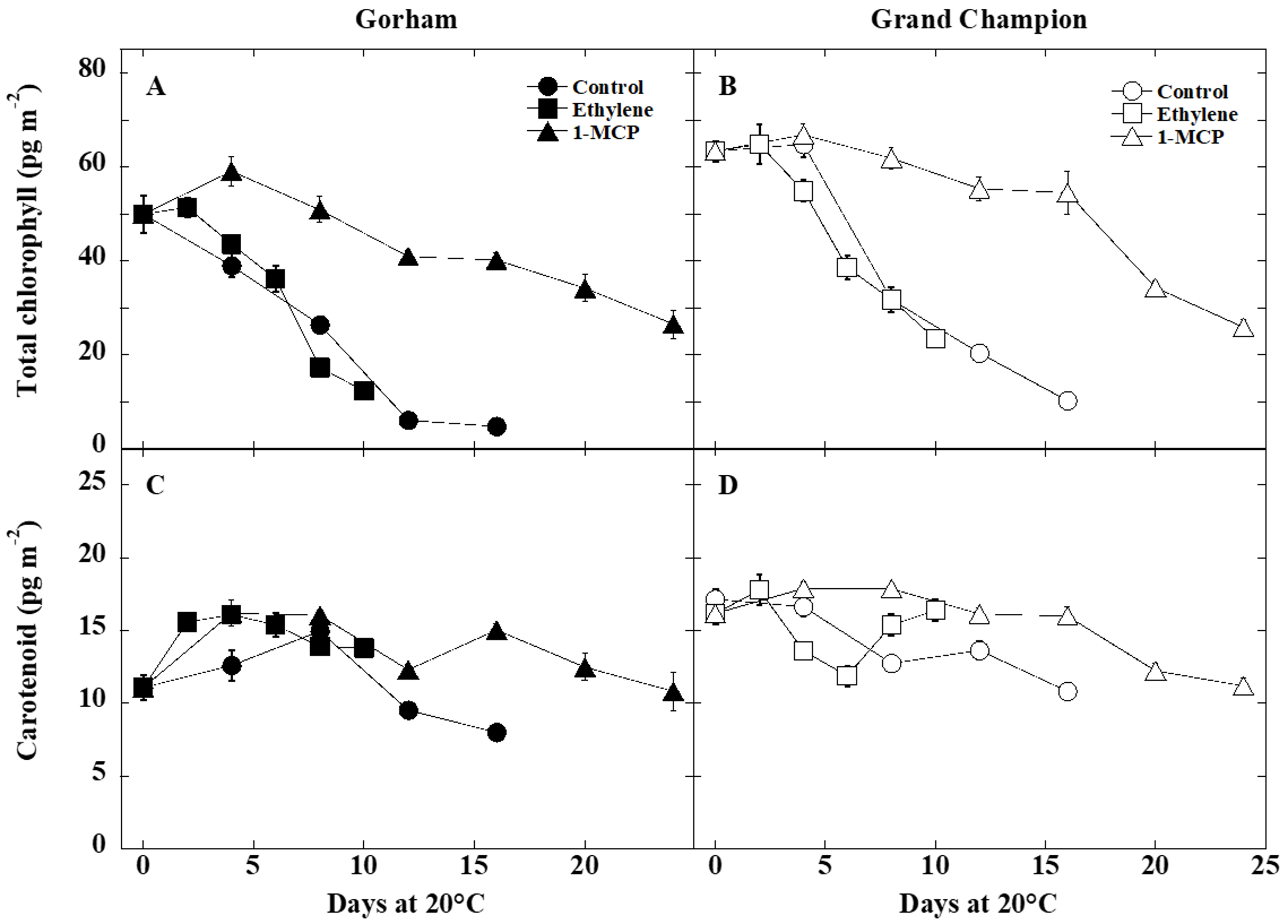
3.2. Color and Pigment Changes
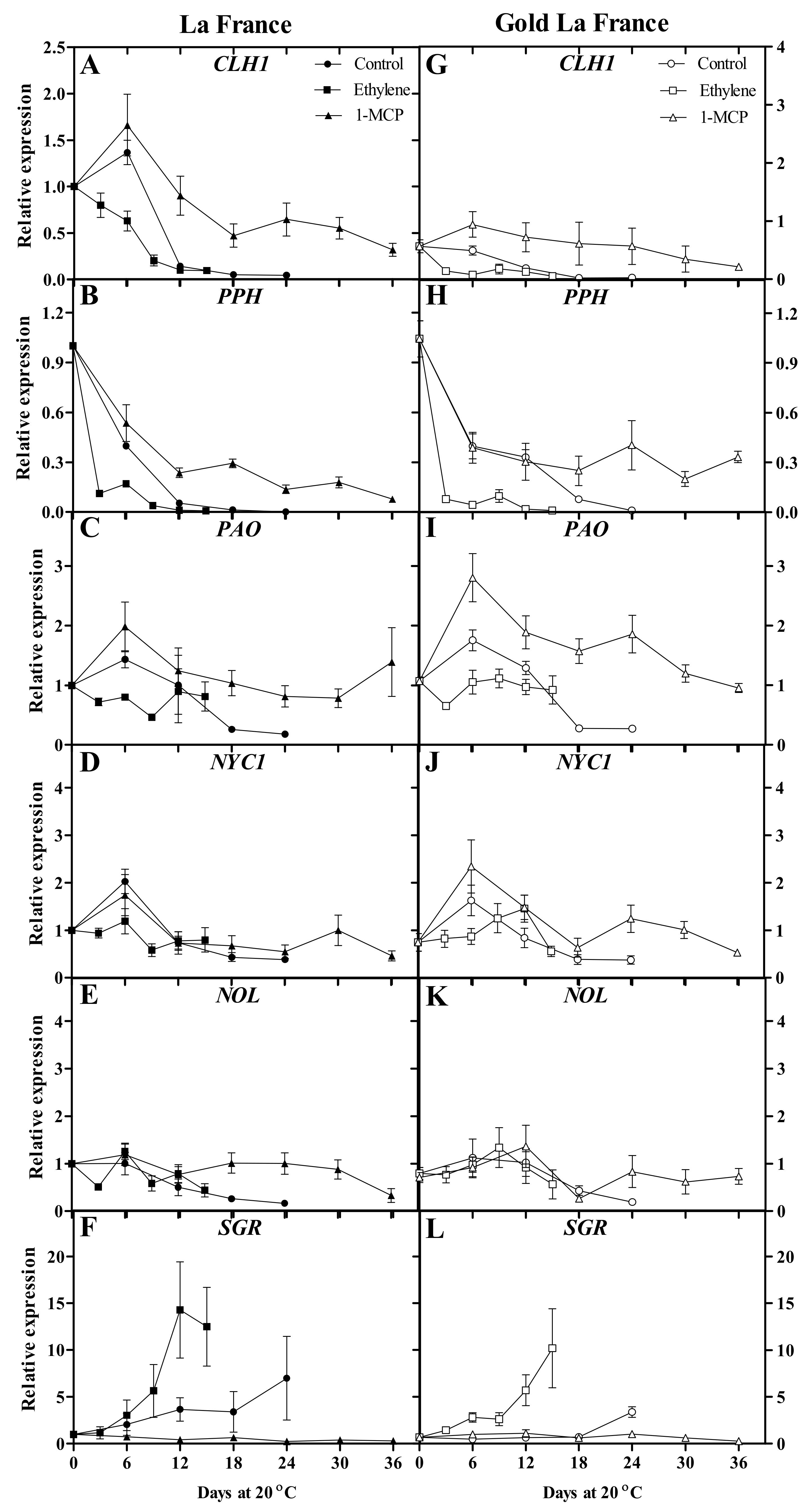
3.3. Chlorophyll-Degradation-Related Gene Expression
4. Discussion
4.1. Ethylene Production and Fruit Softening
4.2. Fruit Color and Pigment Changes
4.3. Chlorophyll-Degradation-Related Genes
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gross, J. Chlorophyll and carotenoids in the peel of 2 pear cultivars. Gartenbauwissenschaft 1984, 49, 128–131. [Google Scholar] [CrossRef]
- Matile, P.; Hortensteiner, S.; Thomas, H. Chlorophyll degradation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 67–95. [Google Scholar] [CrossRef] [PubMed]
- Charoenchongsuk, N.; Ikeda, K.; Itai, A.; Oikawa, A.; Murayama, H. Comparison of the expression of chlorophyll-degradation-related genes during ripening between stay-green and yellow-pear cultivars. Sci. Hortic. 2015, 181, 89–94. [Google Scholar] [CrossRef]
- Soler, M.; Serra, O.; Molinas, M.; Huguet, G.; Fluch, S.; Figueras, M. A genomic approach to suberin biosynthesis and cork differentiation. Plant Physiol. 2007, 144, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Pollard, M.; Beisson, F.; Li, Y.H.; Ohlrogge, J.B. Building lipid barriers: Biosynthesis of cutin and suberin. Trends Plant Sci. 2008, 13, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, L.; Franke, R.; Hartmann, K. Wax and suberin development of native and wound periderm of potato (Solanum tuberosum L.) and its relation to peridermal transpiration. Planta 2005, 220, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Kikuti, A. On the origin of Japanese pear and the inheritance of the skin colours of their fruits. Jpn. J. Genet. 1924, 3, 1–21. [Google Scholar] [CrossRef]
- Inoue, E.; Kasumi, M.; Sakuma, F.; Anzai, H.; Amano, K.; Hara, H. Identification of RAPD marker linked to fruit skin color in Japanese pear (Pyrus pyrifolia Nakai). Sci. Hortic. 2006, 107, 254–258. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Dai, M.S.; Zhang, S.J.; Shi, Z.B. Exploring Candidate genes for pericarp russet pigmentation of sand pear (Pyrus pyrifolia) via RNA-seq data in two genotypes contrasting for pericarp color. PLoS ONE 2014, 9, e83675. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Zhang, S.J.; Dai, M.S.; Shi, Z.B. Pigmentation in sand pear (Pyrus pyrifolia) fruit: Biochemical characterization, gene discovery and expression analysis with exocarp pigmentation mutant. Plant Mol. Biol. 2014, 85, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Sugar, D.; Basile, S.R. Color and russet variation among selections of ‘Bosc’ pear. J. Am. Pomol. Soc. 2008, 62, 77–81. [Google Scholar]
- Barcelo-Vidal, C.; Bonany, J.; Martin-Fernandez, J.A.; Carbo, J. Modelling of weather parameters to predict russet on ‘Golden Delicious’ apple. J. Hortic. Sci. Biotechnol. 2013, 88, 624–630. [Google Scholar] [CrossRef]
- Heidenreich, M.C.M.; CorralGarcia, M.R.; Momol, E.A.; Burr, T.J. Russet of apple fruit caused by Aureobasidium pullulans and Rhodotorula glutinis. Plant Dis. 1997, 81, 337–342. [Google Scholar] [CrossRef]
- Spotts, R.A.; Cervantes, L.A. Involvement of Aureobasidium pullulans and Rhodotorula glutinis in russet of d’Anjou pear fruit. Plant Dis. 2002, 86, 625–628. [Google Scholar] [CrossRef]
- Looney, N.E.; Granger, R.L.; Chu, C.L.; McArtney, S.J.; Mander, L.N.; Pharis, R.P. Influences of gibberellins A4, A4+7, and A4+Iso-A7 on apple fruit-quality and tree productivity. I. Effects on fruit russet and tree yield components. J. Hortic. Sci. 1992, 67, 613–618. [Google Scholar] [CrossRef]
- Sugar, D.; Powers, K.A.; Basile, S.R. Mancozeb and kaolin applications can reduce russet of ‘Comice’ pear. HortTechnology 2005, 15, 272–275. [Google Scholar]
- Barry, C.S.; Giovannoni, J.J. Ethylene and fruit ripening. J. Plant Growth Regul. 2007, 26, 143–159. [Google Scholar] [CrossRef]
- Sisler, E.C.; Serek, M. Inhibitors of ethylene responses in plants at the receptor level: Recent developments. Physiol. Plant 1997, 100, 577–582. [Google Scholar] [CrossRef]
- Blankenship, S.M.; Dole, J.M. 1-methylcyclopropene: A review. Postharvest Biol. Technol. 2003, 28, 1–25. [Google Scholar] [CrossRef]
- Watkins, C.B. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef] [PubMed]
- Isidoro, N.; Almeida, D.P.F. Alpha-farnesene, conjugated trienols, and superficial scald in ‘Rocha’ pear as affected by 1-methylcyclopropene and diphenylarnine. Postharvest Biol. Technol. 2003, 42, 49–56. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, J.; Wang, Y. Initiation of ripening capacity in 1-MCP treated green and red ‘Anjou’ pears and associated expression of genes related to ethylene biosynthesis and perception following cold storage and post-storage ethylene conditioning. Postharvest Biol. Technol. 2016, 111, 140–149. [Google Scholar] [CrossRef]
- Zhi, H.; Dong, Y. Effect of 1-methylcyclopropene on superficial scald associated with ethylene production, α-farnesene catabolism, and antioxidant system of over-mature ‘d’Anjou’ pears after long-term storage. Food Bioprocess Technol. 2018, 11, 1775–1786. [Google Scholar] [CrossRef]
- Bower, J.H.; Blasi, W.V.; Mitcham, E.J. Effect of ethylene in the storage environment on quality of ‘Bartlett pears’. Postharvest Biol. Technol. 2003, 28, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.D.; Guan, J.F. Involvement of pheophytinase in ethylene-mediated chlorophyll degradation in the peel of harvested ‘Yali’ pear. J. Plant Growth Regul. 2014, 33, 364–372. [Google Scholar] [CrossRef]
- Cheng, Y.D.; Dong, Y.; Yan, H.B.; Ge, W.Y.; Shen, C.G.; Guan, J.F.; Liu, L.Q.; Zhang, Y.Y. Effects of 1-MCP on chlorophyll degradation pathway-associated genes expression and chloroplast ultrastructure during the peel yellowing of Chinese pear fruits in storage. Food Chem. 2012, 135, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxydase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Kuang, J.F.; Chen, L.; Xie, H.; Peng, H.H.; Xiao, Y.Y.; Li, X.P.; Chen, W.X.; He, Q.G.; Chen, J.Y.; et al. Molecular characterization of banana NAC transcription factors and their interactions with ethylene signalling component EIL during fruit ripening. J. Exp. Bot. 2012, 63, 5171–5187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, X.R.; Chen, K.S.; Allan, A.C.; Wu, R.M.; Zhang, B.; Lallu, N.; Ferguson, I.B. Ethylene-induced modulation of genes associated with the ethylene signalling pathway in ripening kiwifruit. J. Exp. Bot. 2008, 59, 2097–2108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamrasni, D.; Ben-Arie, R.; Goldway, M. 1-Methylcyclopropene (1-MCP) application to Spadona pears at different stages of ripening to maximize fruit quality after storage. Postharvest Biol. Technol. 2010, 58, 104–112. [Google Scholar] [CrossRef]
- Villalobos-Acuna, M.G.; Biasi, W.V.; Flores, S.; Jiang, C.Z.; Reid, M.S.; Willits, N.H.; Mitcham, E.J. Effect of maturity and cold storage on ethylene biosynthesis and ripening in ‘Bartlett’ pears treated after harvest with 1-MCP. Postharvest Biol. Technol. 2011, 59, 1–9. [Google Scholar] [CrossRef]
- Pech, J.C.; Bouzayen, M.; Latché, A. Climacteric fruit ripening: Ethylene-dependent and independent regulation of ripening pathways in melon fruit. Plant Sci. 2008, 175, 114–120. [Google Scholar] [CrossRef] [Green Version]
- Guis, M.; Botondi, R.; Ben-Amor, M.; Ayub, R.; Bouzayen, M.; Pech, J.-C.; Latché, A. Ripening-associated biochemical traits of Cantaloupe Charentais melons expressing an antisense ACC oxidase transgene. J. Am. Soc. Hortic. Sci. 1997, 122, 748–751. [Google Scholar]
- Hiwasa, K.; Kinugasa, Y.; Amano, S.; Hashimoto, A.; Nakano, R.; Inaba, A.; Kubo, Y. Ethylene is required for both the initiation and progression of softening in pear (Pyrus communis L.) fruit. J. Exp. Bot. 2003, 54, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Grunenfelder, L.; Hiller, L.K.; Knowles, N.R. Color indices for the assessment of chlorophyll development and greening of fresh market potatoes. Postharvest Biol. Technol. 2006, 40, 73–81. [Google Scholar] [CrossRef]
- Porat, R.; Weiss, B.; Cohen, L.; Daus, A.; Goren, R.; Droby, S. Effects of ethylene and 1-methylcyclopropene on the postharvest qualities of ‘Shamouti’ oranges. Postharvest Biol. Technol. 1999, 15, 155–163. [Google Scholar] [CrossRef]
- Trebitsh, T.; Goldschmidt, E.E.; Riov, J. Ethylene induces de-novo synthesis of chlorophyllase, a chlorophyll degrading enzyme, in citrus-fruit peel. Proc. Natl. Acad. Sci. USA 1993, 90, 9441–9445. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Blankenship, S.M.; Mattheis, J. 1-Methylcyclopropene inhibits apple ripening. J. Am. Soc. Hortic. Sci. 1999, 124, 690–695. [Google Scholar]
- Watkins, C.B.; Nock, J.F.; Whitaker, B.D. Responses of early, mid and late season apple cultivars to postharvest application of 1-methylcyclopropene (1-MCP) under air and controlled atmosphere storage conditions. Postharvest Biol. Technol. 2000, 19, 17–23. [Google Scholar] [CrossRef]
- Mir, N.A.; Curell, E.; Khan, N.; Whitaker, M.; Beaudry, R.M. Harvest maturity, storage temperature, and 1-MCP application frequency alter firmness retention and chlorophyll fluorescence of ‘Redchief Delicious’ apples. J. Am. Soc. Hortic. Sci. 2001, 126, 618–624. [Google Scholar]
- Sakuraba, Y.; Schelbert, S.; Park, S.-Y.; Han, S.-H.; Lee, B.-D.; Andrès, C.B.; Kessler, F.; Hörtensteiner, S.; Paek, N.C. STAY-GREEN and chlorophyll catabolic enzymes interact at light-harvesting complex II for chlorophyll detoxification during leaf senescence in Arabidopsis. Plant Cell 2012, 24, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Barry, C.S.; McQuinn, R.P.; Chung, M.-Y.; Besuden, A.; Giovannoni, J.J. Amino Acid Substitutions in homologs of the STAY-GREEN protein are responsible for the green-flesh and chlorophyll retainer mutations of tomato and pepper. Plant Physiol. 2008, 147, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Borovsky, Y.; Paran, I. Chlorophyll breakdown during pepper fruit ripening in the chlorophyll retainer mutation is impaired at the homolog of the senescence-inducible stay-green gene. Theor. Appl. Genet. 2008, 117, 235–240. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charoenchongsuk, N.; Matsumoto, D.; Itai, A.; Murayama, H. Ripening Characteristics and Pigment Changes in Russeted Pear Fruit in Response to Ethylene and 1-MCP. Horticulturae 2018, 4, 22. https://doi.org/10.3390/horticulturae4030022
Charoenchongsuk N, Matsumoto D, Itai A, Murayama H. Ripening Characteristics and Pigment Changes in Russeted Pear Fruit in Response to Ethylene and 1-MCP. Horticulturae. 2018; 4(3):22. https://doi.org/10.3390/horticulturae4030022
Chicago/Turabian StyleCharoenchongsuk, Nongluk, Daiki Matsumoto, Akihiro Itai, and Hideki Murayama. 2018. "Ripening Characteristics and Pigment Changes in Russeted Pear Fruit in Response to Ethylene and 1-MCP" Horticulturae 4, no. 3: 22. https://doi.org/10.3390/horticulturae4030022
APA StyleCharoenchongsuk, N., Matsumoto, D., Itai, A., & Murayama, H. (2018). Ripening Characteristics and Pigment Changes in Russeted Pear Fruit in Response to Ethylene and 1-MCP. Horticulturae, 4(3), 22. https://doi.org/10.3390/horticulturae4030022



