Abstract
In recent years, the addition of microorganisms such as Plant Growth-Promoting Bacteria (PGPB) and mycorrhiza are becoming more popular, both in research as well as in practical use. While inoculants are usually not necessary for plants cultivated outdoors on biologically active soil, they can be useful on sterile substrates, newly created artificial landscapes, and also in soils that have been managed using non-selective sterilization methods, such as fumigation. In a multi-year lysimeter experiment, we investigated the influence of a commercial mycorrhizal inoculum on water use efficiency and biomass production of maize (Zea mays), sunflower (Helianthus annuus), sweet clover (Melilotus officinalis), sweet sorghum (Sorghum bicolor), cup-plant (Silphium perfoliatum) and tall wheatgrass (Elymus elongatus subsp. ponticus cv. Szarvasi-1) when exposed to high or low ground-water levels. Results showed that all plants benefited from the mycorrhizal association. Mycorrhizal-inoculated plants were more successful in terms of dry matter production and water use than the non-mycorrhizal plants. The source of the mycorrhiza—autochthonous or introduced—made no significant difference. The results indicate that inoculation with mycorrhiza and promotion of the naturally abundant mycorrhiza in agricultural production systems can significantly contribute to a sustainable production of crops. Effects depended on plant species, cultivar, soil type, ground-water level and the mycotrophy of the individual crop species.
1. Introduction
Since 1992, there are horticultural potting soils available that contain Bacillus subtilis and commercial endomycorrhiza inoculum (predominantly Funneliformis mosseae and Rhizophagus irregularis). Sustainable and resource conserving management of horticultural production systems includes efficient management of soil microorganisms such as mycorrhizas [1,2,3,4]. El Husseini et al. [5] and Chowdhury et al. [6] demonstrated the beneficial impact of organic fertilizer containing Bacillus subtilis (FZB24®) or Bacillus amyloliquefaciens (RhizoVital® 42), respectively on crop production systems. The addition of Enterobacter radicincitans and commercially produced mycorrhizal fungi also resulted in a promotion of plant growth [7,8].
Usually, a commercial endomycorrhiza inoculum is produced in greenhouses on expanded clay, with proliferation of mycorrhizal fungi on suitable host plants such as Zea mays cv. Badischer Landmais, a cultivar with a high level of mycotrophy and Tagetes spp. At the end of the production cycle, the expanded clay contains between 50 and 150 spores per mL of clay volume. Twenty kilogram (60 L) of the inoculated expanded clay is mixed with one ton of organic fertilizer. This fertilizer called “MYKO-AKTIV” (Cuxin DCM, Telgte, Germany) is used for the preparation of different horticultural potting soil. One cubic metre of horticultural potting soil and 4 to 6 kg of MYKO-AKTIV fertilizer are mixed. Currently there are about 60 different nursery substrates and horticultural potting soils marketed in Europe.
Arbuscular mycorrhiza (AM), the symbiotic association between soil fungi and plant roots, are known to protect host plants from the harmful effects of drought [7,9,10,11,12] and can improve the nutrient uptake and growth of plants under water stress conditions. Various experiments under controlled and field conditions have shown that mycorrhizal colonisation of roots increased drought tolerance of different crops such as maize [13,14] wheat [15], soybean [16], onion [17], lettuce [18,19,20,21], and red clover [22]. One of the mechanisms of the mycorrhizal symbiosis on host plant water balance is increased root biomass and, subsequently, plant size. In particular, the mobilization and uptake of phosphorus is often related to an increase in plant size [23].
This study focused on the influence of a commercial mycorrhizal inoculum on water use efficiency and biomass production of maize, sunflower, sweet clover, sweet sorghum, cup-plant (Silphium perfoliatum), and tall wheatgrass (Elymus elongatus subsp. ponticus) when exposed to high or low ground-water levels in lysimeter experiments.
2. Experimental Section
Site Characteristics and Experimental Setup
The experiment was carried out in 24 lysimeters at the Lysimeter Station Paulinenaue in northeast Germany with 515 mm precipitation/year (30 year average). The average precipitation during the crop season is 318 mm, and the average annual temperature is 8.9 °C.
Stainless steel lysimeter vessels were filled in 1968 with undisturbed hydromorphic mineral soil monoliths of low-level moors, half-bogs, humus gley, and sand gley, as well as loamy substrates. The vessels have a surface area of 1 m2 and are 1.5 m deep with fully adjustable ground water levels at 40, 70, 100, and 120 cm below the surface (Figure 1). The latter three groundwater levels simulate drought conditions.
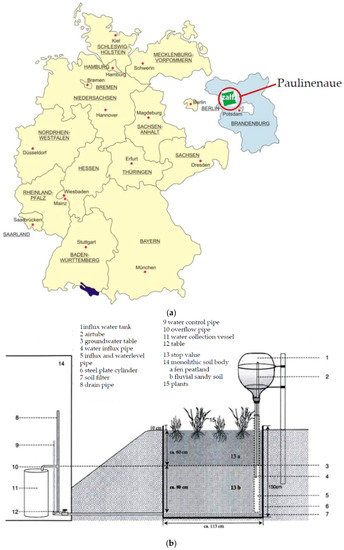
Figure 1.
Map of Germany with the ZALF experimental station in Paulinenaue in the state Brandenburg (a) and lysimeter design (b).
The maize cultivar Nolween (at 30 kg seeds/ha), sunflower cultivar Aloa (at 7 seeds/m²), sweet clover (at 22 kg/ha), grain sorghum cultivar Lussi (at 30 kg/ha), sudangrass cultivar Nutri Honey (at 30 kg/ha), cup-plant (at 5 plants/m2), and tall wheatgrass cultivar Szarvazi-1 (at 20 kg/ha) were planted at two different ground water levels (40 or 100 cm), and at more in some instances.
A commercial mycorrhizal product with two different species of mycorrhizal fungi (Rhizophagus interadices and Claroideoglomus etunicatum) was applied to each treatment according to the producer’s recommendation (75–100 g/m2) incorporated into the upper 20 cm of the soil. Lysimeters without mycorrhiza application served as control. The planted lysimeters are shown in Figure 2.

Figure 2.
Experimental setup of the crop lysimeter experiment (Photo: Marion Tauschke 2014).
Root colonisation was monitored by staining fresh roots with a lacto glycerol-trypan blue solution (0.05% w/v) to determine colonization progress [24]. The mean percentage of root colonization was counted by the gridline intersection method (Brundrett et al.) [25]. A total of 100 root segments were observed for each plant. In the 4 year experiment (2010–2014), 163 measurements were performed for all species, for maize 43. The “deeper water level” and the mycorrhizal inoculation were compared to control, with multifactorial ANOVA. The averages were determined by post hoc comparison using Fisher’s least significant difference test (p = 0.95).
3. Results and Discussion
3.1. Inoculation Success
Root staining revealed that inoculated as well as control (non-inoculated) plants were colonised with mycorrhizal fungi. The mycorrhizal colonisation of inoculated plants varied between 34% and 70% and was not significantly different from that of control plants over the entire monitoring period of four years (data not shown). Because of the colonisation potential of the naturally abundant mycorrhizal fungi population present in the lysimeters, the mycorrhizal colonisation of control plant roots was as high as 68%, e.g., in maize in 2011 and cup-plant in 2013.
The percentage of colonised root was not affected by the addition of the commercial inoculum (Figure 3). However, in maize the colonization of roots was significantly increased (r = 0.53; p < 0.05) after inoculation with the commercial inoculum in almost all treatments (Figure 4).
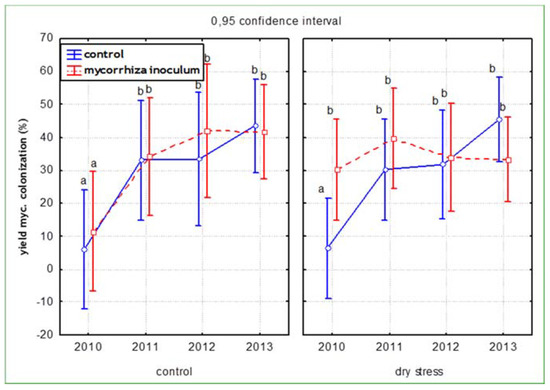
Figure 3.
Mycorrhizal colonisation of control and inoculated plants across all species.
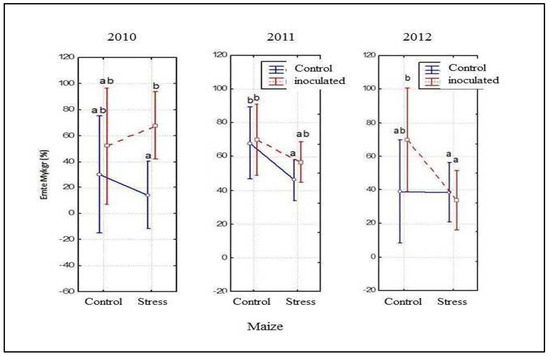
Figure 4.
Mycorrhizal colonisation of maize plants.
3.2. Dry Matter
Although for most plant species there was no additional colonisation after application of the commercial inoculum, the effect of colonisation by autochthonous populations and applied mycorrhiza on plant development could be seen. The degree of mycorrhizal colonisation of the different plant species was directly correlated (r = 0.71; p < 0.05) to dry matter production/m2. Plants with mycorrhizal root colonisation of 60% formed 30% more dry matter than plants with only 30% mycorrhizal colonisation (Figure 5). Moreover, the P and N content was significantly correlated (p < 0.05) with the mycorrhizal colonisation of the roots with r = 0.68, and r = 0.66, respectively.
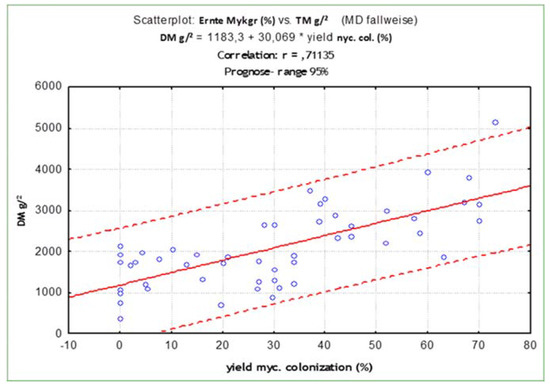
Figure 5.
Correlation of mycorrhizal colonisation with plant dry matter across all species and years.
3.3. Water Use Efficiency
Except for grain sorghum, the specific water use efficiency was improved for all plants with a high percentage of mycorrhizal colonisation (r = 0.46). Mycorrhizal plants required less water than non-colonised plants to produce 1 kg of dry matter. Plants with 60% root colonisation required about 25% less water to produce 30% more dry matter than plants with 30% colonisation (Figure 6), showing a higher water use efficiency. Regardless of the degree of mycotrophy in the analysed plant species this correlation was not affected.
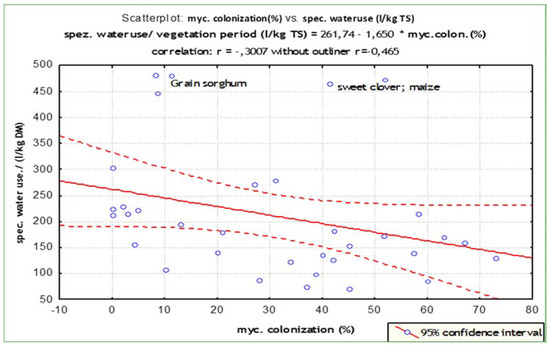
Figure 6.
Correlation of root mycorrhizal colonisation and water use efficiency.
4. Conclusions
In our experiments all plant species benefited from mycorrhizal association. Colonised plants of all species had greater dry matter production and less water use than non-mycorrhizal plants, independent of the fungal origin-applied or autochthonous. The different species varied in the degree of their reaction to the mycorrhiza. The effect was more pronounced the lower the ground water level. Results showed that mycorrhiza can not only improve the growth of high performance crops, but also promote their adaptation to different environmental conditions such as deep groundwater levels. The results also indicated that inoculation with mycorrhiza in biologically active soils is not necessary. However, they may be useful in sterile substrates, newly-created artificial landscapes, and also in soil that has been managed using non-selective sterilization methods, such as fumigation.
Acknowledgments
This work was supported by the Federal Ministry of Food and Agriculture of Germany (BMEL) and by the Royal Society of New Zealand.
Author Contributions
Frank Eulenstein is the producer of mycorrhiza inoculum, prepared the manuscript and was the Initiator of the project. Marion Tauschke designed the mycorrhiza experiments detected mycorrhiza in soil and roots and assisted in statistical analysis and preparation of the manuscript. Axel Behrendt implemented the lysimeter experiments, the soil- and plant-sampling and the groundwater analysis. Jana Monk stained the roots, evaluated the mycorrhizal colonization, counted the spores and provided assistance with the manuscript. Shaun Monk produced the mycorrhiza inoculum, advised the soil- plant sampling and the groundwater analysis. Marcos A. Lana calculated the water balances and assisted in the preparation of the manuscript. Uwe Schindler designed the lysimeter experiments and calculated the water balances.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jeffries, P.; Gianinazzi, S.; Perotto, S.; Turnau, K.; Barea, J.M. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fertil. Soils 2003, 37, 1–16. [Google Scholar]
- Bunemann, E.K.; Schwenke, G.D.; Van Zwieten, L. Impact of agricultural inputs on soil organism—A review. Aust. J. Soil Res. 2006, 44, 379–406. [Google Scholar] [CrossRef]
- Vosátka, M.; Albrechtová, J. Microbial strategies for crop improvement. In Benefits of Arbuscular Mycorrhizal Fungi to Sustainable Crop Production; Khan, M.S., Zaidi, A., Musarrat, J., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 205–225. [Google Scholar]
- Gianinazzi, S.; Gollotte, A.; Binet, M.N.; van Tuinen, D.; Redecker, D.; Wipf, D. Agroecology: The key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 2010, 20, 519–530. [Google Scholar] [CrossRef] [PubMed]
- El Husseini, M.M.; Bochow, H.; Junge, H. The biofertilising effect of seed dressing with PGPR Bacillus amyloliquefaciens FZB 42 combined with two levels of mineral fertilising in African cotton production. Arch. Phytopathol. Plant Prot. 2012, 45, 2261–2271. [Google Scholar] [CrossRef]
- Chowdhury, S.P.; Dietel, K.; Rändler, M.; Schmid, M.; Junge, H.; Borriss, R.; Hartmann, A.; Grosch, R. Effects of Bacillus amyloliquefaciens FZB42 on lettuce growth and health under pathogen pressure and its impact on the rhizosphere bacterial community. PLoS ONE 2013. [Google Scholar] [CrossRef] [PubMed]
- Brock, A.K.; Berger, B.; Mewis, I.; Ruppel, S. Impact of the PGPB Enterobacter radicincitans DSM 16656 on growth, glucosinolate profile, and immune responses of Arabidopsis thaliana. Microb. Ecol. 2013, 65, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Auge, R.M. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza 2003, 13, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Boomsma, C.R.; Vyn, T.J. Maize drought tolerance: Potential improvements through arbuscular mycorrhizal symbiosis? Field Crops Res. 2008, 108, 14–31. [Google Scholar] [CrossRef]
- Tauschke, M.; Behrendt, A.; Monk, J.; Lentzsch, P.; Eulenstein, F.; Monk, S. Improving the water use efficiency of crop plants by application of mycorrhizal fungi. In Moving Farm Systems to Improved Nutrient Attenuation; Currie, L., Burkitt, K.L., Eds.; Fertilizer and Lime Research Centre, Massey University: Palmerston North, New Zealand, 2015; pp. 1–8. [Google Scholar]
- Sylvia, D.E.; Hammond, L.C.; Bennet, J.M.; Hass, J.H.; Linda, S.B. Field response of maize to a VAM fungus and water management. Agron. J. 1993, 85, 193–198. [Google Scholar] [CrossRef]
- Subramanian, K.S.; Charest, C. Influence of arbuscular mycorrhizae on the metabolism of maize under drought stress. Mycorrhiza 1995, 5, 273–278. [Google Scholar] [CrossRef]
- Bryla, D.R.; Eissenstat, D.M. Respiratory costs of mycorrhizal associations. In Plant Respiration: From Cell to Ecosystem. Advances in Photosynthesis and Respiration; Lambers, H., Ribas-Carbo, M., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 207–224. [Google Scholar]
- Bethlenfalvay, G.J.; Schuepp, H. Arbuscular mycorrhizas and agroecosystem stability. In Impact of Arbuscular Mycorrhizas on Sustainable Agriculture and Natural Ecosystems; Gianinazzi, S., Schuepp, H., Eds.; Birkhäuser Verlag: Basel, Switzerland, 1994; pp. 117–131. [Google Scholar]
- Azcon, R.; Tobar, R.M. Activity of nitrate reductase and glutamine synthetase in shoot and root of mycorrhizal Allium cepa. Effects of drought stress. Plant Sci. 1998, 133, 1–8. [Google Scholar] [CrossRef]
- Tobar, R.M.; Azcon, R.; Barea, J.M. Improved nitrogen uptake and transport from 15N- labeled nitrate by external hyphae of arbuscular mycorrhizae under water-stressed conditions. New Phytol. 1994, 126, 119–122. [Google Scholar] [CrossRef]
- Tobar, R.M.; Azcon, R.; Barea, J.M. The improvement of plant N acquisition from an ammonium-treated, drought-stressed soil by the fungal symbiont in arbuscular mycorrhizae. Mycorrhiza 1994, 4, 105–108. [Google Scholar] [CrossRef]
- Azcon, R.; Gomez, M.; Tobar, R. Physiological and nutritional responses by Lactuca sativa L. to nitrogen sources and mycorrhizal fungi under drought conditions. Biol. Fertil. Soils 1996, 22, 156–161. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Azcon, R. Mycorrhizal colonization and drought stress exposition as factors affecting nitrate reductase activity in lettuce plants. Agric. Ecosyst. Environ. 1996, 60, 175–181. [Google Scholar] [CrossRef]
- Fitter, A.H. Water relations of red clover Trifolium pratense L. as affected by VA mycorrhizal infection and phosphorus supply before and during drought. J. Exp. Bot. 1988, 39, 595–604. [Google Scholar] [CrossRef]
- Subramanian, K.S.; Santhanakrishnan, P.; Balasubramanian, P. Responses of field grown tomato plants to arbuscular mycorrhizal fungal colonization under varying intensities of drought stress. Sci. Hortic. 2006, 107, 245–253. [Google Scholar] [CrossRef]
- Phillips, I.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970. [Google Scholar] [CrossRef]
- Brundrett, M.; Bougher, N.; Dell, B.; Grove, T.; Malajczuk, N. Working with Mycorrhizas in Forestry and Agriculture; Australian Centre for International Agricultural Research (ACIAR): Canberra, Australia, 1996; p. 374.
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).