Ripening Characteristics and Pigment Changes in Russeted Pear Fruit in Response to Ethylene and 1-MCP
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Measurement of Ethylene Production and Flesh Firmness
2.3. Pigment Analysis
2.4. RNA Isolation and Quantitative Polymerase Chain Reaction (qRT-PCR)
2.5. Statistical Analyses
3. Results
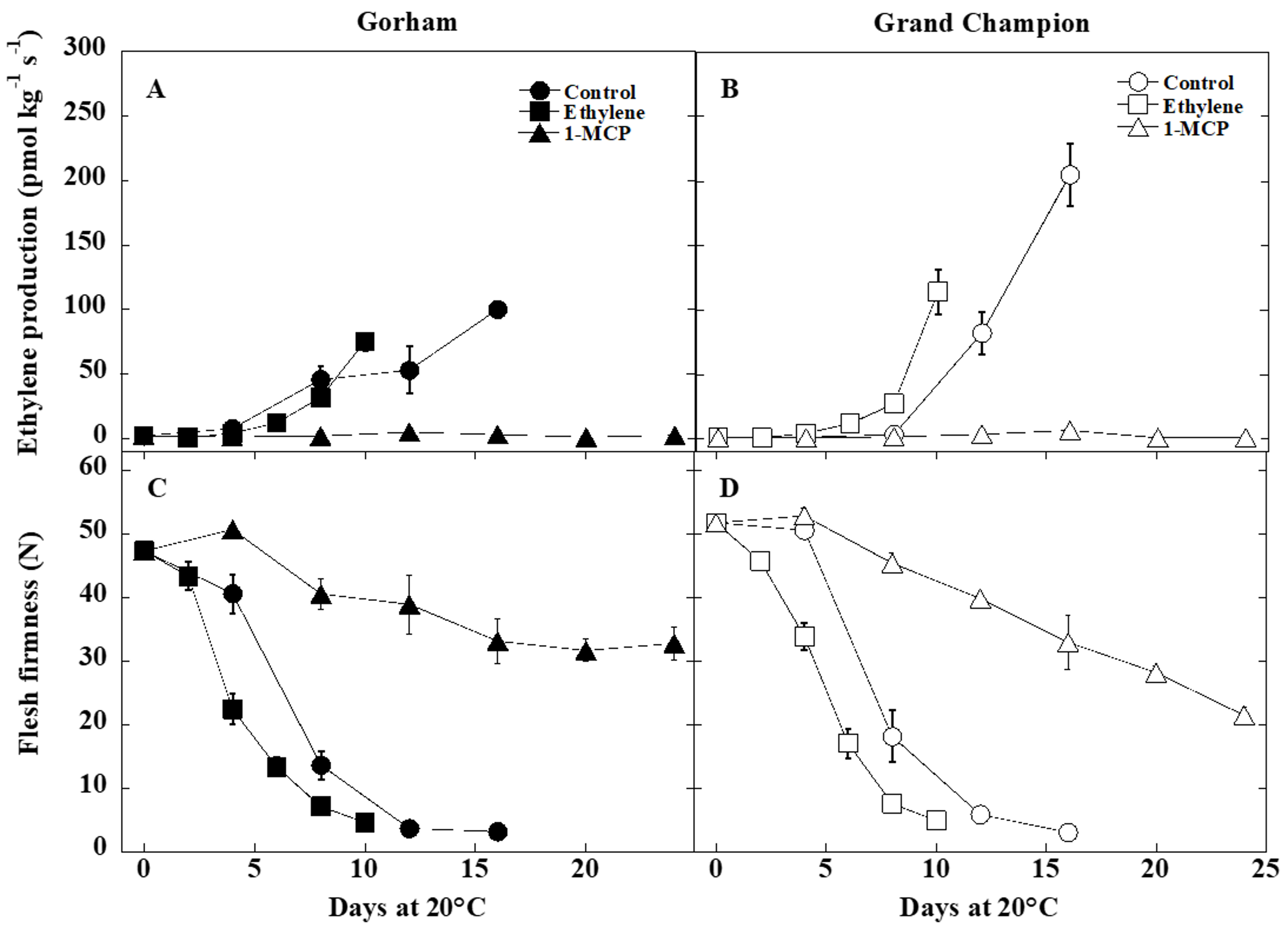
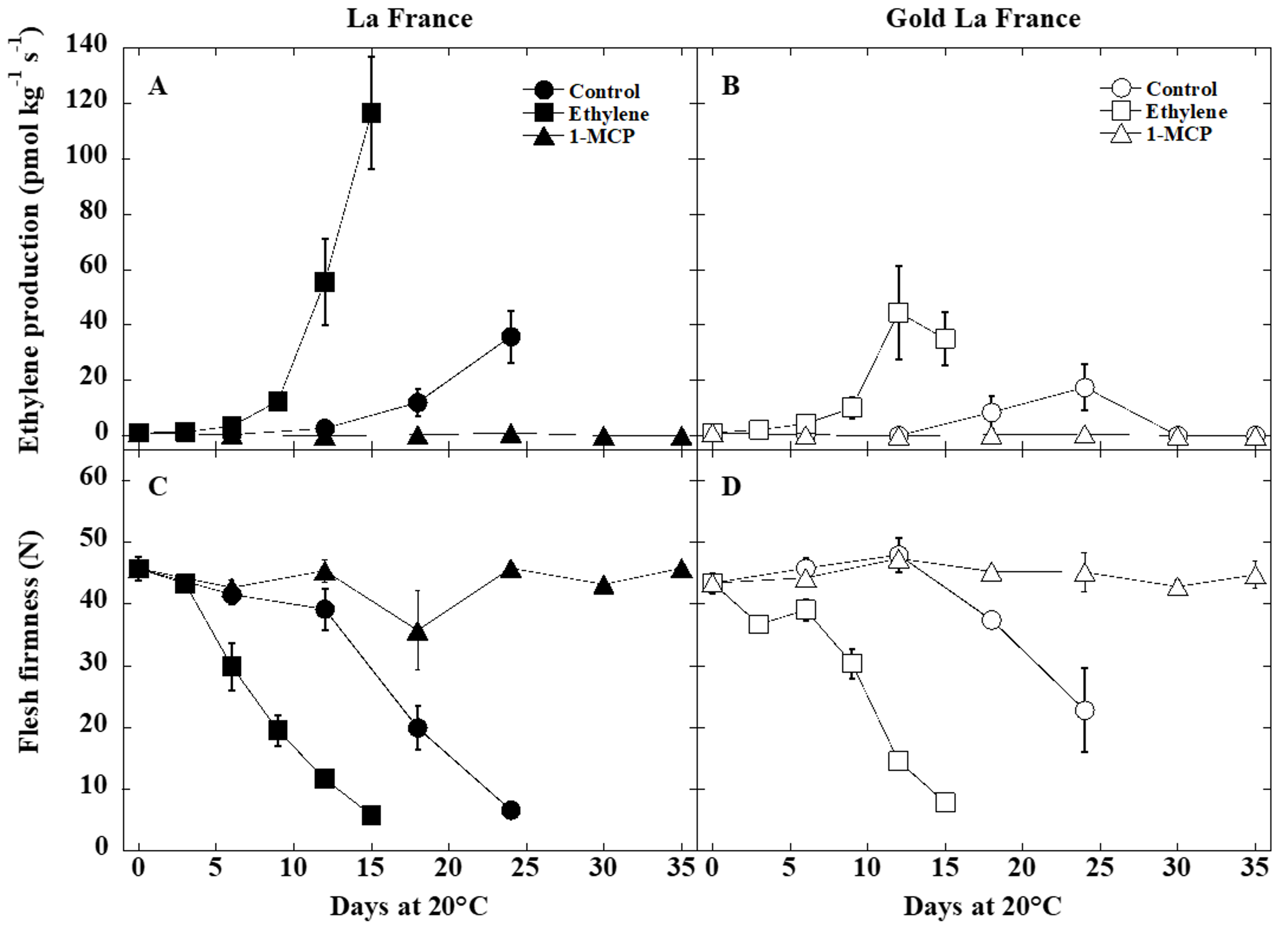
3.1. Ethylene Production and Fruit Softening
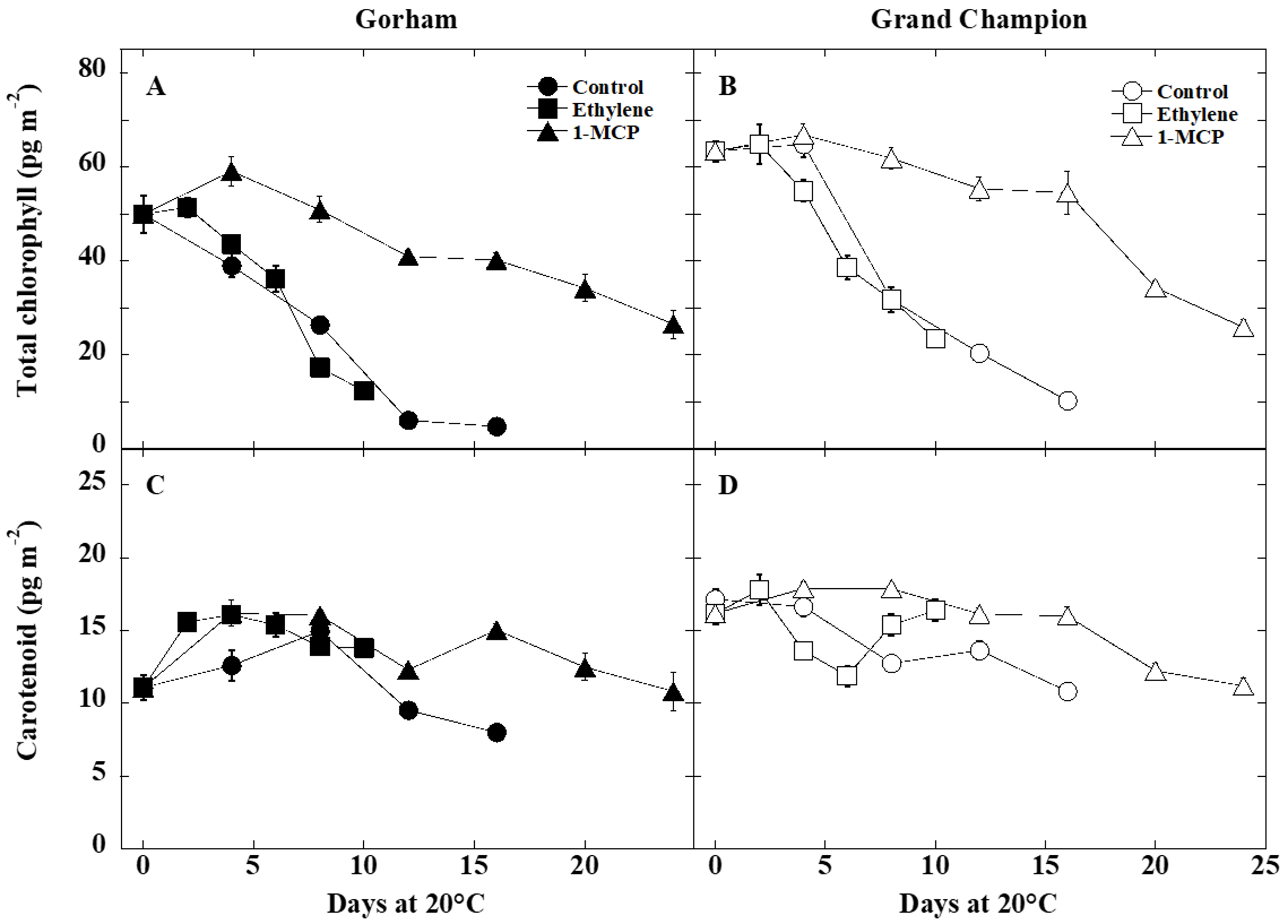
3.2. Color and Pigment Changes
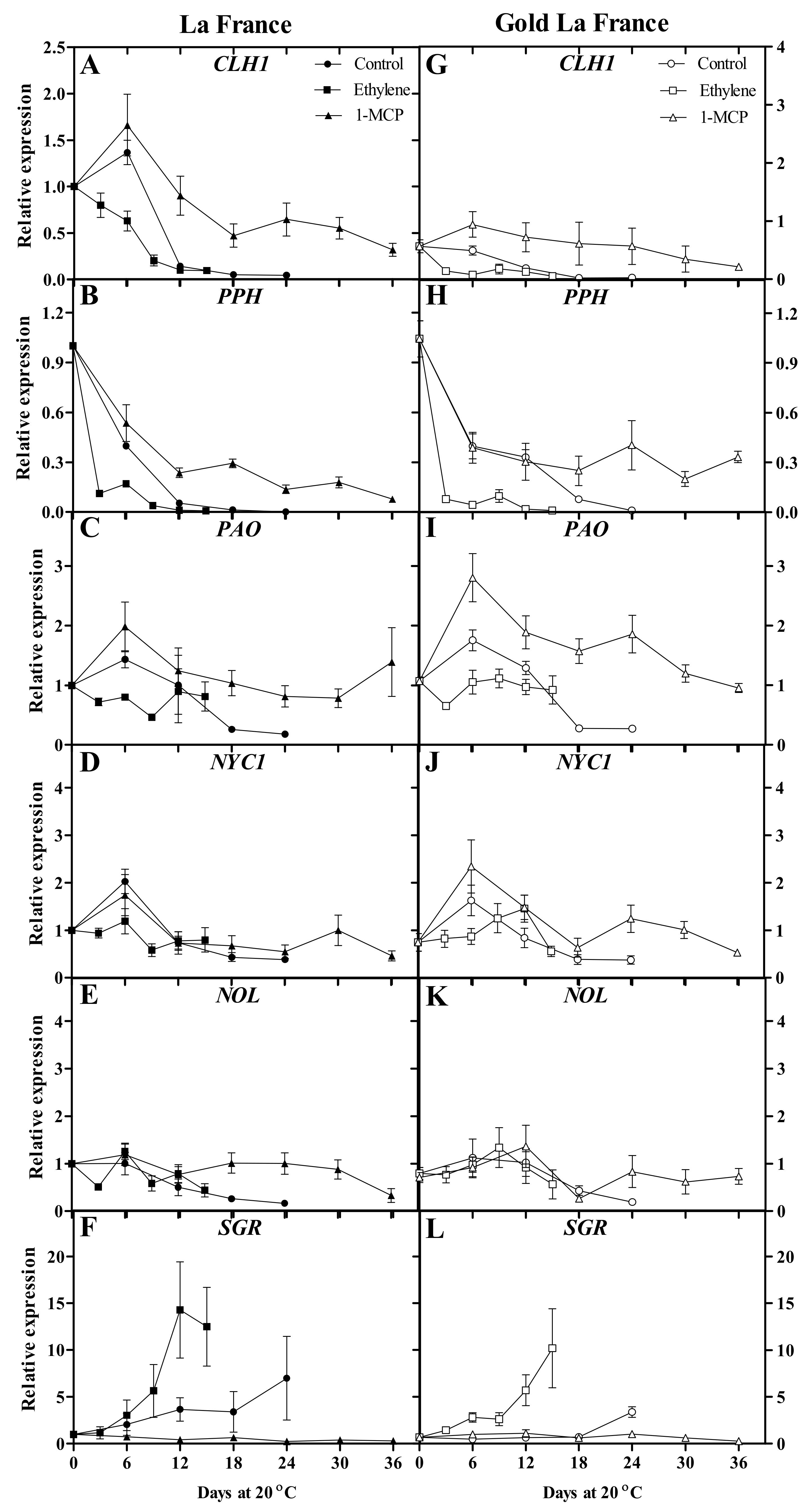
3.3. Chlorophyll-Degradation-Related Gene Expression
4. Discussion
4.1. Ethylene Production and Fruit Softening
4.2. Fruit Color and Pigment Changes
4.3. Chlorophyll-Degradation-Related Genes
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gross, J. Chlorophyll and carotenoids in the peel of 2 pear cultivars. Gartenbauwissenschaft 1984, 49, 128–131. [Google Scholar] [CrossRef]
- Matile, P.; Hortensteiner, S.; Thomas, H. Chlorophyll degradation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 67–95. [Google Scholar] [CrossRef] [PubMed]
- Charoenchongsuk, N.; Ikeda, K.; Itai, A.; Oikawa, A.; Murayama, H. Comparison of the expression of chlorophyll-degradation-related genes during ripening between stay-green and yellow-pear cultivars. Sci. Hortic. 2015, 181, 89–94. [Google Scholar] [CrossRef]
- Soler, M.; Serra, O.; Molinas, M.; Huguet, G.; Fluch, S.; Figueras, M. A genomic approach to suberin biosynthesis and cork differentiation. Plant Physiol. 2007, 144, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Pollard, M.; Beisson, F.; Li, Y.H.; Ohlrogge, J.B. Building lipid barriers: Biosynthesis of cutin and suberin. Trends Plant Sci. 2008, 13, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, L.; Franke, R.; Hartmann, K. Wax and suberin development of native and wound periderm of potato (Solanum tuberosum L.) and its relation to peridermal transpiration. Planta 2005, 220, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Kikuti, A. On the origin of Japanese pear and the inheritance of the skin colours of their fruits. Jpn. J. Genet. 1924, 3, 1–21. [Google Scholar] [CrossRef]
- Inoue, E.; Kasumi, M.; Sakuma, F.; Anzai, H.; Amano, K.; Hara, H. Identification of RAPD marker linked to fruit skin color in Japanese pear (Pyrus pyrifolia Nakai). Sci. Hortic. 2006, 107, 254–258. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Dai, M.S.; Zhang, S.J.; Shi, Z.B. Exploring Candidate genes for pericarp russet pigmentation of sand pear (Pyrus pyrifolia) via RNA-seq data in two genotypes contrasting for pericarp color. PLoS ONE 2014, 9, e83675. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Zhang, S.J.; Dai, M.S.; Shi, Z.B. Pigmentation in sand pear (Pyrus pyrifolia) fruit: Biochemical characterization, gene discovery and expression analysis with exocarp pigmentation mutant. Plant Mol. Biol. 2014, 85, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Sugar, D.; Basile, S.R. Color and russet variation among selections of ‘Bosc’ pear. J. Am. Pomol. Soc. 2008, 62, 77–81. [Google Scholar]
- Barcelo-Vidal, C.; Bonany, J.; Martin-Fernandez, J.A.; Carbo, J. Modelling of weather parameters to predict russet on ‘Golden Delicious’ apple. J. Hortic. Sci. Biotechnol. 2013, 88, 624–630. [Google Scholar] [CrossRef]
- Heidenreich, M.C.M.; CorralGarcia, M.R.; Momol, E.A.; Burr, T.J. Russet of apple fruit caused by Aureobasidium pullulans and Rhodotorula glutinis. Plant Dis. 1997, 81, 337–342. [Google Scholar] [CrossRef]
- Spotts, R.A.; Cervantes, L.A. Involvement of Aureobasidium pullulans and Rhodotorula glutinis in russet of d’Anjou pear fruit. Plant Dis. 2002, 86, 625–628. [Google Scholar] [CrossRef]
- Looney, N.E.; Granger, R.L.; Chu, C.L.; McArtney, S.J.; Mander, L.N.; Pharis, R.P. Influences of gibberellins A4, A4+7, and A4+Iso-A7 on apple fruit-quality and tree productivity. I. Effects on fruit russet and tree yield components. J. Hortic. Sci. 1992, 67, 613–618. [Google Scholar] [CrossRef]
- Sugar, D.; Powers, K.A.; Basile, S.R. Mancozeb and kaolin applications can reduce russet of ‘Comice’ pear. HortTechnology 2005, 15, 272–275. [Google Scholar]
- Barry, C.S.; Giovannoni, J.J. Ethylene and fruit ripening. J. Plant Growth Regul. 2007, 26, 143–159. [Google Scholar] [CrossRef]
- Sisler, E.C.; Serek, M. Inhibitors of ethylene responses in plants at the receptor level: Recent developments. Physiol. Plant 1997, 100, 577–582. [Google Scholar] [CrossRef]
- Blankenship, S.M.; Dole, J.M. 1-methylcyclopropene: A review. Postharvest Biol. Technol. 2003, 28, 1–25. [Google Scholar] [CrossRef]
- Watkins, C.B. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef] [PubMed]
- Isidoro, N.; Almeida, D.P.F. Alpha-farnesene, conjugated trienols, and superficial scald in ‘Rocha’ pear as affected by 1-methylcyclopropene and diphenylarnine. Postharvest Biol. Technol. 2003, 42, 49–56. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, J.; Wang, Y. Initiation of ripening capacity in 1-MCP treated green and red ‘Anjou’ pears and associated expression of genes related to ethylene biosynthesis and perception following cold storage and post-storage ethylene conditioning. Postharvest Biol. Technol. 2016, 111, 140–149. [Google Scholar] [CrossRef]
- Zhi, H.; Dong, Y. Effect of 1-methylcyclopropene on superficial scald associated with ethylene production, α-farnesene catabolism, and antioxidant system of over-mature ‘d’Anjou’ pears after long-term storage. Food Bioprocess Technol. 2018, 11, 1775–1786. [Google Scholar] [CrossRef]
- Bower, J.H.; Blasi, W.V.; Mitcham, E.J. Effect of ethylene in the storage environment on quality of ‘Bartlett pears’. Postharvest Biol. Technol. 2003, 28, 371–379. [Google Scholar] [CrossRef]
- Cheng, Y.D.; Guan, J.F. Involvement of pheophytinase in ethylene-mediated chlorophyll degradation in the peel of harvested ‘Yali’ pear. J. Plant Growth Regul. 2014, 33, 364–372. [Google Scholar] [CrossRef]
- Cheng, Y.D.; Dong, Y.; Yan, H.B.; Ge, W.Y.; Shen, C.G.; Guan, J.F.; Liu, L.Q.; Zhang, Y.Y. Effects of 1-MCP on chlorophyll degradation pathway-associated genes expression and chloroplast ultrastructure during the peel yellowing of Chinese pear fruits in storage. Food Chem. 2012, 135, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxydase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Kuang, J.F.; Chen, L.; Xie, H.; Peng, H.H.; Xiao, Y.Y.; Li, X.P.; Chen, W.X.; He, Q.G.; Chen, J.Y.; et al. Molecular characterization of banana NAC transcription factors and their interactions with ethylene signalling component EIL during fruit ripening. J. Exp. Bot. 2012, 63, 5171–5187. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.R.; Chen, K.S.; Allan, A.C.; Wu, R.M.; Zhang, B.; Lallu, N.; Ferguson, I.B. Ethylene-induced modulation of genes associated with the ethylene signalling pathway in ripening kiwifruit. J. Exp. Bot. 2008, 59, 2097–2108. [Google Scholar] [CrossRef] [PubMed]
- Gamrasni, D.; Ben-Arie, R.; Goldway, M. 1-Methylcyclopropene (1-MCP) application to Spadona pears at different stages of ripening to maximize fruit quality after storage. Postharvest Biol. Technol. 2010, 58, 104–112. [Google Scholar] [CrossRef]
- Villalobos-Acuna, M.G.; Biasi, W.V.; Flores, S.; Jiang, C.Z.; Reid, M.S.; Willits, N.H.; Mitcham, E.J. Effect of maturity and cold storage on ethylene biosynthesis and ripening in ‘Bartlett’ pears treated after harvest with 1-MCP. Postharvest Biol. Technol. 2011, 59, 1–9. [Google Scholar] [CrossRef]
- Pech, J.C.; Bouzayen, M.; Latché, A. Climacteric fruit ripening: Ethylene-dependent and independent regulation of ripening pathways in melon fruit. Plant Sci. 2008, 175, 114–120. [Google Scholar] [CrossRef]
- Guis, M.; Botondi, R.; Ben-Amor, M.; Ayub, R.; Bouzayen, M.; Pech, J.-C.; Latché, A. Ripening-associated biochemical traits of Cantaloupe Charentais melons expressing an antisense ACC oxidase transgene. J. Am. Soc. Hortic. Sci. 1997, 122, 748–751. [Google Scholar]
- Hiwasa, K.; Kinugasa, Y.; Amano, S.; Hashimoto, A.; Nakano, R.; Inaba, A.; Kubo, Y. Ethylene is required for both the initiation and progression of softening in pear (Pyrus communis L.) fruit. J. Exp. Bot. 2003, 54, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Grunenfelder, L.; Hiller, L.K.; Knowles, N.R. Color indices for the assessment of chlorophyll development and greening of fresh market potatoes. Postharvest Biol. Technol. 2006, 40, 73–81. [Google Scholar] [CrossRef]
- Porat, R.; Weiss, B.; Cohen, L.; Daus, A.; Goren, R.; Droby, S. Effects of ethylene and 1-methylcyclopropene on the postharvest qualities of ‘Shamouti’ oranges. Postharvest Biol. Technol. 1999, 15, 155–163. [Google Scholar] [CrossRef]
- Trebitsh, T.; Goldschmidt, E.E.; Riov, J. Ethylene induces de-novo synthesis of chlorophyllase, a chlorophyll degrading enzyme, in citrus-fruit peel. Proc. Natl. Acad. Sci. USA 1993, 90, 9441–9445. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Blankenship, S.M.; Mattheis, J. 1-Methylcyclopropene inhibits apple ripening. J. Am. Soc. Hortic. Sci. 1999, 124, 690–695. [Google Scholar]
- Watkins, C.B.; Nock, J.F.; Whitaker, B.D. Responses of early, mid and late season apple cultivars to postharvest application of 1-methylcyclopropene (1-MCP) under air and controlled atmosphere storage conditions. Postharvest Biol. Technol. 2000, 19, 17–23. [Google Scholar] [CrossRef]
- Mir, N.A.; Curell, E.; Khan, N.; Whitaker, M.; Beaudry, R.M. Harvest maturity, storage temperature, and 1-MCP application frequency alter firmness retention and chlorophyll fluorescence of ‘Redchief Delicious’ apples. J. Am. Soc. Hortic. Sci. 2001, 126, 618–624. [Google Scholar]
- Sakuraba, Y.; Schelbert, S.; Park, S.-Y.; Han, S.-H.; Lee, B.-D.; Andrès, C.B.; Kessler, F.; Hörtensteiner, S.; Paek, N.C. STAY-GREEN and chlorophyll catabolic enzymes interact at light-harvesting complex II for chlorophyll detoxification during leaf senescence in Arabidopsis. Plant Cell 2012, 24, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Barry, C.S.; McQuinn, R.P.; Chung, M.-Y.; Besuden, A.; Giovannoni, J.J. Amino Acid Substitutions in homologs of the STAY-GREEN protein are responsible for the green-flesh and chlorophyll retainer mutations of tomato and pepper. Plant Physiol. 2008, 147, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Borovsky, Y.; Paran, I. Chlorophyll breakdown during pepper fruit ripening in the chlorophyll retainer mutation is impaired at the homolog of the senescence-inducible stay-green gene. Theor. Appl. Genet. 2008, 117, 235–240. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charoenchongsuk, N.; Matsumoto, D.; Itai, A.; Murayama, H. Ripening Characteristics and Pigment Changes in Russeted Pear Fruit in Response to Ethylene and 1-MCP. Horticulturae 2018, 4, 22. https://doi.org/10.3390/horticulturae4030022
Charoenchongsuk N, Matsumoto D, Itai A, Murayama H. Ripening Characteristics and Pigment Changes in Russeted Pear Fruit in Response to Ethylene and 1-MCP. Horticulturae. 2018; 4(3):22. https://doi.org/10.3390/horticulturae4030022
Chicago/Turabian StyleCharoenchongsuk, Nongluk, Daiki Matsumoto, Akihiro Itai, and Hideki Murayama. 2018. "Ripening Characteristics and Pigment Changes in Russeted Pear Fruit in Response to Ethylene and 1-MCP" Horticulturae 4, no. 3: 22. https://doi.org/10.3390/horticulturae4030022
APA StyleCharoenchongsuk, N., Matsumoto, D., Itai, A., & Murayama, H. (2018). Ripening Characteristics and Pigment Changes in Russeted Pear Fruit in Response to Ethylene and 1-MCP. Horticulturae, 4(3), 22. https://doi.org/10.3390/horticulturae4030022



