Effects of Drying and Blanching on the Retention of Bioactive Compounds in Ginger and Turmeric
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ginger and Turmeric Samples
2.2. Sample Preparation
2.3. Drying Treatments
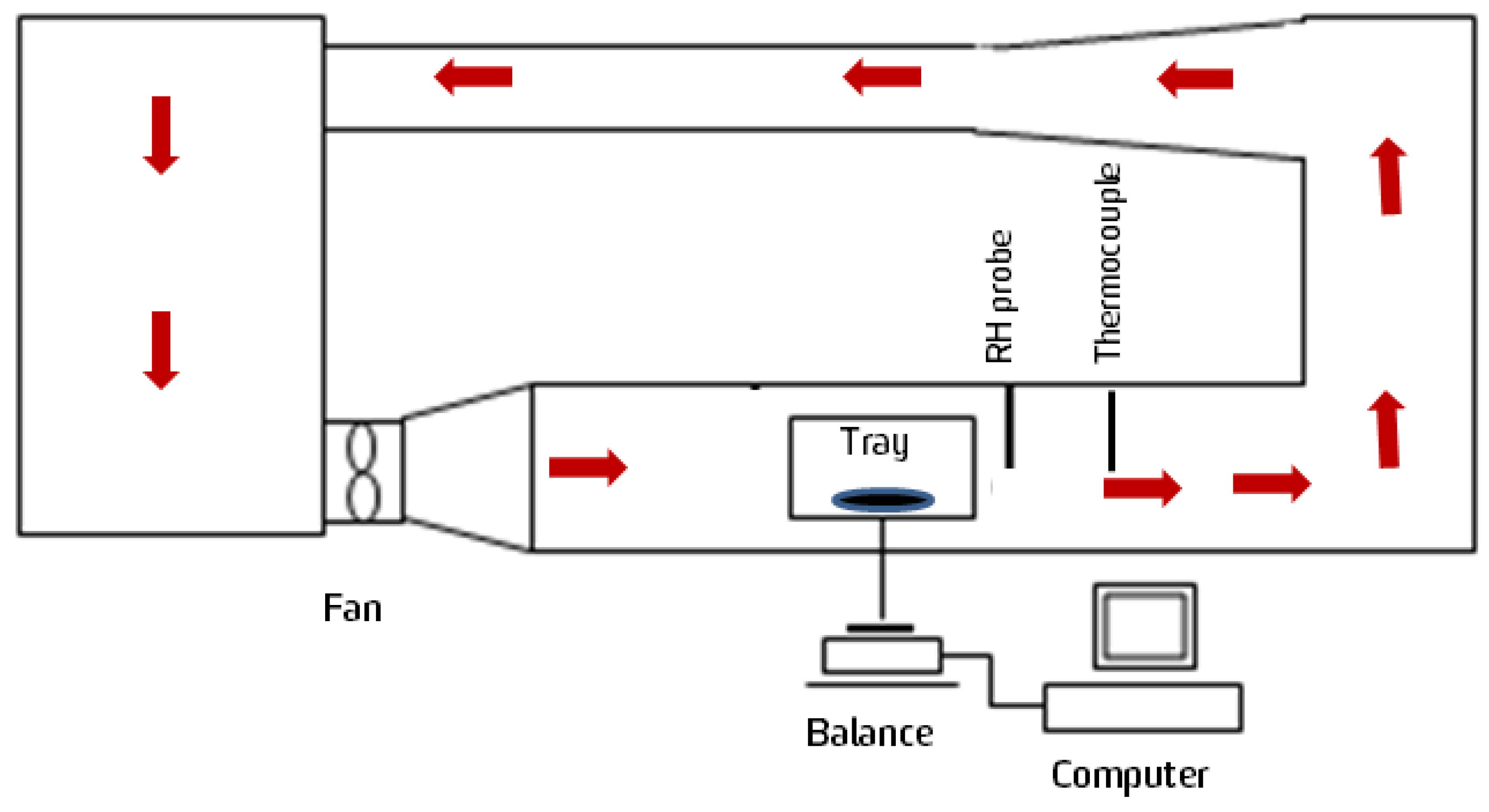
2.3.1. Dryer
2.3.2. Drying Conditions
Ginger
Turmeric
2.4. Determination of Bioactive Compounds
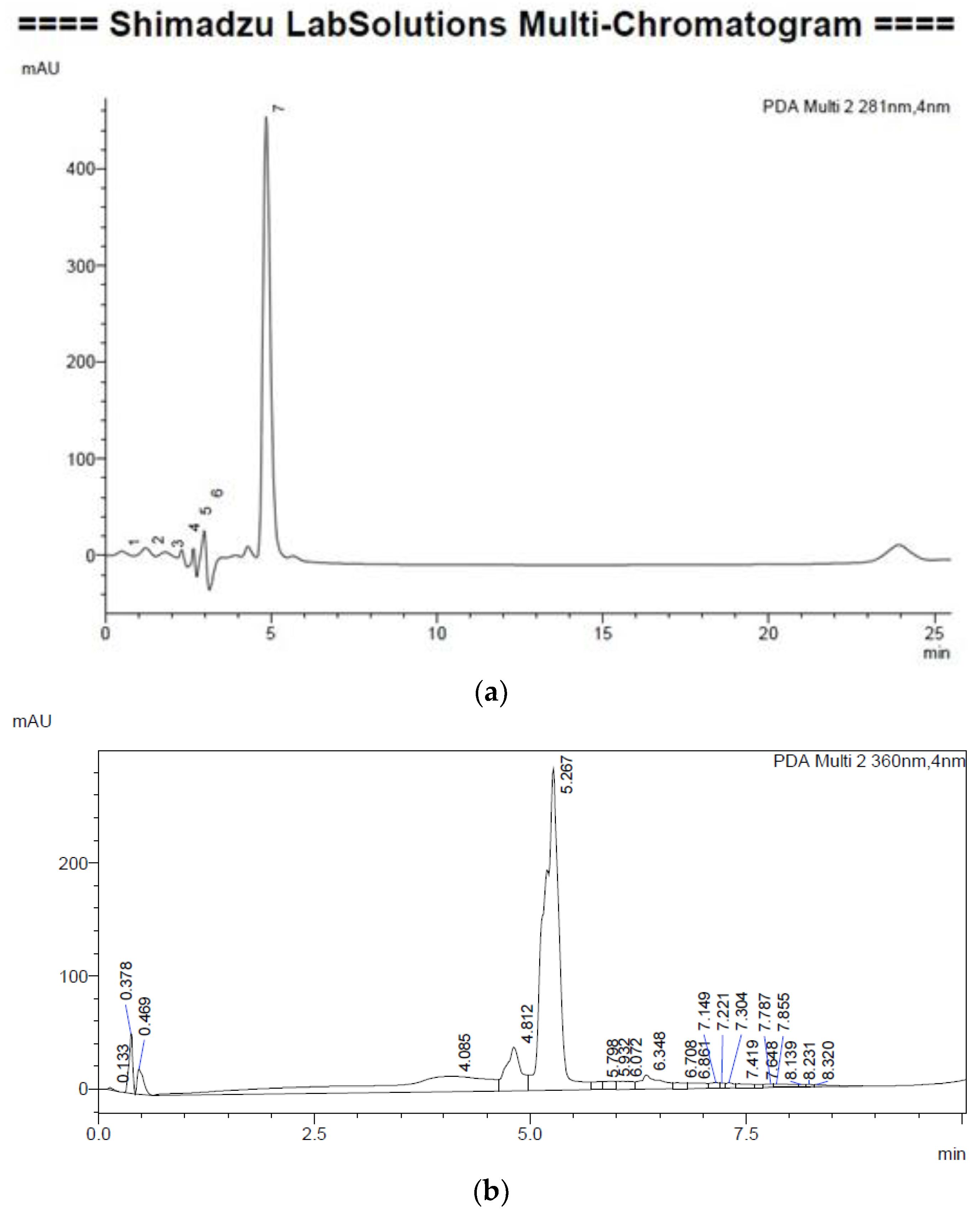
2.4.1. Gingerol
Extraction
Instrumentation and Chromatographic Conditions
2.4.2. Curcumin
Extraction
Instrumentation and Chromatographic Conditions
3. Results
3.1. Drying
3.1.1. Ginger
3.1.2. Turmeric
3.1.3. Retention of 6-Gingerol in Ginger
3.1.4. Retention of Curcumin in Turmeric
4. Conclusions
- Increasing drying temperature decreased 6-gingerol content in the dried product.
- Changing drying conditions significantly increased the concentration of 6-gingerol.
- Blanching treatment had a significant negative effect on 6-gingerol retention.
- Increasing drying temperature at low RH tended to increase curcumin retention.
- Blanching for 15 min favoured curcumin retention in the dried product.
Author Contributions
Conflicts of Interest
References
- Phoungchandang, S.; Saentaweesuk, S. Effect of two stage, tray and heat pump assisted-dehumidified drying on drying characteristics and qualities of dried ginger. Food Bioprod. Process. 2010, 89, 429–437. [Google Scholar] [CrossRef]
- Gan, H. Effects of Air Drying Treatments on the Concentration of the Key Bioactive Compound 6-Gingerol in Ginger. Master’s Thesis, School of Chemical Engineering, The University of New South Wales, Sydney, Australia, 15 December 2013. [Google Scholar]
- Stoilova, I.; Krastanov, A.; Stoyanova, A.; Denev, P.; Gargova, S. Antioxidant activity of a ginger extract (Zingiber officinale). Food Chem. 2007, 102, 764–770. [Google Scholar] [CrossRef]
- Sakulnarmrat, K.; Srzednicki, G.; Konczak, I. Antioxidant, enzyme inhibitory and antiproliferative activity of polyphenolic-rich fraction of commercial dry ginger powder. Int. J. Food Sci. Tech. 2015. [CrossRef]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative antioxidant and anti-inflammatory effects of 6-gingerol, 8-gingerol, 10-gingerol and 6-shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Charters, E. The Effects of Drying and Blanching on Curcumin Yields of Root Turmeric. Bachelor’s Thesis, School of Chemical Engineering, The University of New South Wales, Sydney, Australia, 31 October 2014. [Google Scholar]
- Parthasarathy, V.A.; Chempakam, B.; Zachariah, T.J. Chemistry of Spices; Centre for Agriculture and Bioscience International (CABI): Wallingford, CO, USA, 2008. [Google Scholar]
- Chan, E.; Lim, Y.Y.; Wong, S.K.; Lim, K.K.; Tan, S.P.; Lianto, F.S.; Yong, M.Y. Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem. 2009, 113, 166–172. [Google Scholar] [CrossRef]
- Chen, C.C.; Ho, C.T. Volatile compounds in ginger oil generated by thermal treatment. ACS Symp. Ser. 1989, 409, 366–375. [Google Scholar]
- Thuwapanichayanan, R.; Phowong, C.; Jaisut, D.; Štencl, J. Effects of pretreatments and drying temperatures on drying characteristics, antioxidant properties and color of ginger slice. Acta Univ. Agric. Silv. Mendel. Brun. 2014, 62, 1125–1134. [Google Scholar] [CrossRef]
- Chen, L.; Bai, G.; Yang, S.; Yang, R.; Zhao, G.; Xu, C.; Leung, W. Encapsulation of curcumin in recombinant human H-chain ferritin increases it water-solubility and stability. Food Res. Int. 2014, 62, 1147–1153. [Google Scholar] [CrossRef]
- Blasco, M.; Garcia-Perez, J.V.; Bon, J.; Carreres, J.E.; Mulet, A. Effect of blanching and air flow rate on turmeric drying. Food Sci. Technol. Int. 2006, 12, 315–323. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). AOAC Official Methods of Analysis of AOAC International. AOAC Official Method 934.06, Moisture in Dried Fruits; Association of Official Analytical Chemists (AOAC): Rockville, MD, USA, 1997. [Google Scholar]
| S/N | Strategy | Temperature (°C) | Relative Humidity (%) | Blanching (min) |
|---|---|---|---|---|
| 1 | Temperature control only | 40 | 9–12 | 0 |
| 2 | 40 | 9–12 | 5 | |
| 3 | 40 | 9–12 | 15 | |
| 4 | 40 | 9–12 | 30 | |
| 5 | 50 | 6–8 | 0 | |
| Temperature & RH Control | ||||
| 6 | Constant conditions | 30 | 30 | 0 |
| 7 | 40 | 10 | 0 | |
| 8 | 40 | 30 | 0 | |
| 9 | 50 | 25 | 0 | |
| 10 | 60 | 15 | 0 | |
| 11 | Changing conditions | 40 then 60 | 30 then 15 | 0 |
| 12 | 60 then 40 | 15 then 30 | 0 | |
| 13 | 40 then 30 | 10 then 30 | 0 | |
| 14 | 30 then 40 | 30 then 10 | 0 | |
| 15 | 50 then 60 | 25 then 15 | 0 | |
| 16 | 60 then 50 | 15 then 25 | 0 | |
| S/N | Temperature (°C) | Relative Humidity (%) | Blanching (min) |
|---|---|---|---|
| 1 | 40 | 20 | 0 |
| 2 | 40 | 40 | 0 |
| 3 | 50 | 20 | 0 |
| 4 | 50 | 20 | 5 |
| 5 | 50 | 20 | 15 |
| 6 | 50 | 20 | 30 |
| 7 | 60 | 20 | 0 |
| 8 | 60 | 40 | 0 |
| 9 | 70 | 20 | 0 |
| Step | Time (min) | A% | B% | Flow Rate (mL/min) |
|---|---|---|---|---|
| 0 | 0.00 | 99.5 | 0.5 | 6.0 |
| 1 | 0.34 | 99.5 | 0.5 | 6.0 |
| 2 | 1.40 | 45.0 | 55.0 | 6.0 |
| 3 | 4.50 | 40.0 | 60.0 | 6.0 |
| 4 | 4.80 | 0.5 | 99.5 | 7.5 |
| 5 | 6.60 | 0.5 | 99.5 | 7.5 |
| 6 | 7.00 | 99.5 | 0.5 | 7.5 |
| 7 | 10.00 | 99.5 | 0.5 | 7.5 |
| Treatment | Fresh MC (% db) | Dried MC (% db) |
|---|---|---|
| 40 °C/9–12% RH/No blanching | 904 b ± 3 | 11.9 f ± 0.2 |
| 40 °C/9–12% RH/5 min blanching | 922 a ± 5 | 10.7 g ± 0.3 |
| 40 °C/9–12% RH/15 min blanching | 523 i ± 2 | 9.7 g ± 0.2 |
| 40 °C/9–12% RH/30 min blanching | 519 i ± 1 | 10.3 g ± 0 .2 |
| 50 °C/6%–8% RH | 935 a ± 1 | 8.5 h ± 0.3 |
| 60 °C/15% RH | 536 h ± 4 | 7.5 i ± 0.2 |
| 50 °C/25% RH | 604 f ± 2 | 11.0 f ± 0.2 |
| 40 °C/30% RH | 924 a ± 3 | 18.4 c ± 0.2 |
| 40 °C/10% RH | 666 e ± 3 | 10.2 g ± 0.3 |
| 30 °C/30% RH | 587 f ± 5 | 21.6 a ± 0.2 |
| 60 °C/15% RH to 50 °C/25% RH | 761 d ± 1 | 13.8 e ± 0.2 |
| 50 °C/25% RH to 60 °C/15% RH | 865 c ± 1 | 17.8 d ± 0.2 |
| 60 °C/15% RH to 40 °C/30% RH | 596 f ± 4 | 20.2 b ± 0.2 |
| 40 °C/30% RH to 60 °C/15% RH | 566 g ± 4 | 20.5 b ± 0.2 |
| 40 °C/10% RH to 30 °C/30% RH | 634 e ± 3 | 11.8 c ± 0.2 |
| 30 °C/30% RH to 40 °C/10% RH | 671 b ± 3 | 19.4 f ± 0.2 |
| Treatment | Fresh MC (% db) | Dried MC (% db) |
|---|---|---|
| 40 °C/20% RH | 190 g ± 3 | 7.4 b ± 0.2 |
| 40 °C/40% RH | 335 e ± 3 | 10.5 a ± 0.3 |
| 50 °C/20% RH | 377 d ± 3 | 7.0 c ± 0.1 |
| 50 °C/20% RH/5 min blanching | 530 a ± 3 | 5.7 d ± 0.2 |
| 50 °C/20% RH/15 min blanching | 420 c ± 3 | 7.0 c ± 0.3 |
| 50 °C/20% RH/30 min blanching | 490 b ± 2 | 7.9 b ± 0.3 |
| 60 °C/20% RH | 274 f ± 3 | 5.2 e ± 0.2 |
| 60 °C/40% RH | 256 f ± 2 | 7.6 b ± 0.3 |
| 70 °C/ 20% RH | 325 e ± 4 | 6.1 d ± 0.1 |
| Treatment | Retention Area | 6-Gingerol Concentration (%) | Drying Time (min) |
|---|---|---|---|
| 40 °C/9%–12% RH | 3572383 | 1.273 e ± 0.063 | 343 d ± 3 |
| 40 °C/9%–12% RH/5 min blanching | 2289876 | 0.773 i ± 0.005 | 353 d ± 3 |
| 40 °C/9%–12% RH/15 min blanching | 1196832 | 0.348 j ± 0.012 | 353 d ± 3 |
| 40 °C/9%–12% RH/30 min blanching | 1059796 | 0.294 j ± 0.005 | 831 a ± 4 |
| 50 °C/6%–9% RH | 3037359 | 1.064 h ± 0.010 | 289 e ± 3 |
| 60 °C/15% RH | 2991478 | 1.047 h ± 0.015 | 177 h ± 3 |
| 50 °C/25% RH | 3306548 | 1.169 g ± 0.011 | 233 g ± 4 |
| 40 °C/30% RH | 3659751 | 1.307 d ± 0.067 | 297 e ± 3 |
| 40 °C/10% RH | 3702015 | 1.323 d ± 0.005 | 257 f ± 2 |
| 30 °C/30% RH | 3512811 | 1.250 f ± 0.050 | 489 b ± 3 |
| 60 °C/15% RH to 50 °C/25% RH | 4155793 | 1.500 b ± 0.036 | 185 h ± 4 |
| 50 °C/25% RH to 60 °C/15% RH | 4007405 | 1.442 c ± 0.050 | 203 h ± 3 |
| 60 °C/15% RH to 40 °C/30% RH | 3532484 | 1.257 e ± 0.005 | 193 h ± 3 |
| 40 °C/30% RH to 60 °C/15% RH | 3675330 | 1.313 d ± 0.054 | 257 f ± 2 |
| 30 °C/30% RH to 40 °C/10% RH | 4470837 | 1.623 a ± 0.025 | 425 c ± 2 |
| 40 °C/10% RH to 30 °C/30% RH | 4458393 | 1.618 a ± 0.025 | 305 e ± 3 |
| Treatment | Area under the Curve | Normalised Concentration (%) |
|---|---|---|
| 40 °C/20% RH | 3308511 | 73.7 |
| 40 °C/40% RH | 2646929 | 58.9 |
| 50 °C/20% RH | 3447014 | 76.8 |
| 50 °C/20% RH/5 min blanching | 3506873 | 78.1 |
| 50 °C/20% RH/15 min blanching | 4490862 | 100.0 |
| 50 °C/20% RH/30 min blanching | 3375932 | 75.2 |
| 60 °C/20% RH | 3691512 | 82.2 |
| 60 °C/40% RH | 2953344 | 65.8 |
| 70 °C/20% RH | 3327375 | 74.1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, H.; Charters, E.; Driscoll, R.; Srzednicki, G. Effects of Drying and Blanching on the Retention of Bioactive Compounds in Ginger and Turmeric. Horticulturae 2017, 3, 13. https://doi.org/10.3390/horticulturae3010013
Gan H, Charters E, Driscoll R, Srzednicki G. Effects of Drying and Blanching on the Retention of Bioactive Compounds in Ginger and Turmeric. Horticulturae. 2017; 3(1):13. https://doi.org/10.3390/horticulturae3010013
Chicago/Turabian StyleGan, Haozhe, Erin Charters, Robert Driscoll, and George Srzednicki. 2017. "Effects of Drying and Blanching on the Retention of Bioactive Compounds in Ginger and Turmeric" Horticulturae 3, no. 1: 13. https://doi.org/10.3390/horticulturae3010013
APA StyleGan, H., Charters, E., Driscoll, R., & Srzednicki, G. (2017). Effects of Drying and Blanching on the Retention of Bioactive Compounds in Ginger and Turmeric. Horticulturae, 3(1), 13. https://doi.org/10.3390/horticulturae3010013





