Interactive Effects of Genotype, Irrigation, and Fertilization on Physiological, Biometric, and Biochemical Traits of Runner Bean (Phaseolus coccineus L.)
Abstract
1. Introduction
2. Materials and Methods
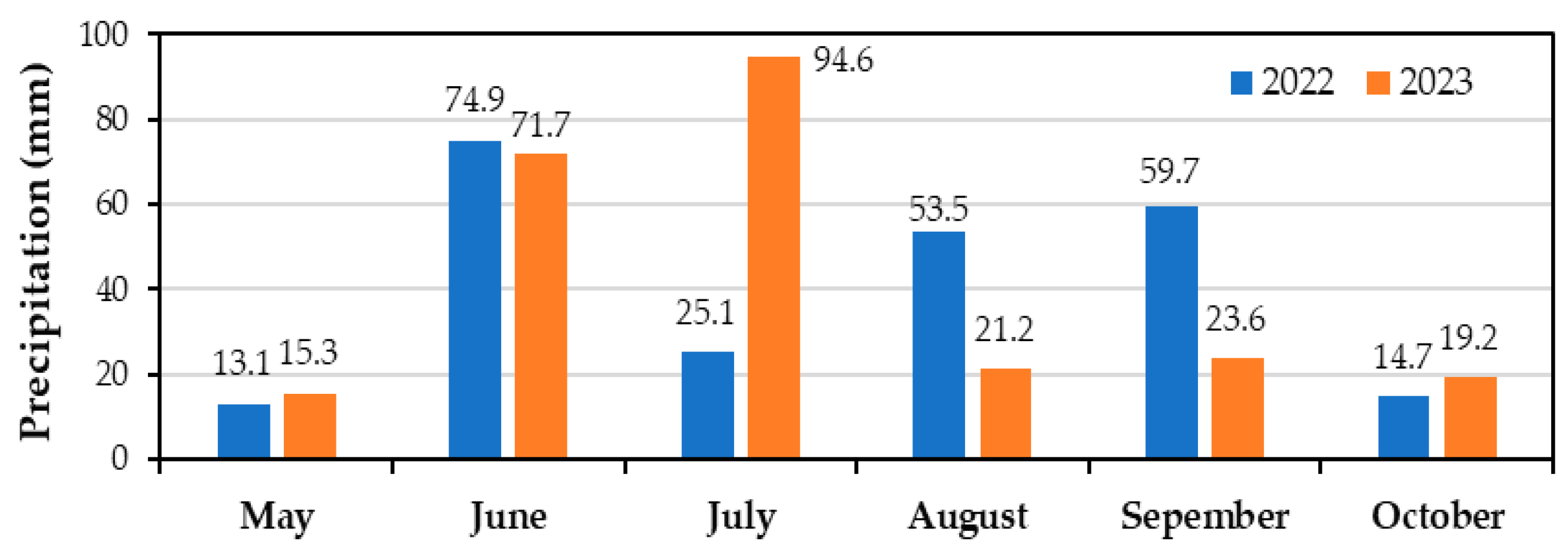
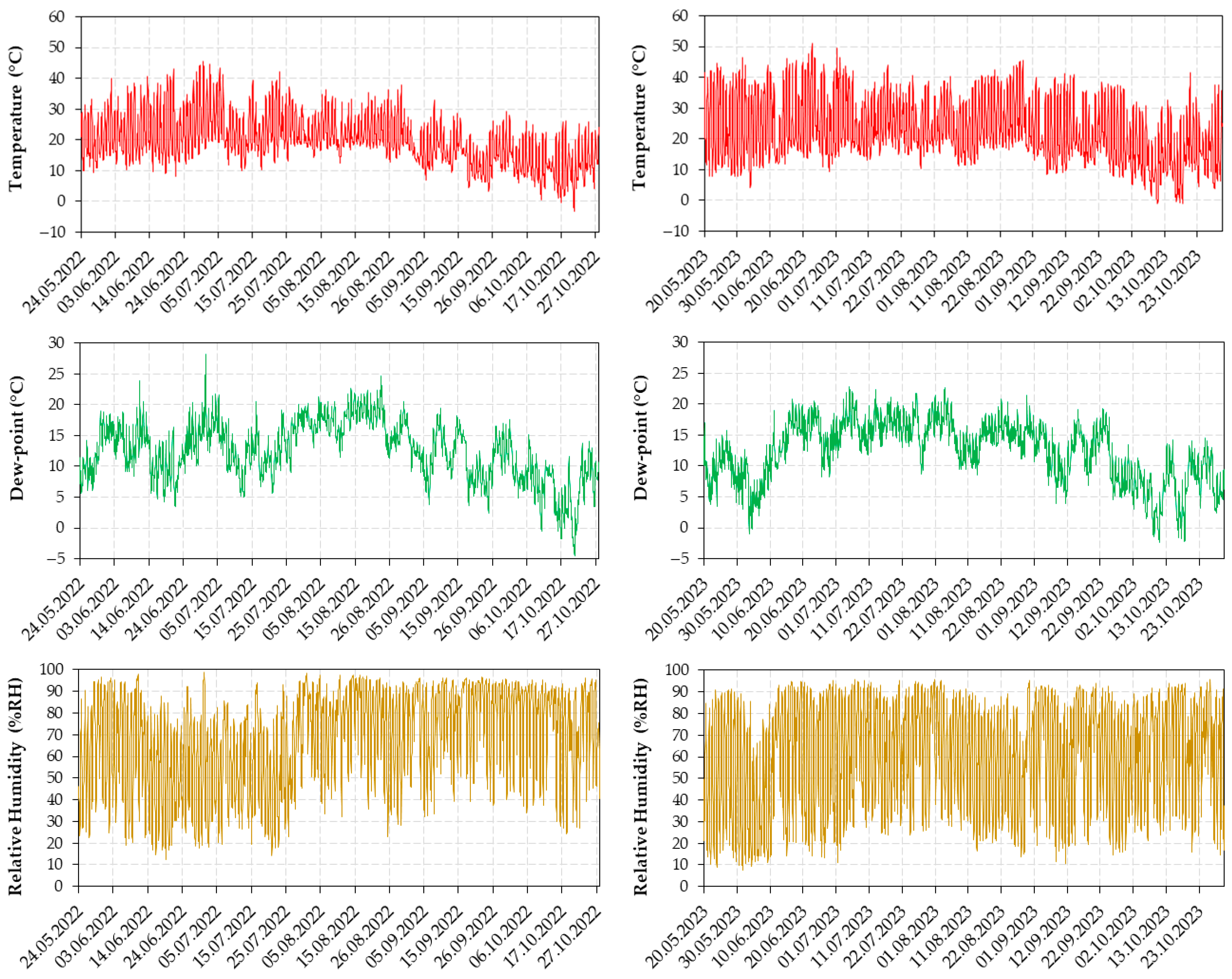
2.1. The Design and the Experimental Protocol
2.2. Methods of Analysis of the Analyzed Parameters
2.3. Statistical Analysis of Results
3. Results
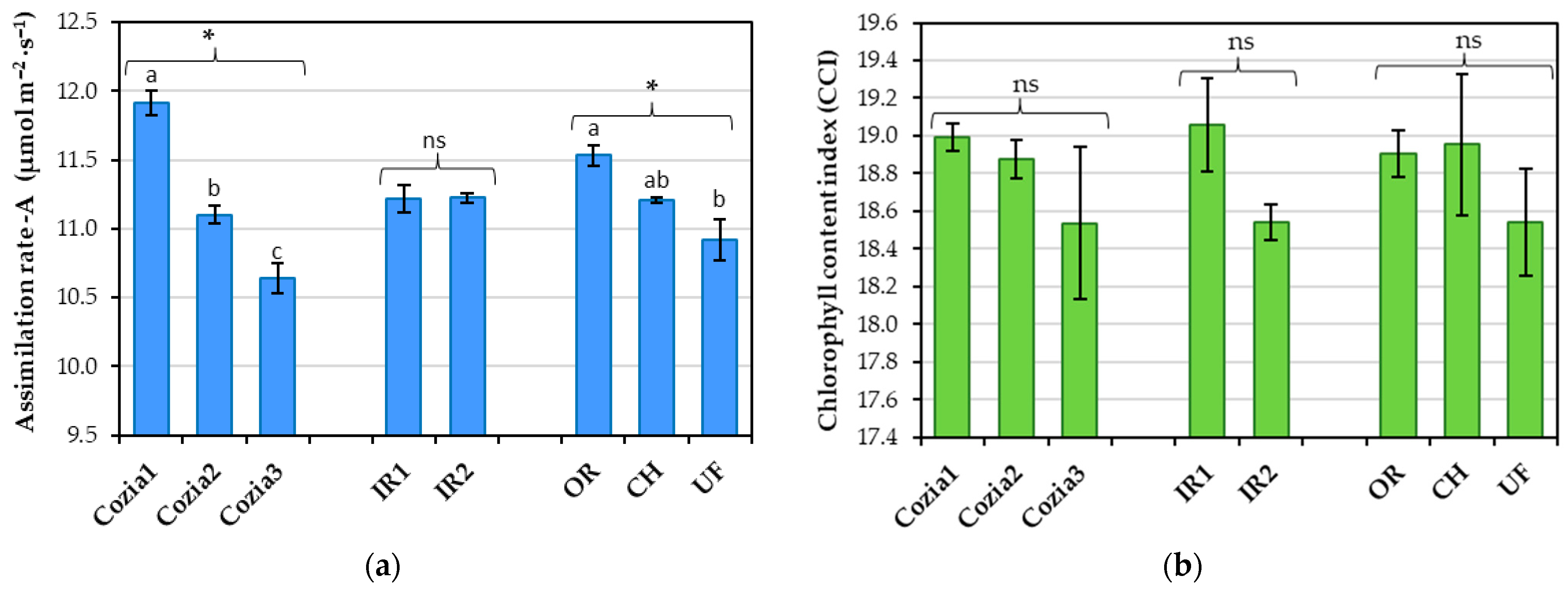
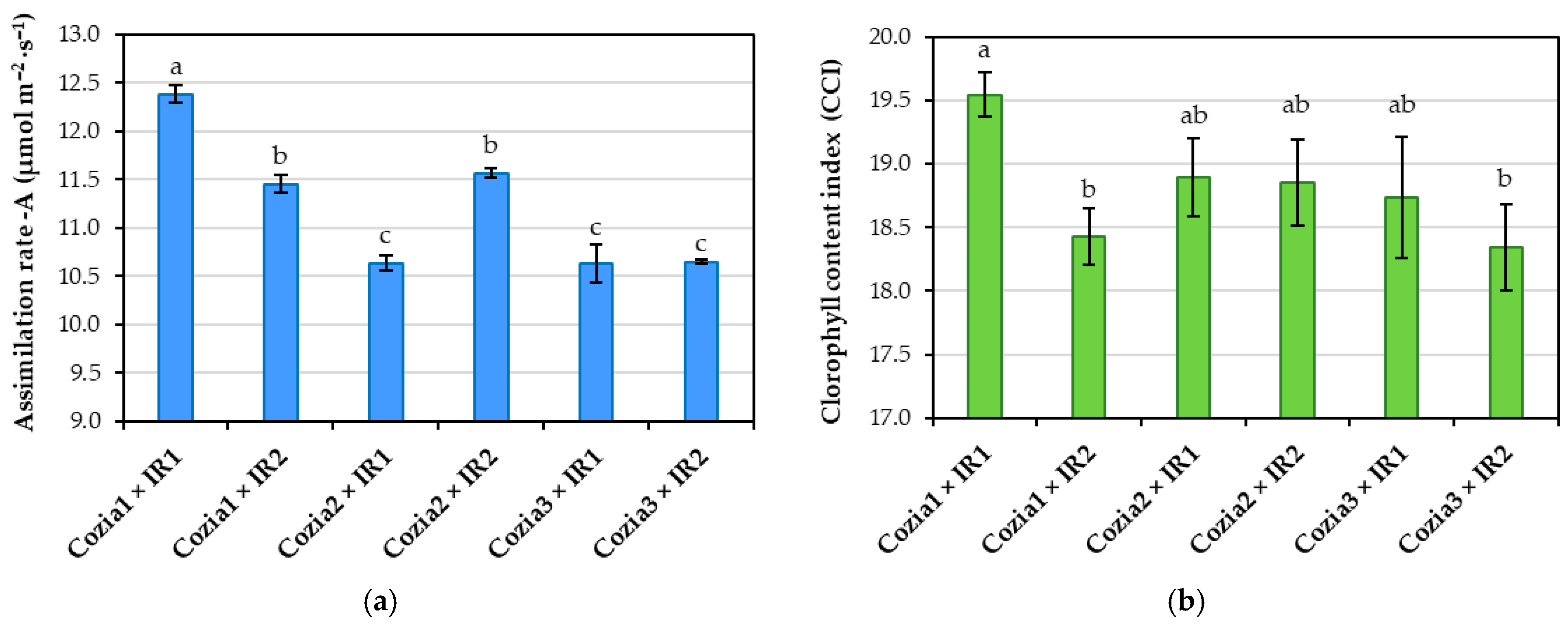
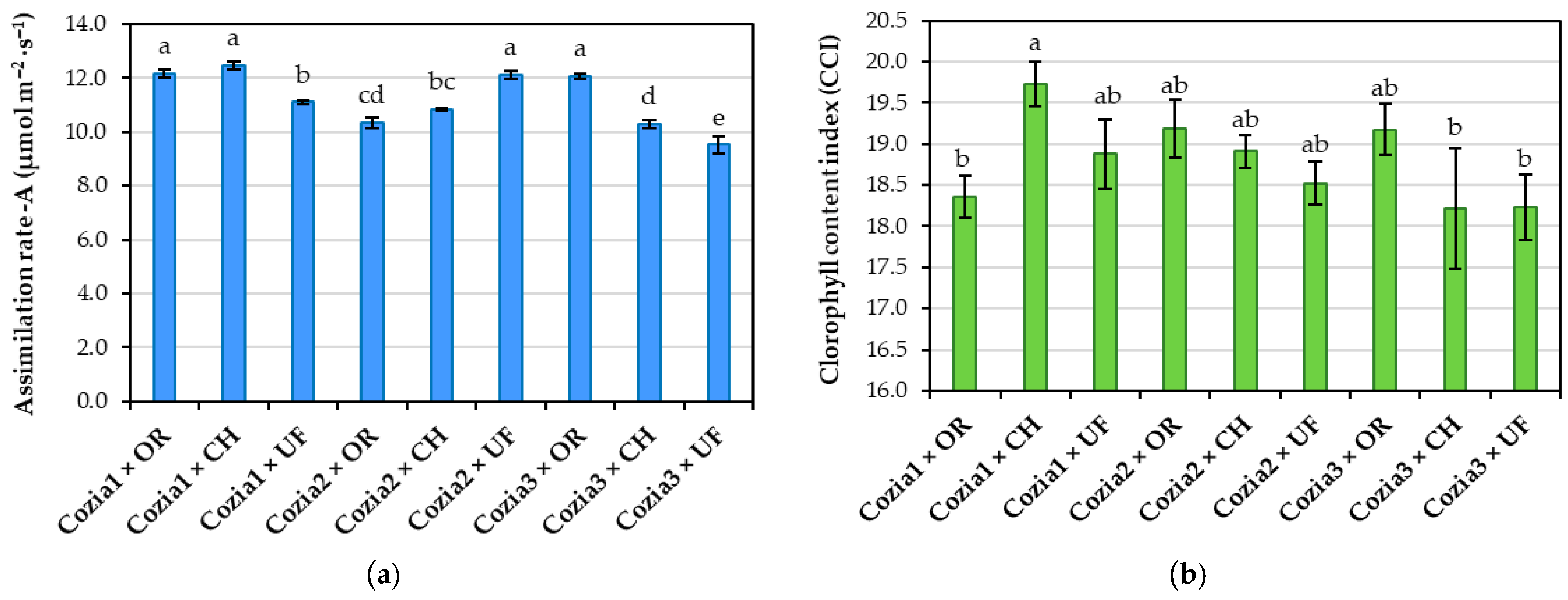
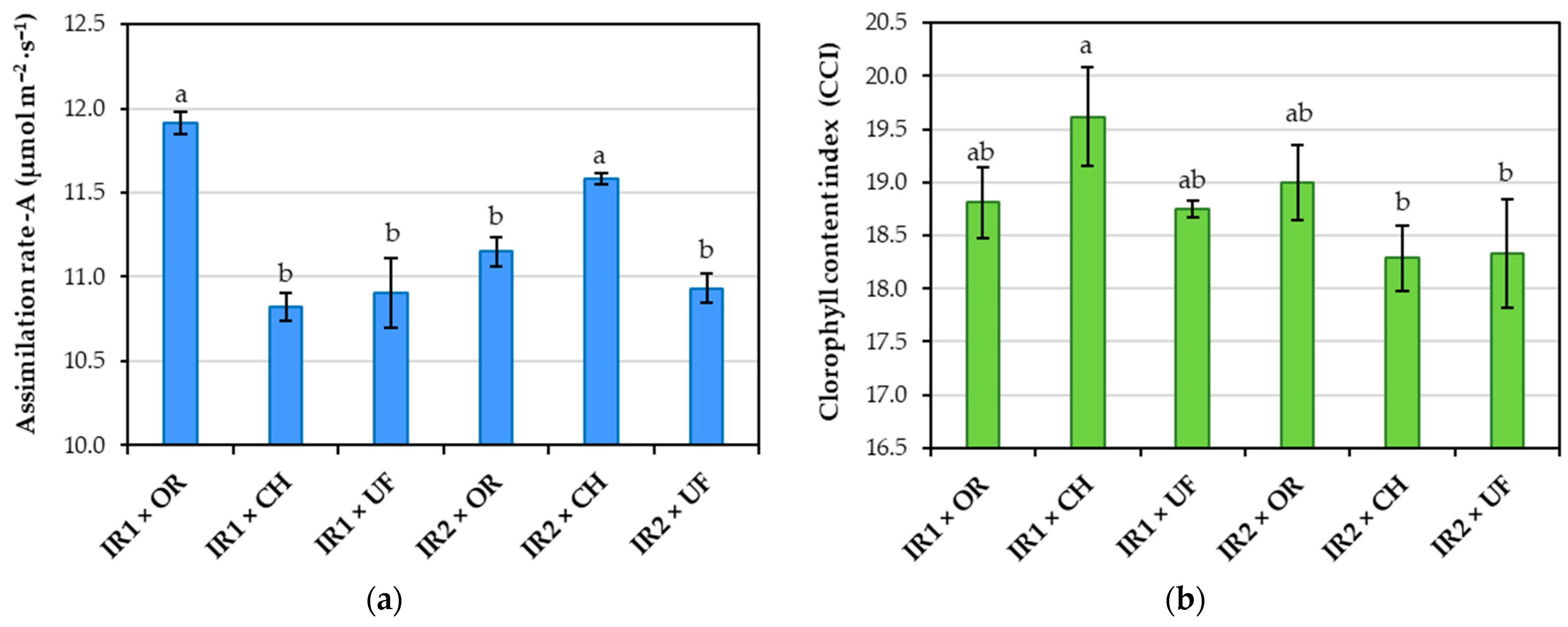
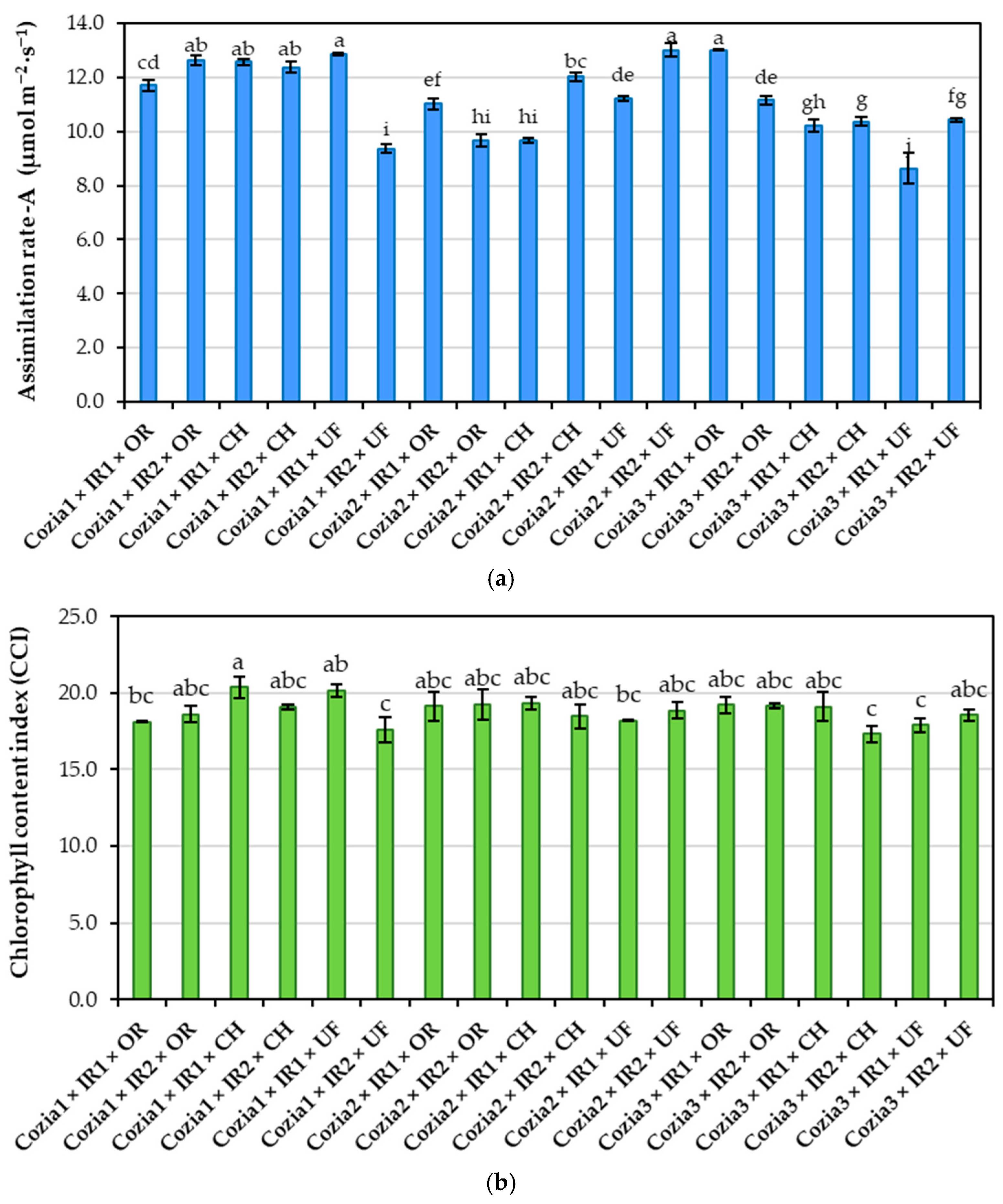
3.1. Influence of Experimental Factors on Physiological Indicators
3.2. Influence of Experimental Factors on Biometric Indicators
3.3. Influence of Experimental Factors on Biochemical Indicators
3.4. Multivariate Relationships Among Traits
4. Discussion
4.1. General Overview of Factor Effects
4.2. Effects of Experimental Factors on Physiological Indicators
4.3. Effects of Experimental Factors on Biometric Indicators
4.4. Effects of Experimental Factors on Biochemical Indicators
4.5. Multivariate Relationships Among Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sánchez-Chino, X.; Jiménez-Martínez, C.; Dávila-Ortiz, G.; Álvarez-González, I.; Madrigal-Bujaidar, E. Nutrient and nonnutrient components of legumes, and its chemopreventive activity: A review. Nutr. Cancer 2015, 67, 401–410. [Google Scholar] [CrossRef]
- Suárez-Martínez, S.E.; Ferriz-Martínez, R.A.; Campos-Vega, R.; Elton-Puente, J.E.; De La Torre Carbot, K.; García-Gasca, T. Bean seeds: Leading nutraceutical source for human health. CyTA—J. Food 2016, 14, 131–137. [Google Scholar] [CrossRef]
- Cardador-Martínez, A.; Castaño-Tostado, E.; Loarca-Piña, G. Antimutagenic activity of natural phenolic compounds present in the common bean (Phaseolus vulgaris) against aflatoxin B1. Food Addit. Contam. 2002, 19, 62–69. [Google Scholar] [CrossRef]
- Alvarado-López, A.N.; Gómez-Oliván, L.M.; Heredia, J.B.; Baeza-Jiménez, R.; Garcia-Galindo, H.S.; Lopez-Martinez, L.X. Nutritional and bioactive characteristics of ayocote bean (Phaseolus coccienus L.): An underutilized legume harvested in mexico. CyTA—J. Food 2019, 17, 199–206. [Google Scholar] [CrossRef]
- Díaz-Batalla, L.; Widholm, J.M.; Fahey, G.C.; Castaño-Tostado, E.; Paredes-López, O. Chemical components with health implications in wild and cultivated Mexican common bean seeds (Phaseolus vulgaris L.). J. Agric. Food Chem. 2006, 54, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- Oomah, B.D.; Corbé, A.; Balasubramanian, P. Antioxidant and anti-inflammatory activities of bean (Phaseolus vulgaris L.) hulls. J. Agric. Food Chem. 2010, 58, 8225–8230. [Google Scholar] [CrossRef] [PubMed]
- Calles, T. The International year of pulses: What are they and why are they important? Agric. Dev. 2016, 27, 40–42. [Google Scholar]
- Acosta-Diaz, E.; Hernandez-Torres, I.; Amador-Ramirez, M.D.; Padilla-Ramirez, J.S.; Zavala-García, F.; Baez-Gonzalez, A.D. Collection and characterization of wild species of Phaseolus (Fabaceae) in Northeastern Mexico for ex situ conservation. Plecevo 2015, 148, 119–127. [Google Scholar] [CrossRef]
- Giurcă, D.M. Morphological and phenological differences between the two species of the Phaseolus genus (Phaseolus vulgaris and Phaseolus coccineus). Cercet. Agron. Mold. 2008, 42, 39–45. [Google Scholar]
- Assunção Neto, W.V.D.; Medeiros, A.M.; Carvalho, L.C.B.; Ferreira, C.D.S.; Lopes, A.C.D.A.; Gomes, R.L.F. Selection of landraces of lima bean for family agriculture. Rev. Caatinga 2022, 35, 137–147. [Google Scholar] [CrossRef]
- Myers, J.R.; Formiga, A.K.; Janick, J. Iconography of beans and related legumes following the columbian exchange. Front. Plant Sci. 2022, 13, 851029. [Google Scholar] [CrossRef]
- Alcázar-Valle, M.; Lugo-Cervantes, E.; Mojica, L.; Morales-Hernández, N.; Reyes-Ramírez, H.; Enríquez-Vara, J.N.; García-Morales, S. Bioactive compounds, antioxidant activity, and antinutritional content of legumes: A comparison between four Phaseolus species. Molecules 2020, 25, 3528. [Google Scholar] [CrossRef]
- Sicard, D.; Nanni, L.; Porfiri, O.; Bulfon, D.; Papa, R. Genetic diversity of Phaseolus vulgaris L. and P. coccineus L. landraces in Central Italy. Plant Breed. 2005, 124, 464–472. [Google Scholar] [CrossRef]
- Romero-Astudillo, M.J.; Tapia, C.; Giménez De Azcárate, J.; Montalvo, D. Diversity of common bean (Phaseolus vulgaris L.) and runner bean (Phaseolus coccineus L.) landraces in rural communities in the Andes Highlands of Cotacachi—Ecuador. Agronomy 2024, 14, 1666. [Google Scholar] [CrossRef]
- Popa, L.-D. Research on Agrobiology of Phaseolus coccineus L., in Order to Optimize the Cultivation; University of Agricultural Sciences and Veterinary Medicine: Iasi, Romania, 2010. [Google Scholar]
- Rodiño, A.P.; Lema, M.; Pérez-Barbeito, M.; Santalla, M.; De Ron, A.M. Assessment of runner bean (Phaseolus coccineus L.) germplasm for tolerance to low temperature during early seedling growth. Euphytica 2007, 155, 63–70. [Google Scholar] [CrossRef]
- Labuda, H. Runner bean (Phaseolus coccineus L.)—Biology and use. Acta Sci. Pol. 2010, 9, 117–132. [Google Scholar]
- Vargas Vázquez, M.L.P.; Muruaga Martínez, J.S.; Lépiz Ildefonso, R.; Pérez Guerrero, A. La colección INIFAP de frijol ayocote (Phaseolus coccineus L.) I. Distribución geográfica de sitios de colecta. Remexca 2012, 3, 1247–1259. [Google Scholar] [CrossRef]
- Munteanu, N. Preliminary study on biodiversity of species Phaseolus coccineus L. Sci. Pap. USAMV Iaşi 2005, 83–92. [Google Scholar]
- Sinkovič, L.; Pipan, B.; Vasić, M.; Antić, M.; Todorović, V.; Ivanovska, S.; Brezeanu, C.; Šuštar-Vozlič, J.; Meglič, V. Morpho-agronomic characterisation of runner bean (Phaseolus coccineus L.) from South-Eastern Europe. Sustainability 2019, 11, 6165. [Google Scholar] [CrossRef]
- Schwember, A.R.; Carrasco, B.; Gepts, P. Unraveling agronomic and genetic aspects of runner bean (Phaseolus coccineus L.). Field Crops Res. 2017, 206, 86–94. [Google Scholar] [CrossRef]
- Rose, T.; Lowe, C.; Miret, J.A.; Walpole, H.; Halsey, K.; Venter, E.; Urban, M.O.; Buendia, H.F.; Kurup, S.; O’Sullivan, D.M.; et al. High temperature tolerance in a novel, high-quality Phaseolus vulgaris breeding line is due to maintenance of pollen viability and successful germination on the stigma. Plants 2023, 12, 2491. [Google Scholar] [CrossRef]
- Karavidas, I.; Ntatsi, G.; Vougeleka, V.; Karkanis, A.; Ntanasi, T.; Saitanis, C.; Agathokleous, E.; Ropokis, A.; Sabatino, L.; Tran, F.; et al. Agronomic practices to increase the yield and quality of common bean (Phaseolus vulgaris L.): A Systematic review. Agronomy 2022, 12, 271. [Google Scholar] [CrossRef]
- Alicandri, E.; Badiani, E.; Paolacci, A.R.; Lo Presti, E.; Caridi, R.; Rea, R.; Pati, F.; Badiani, M.; Ciaffi, M.; Sorgonà, A. Screening for drought tolerance within a common bean (Phaseolus vulgaris L.) landrace accessions core collection from the Lazio Region of Italy. Plants 2024, 13, 3132. [Google Scholar] [CrossRef] [PubMed]
- Field, C.B.; Barros, V.R.; Intergovernmental Panel on Climate Change (Eds.) Climate Change 2014: Impacts, Adaptation, and Vulnerability: Working Group II Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA, 2014; ISBN 978-1-107-64165-5. [Google Scholar]
- Morton, J.F. The impact of climate change on smallholder and subsistence agriculture. Proc. Natl. Acad. Sci. USA 2007, 104, 19680–19685. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.; Liu, G.; Liu, M.; Ji, Y.; He, H.; Gruda, N. Effect of irrigation on growth, yield, and chemical composition of two green bean cultivars. Horticulturae 2018, 4, 3. [Google Scholar] [CrossRef]
- El-Noemani, A.; El-Zeiny, H.; El-Gindy, A.-G.; El-Sahhar, E.; El-Shawadfy, M. Performance of some bean (Phaseolus vulgaris L.) varieties under different irrigation systems and regimes. Aust. J. Basic. Appl. Sci. 2010, 4, 6185–6196. [Google Scholar]
- Sezen, S.M.; Yazar, A.; Akyildiz, A.; Dasgan, H.Y.; Gencel, B. Yield and quality response of drip irrigated green beans under full and deficit irrigation. Sci. Hortic. 2008, 117, 95–102. [Google Scholar] [CrossRef]
- Pereira, L.S.; Cordery, I.; Iacovides, I. Improved indicators of water use performance and productivity for sustainable water conservation and saving. Agric. Water Manag. 2012, 108, 39–51. [Google Scholar] [CrossRef]
- Teliban, G.-C.; Stoleru, V.; Bireescu, G.; Mihalache, G.; Burducea, M.; Munteanu, N.; Țopa, D.; Matei, G.; Rădeanu, G.; Popa, L.-D.; et al. The response of runner bean crop to irrigation and fertilization. Rom. Agric. Res. 2022, 39, 269–281. [Google Scholar] [CrossRef]
- Kawaka, F.; Dida, M.; Opala, P.; Ombori, O.; Maingi, J.; Amoding, A.; Muoma, J. Effect of nitrogen sources on the yield of common bean (Phaseolus vulgaris) in Western Kenya. J. Plant Nutr. 2018, 41, 1652–1661. [Google Scholar] [CrossRef]
- Santosa, M.; Maghfoer, M.D.; Tarno, H. The Influence of organic and inorganic fertilizers on the growth and yield of green bean, Phaseolus vulgaris L. grown in dry and rainy season. Agrivita J. Agr. Sci. 2017, 39, 296–302. [Google Scholar] [CrossRef]
- Kontopoulou, C.-K.; Bilalis, D.; Pappa, V.A.; Rees, R.M.; Savvas, D. Effects of organic farming practices and salinity on yield and greenhouse gas emissions from a common bean crop. Sci. Hortic. 2015, 183, 48–57. [Google Scholar] [CrossRef]
- Sachan, H.K.; Krishna, D. Effect of organic and inorganic fertilization on growth and yield of french bean (Phaseolus vulgaris L.) in Fiji. Legume Res. 2020, 44, 1358–1361. [Google Scholar] [CrossRef]
- Uyanoz, R. The Effects of Different Bio-organic, chemical fertilizers and their combination on yield, macro and micro nutrition content of dry bean (Phaseolus vulgaris L.). Int. J. Agric. Res. 2007, 2, 115–125. [Google Scholar] [CrossRef]
- Karungi, J.; Ekbom, B.; Kyamanywa, S. Effects of Organic versus conventional fertilizers on insect pests, natural enemies and yield of Phaseolus vulgaris. Agric. Ecosyst. Environ. 2006, 115, 51–55. [Google Scholar] [CrossRef]
- Prabhakar, M.; Hebbar, S.S.; Nair, A.K. Growth and yield of french bean (Phaseolus vulgaris L.) under organic farming. J. Appl. Hortic. 2011, 13, 72–73. [Google Scholar] [CrossRef]
- Fernández-Luqueño, F.; Reyes-Varela, V.; Martínez-Suárez, C.; Salomón-Hernández, G.; Yáñez-Meneses, J.; Ceballos-Ramírez, J.M.; Dendooven, L. Effect of different nitrogen sources on plant characteristics and yield of common bean (Phaseolus vulgaris L.). Bioresour. Technol. 2010, 101, 396–403. [Google Scholar] [CrossRef]
- Amalfitano, C.; Gomez, L.D.; Frendo, P.; De Pascale, S.; Pepe, O.; Simister, R.; Ventorino, V.; Agrelli, D.; Borrelli, C.; McQueen-Mason, S.J.; et al. Plant-Rhizobium symbiosis, seed nutraceuticals, and waste quality for energy production of Vicia faba L. as affected by crop management. Chem. Biol. Technol. Agric. 2018, 5, 15. [Google Scholar] [CrossRef]
- Carbas, B.; Machado, N.; Oppolzer, D.; Ferreira, L.; Queiroz, M.; Brites, C.; Rosa, E.A.; Barros, A.I. Nutrients, antinutrients, phenolic composition, and antioxidant activity of common bean cultivars and their potential for food applications. Antioxidants 2020, 9, 186. [Google Scholar] [CrossRef]
- Yang, Q.-Q.; Farha, A.K.; Cheng, L.; Kim, G.; Zhang, T.; Corke, H. Phenolic content and in vitro antioxidant activity in common beans (Phaseolus vulgaris L.) Are not directly related to anti-proliferative activity. Food Biosci. 2020, 36, 100662. [Google Scholar] [CrossRef]
- Yaneva, T.G.; Wiczkowski, W.; Marchev, A.S.; Iserliyska, D.; Georgiev, M.I.; Tomlekova, N.B. Evaluation of polyphenols synthesized in mature seeds of common bean (Phaseolus vulgaris L.) advanced mutant lines. Int. J. Mol. Sci. 2024, 25, 5638. [Google Scholar] [CrossRef]
- García-Díaz, Y.D.; Aquino-Bolaños, E.N.; Chávez-Servia, J.L.; Vera-Guzmán, A.M.; Carrillo-Rodríguez, J.C.; García-Díaz, Y.D.; Aquino-Bolaños, E.N.; Chávez-Servia, J.L.; Vera-Guzmán, A.M.; Carrillo-Rodríguez, J.C. Bioactive compounds and antioxidant activity in the common bean are influenced by cropping season and genotype. Chil. J. Agric. Res. 2018, 78, 255–265. [Google Scholar] [CrossRef]
- Kivu, B.E.; Ion, V.A.; Barcanu, E.; Agapie, O.L.; Gherase, I.; Dobre, G.; Moț, A.; Asănică, A. Comparative study of the chemical composition of some bean accessions. Acta Hortic. 2024, 1391, 247–252. [Google Scholar] [CrossRef]
- Nadezkin, S.M.; Ushakov, V.A.; Pronina, E.P.; Antoshkin, A.A.; Molchanova, A.V. The Effectiveness of applying mineral fertilizers in the cultivation of green bean for seeds. IOP Conf. Ser. Earth Environ. Sci. 2021, 650, 012070. [Google Scholar] [CrossRef]
- Bomers, S.; Sehr, E.M.; Adam, E.; Von Gehren, P.; Hansel-Hohl, K.; Prat, N.; Ribarits, A. Towards heat tolerant runner bean (Phaseolus coccineus L.) by utilizing plant genetic resources. Agronomy 2022, 12, 612. [Google Scholar] [CrossRef]
- Blair, M.W.; González, L.F.; Kimani, P.M.; Butare, L. Genetic diversity, inter-gene pool introgression and nutritional quality of common beans (Phaseolus vulgaris L.) from Central Africa. Theor. Appl. Genet. 2010, 121, 237–248. [Google Scholar] [CrossRef]
- Brandão, D.; de Freitas, P.S.L.; Rezende, R.; Dallacort, R.; Garcia, M.M. Effects of water stress on photosynthesis and evapotranspiration in common bean plants. J. Food Agric. Environ. 2013, 11, 383–388. [Google Scholar]
- Elhawary, S.M.A.; Ordóñez-Díaz, J.L.; Nicolaie, F.; Montenegro, J.C.; Teliban, G.-C.; Cojocaru, A.; Moreno-Rojas, J.M.; Stoleru, V. Quality responses of sweet pepper varieties under irrigation and fertilization regimes. Horticulturae 2025, 11, 128. [Google Scholar] [CrossRef]
- Meteostat.net. Iaşi|Weather History & Climate. Available online: https://meteostat.net/en/place/ro/iasi (accessed on 28 July 2025).
- Stoleru, V.; Inculet, S.-C.; Mihalache, G.; Cojocaru, A.; Teliban, G.-C.; Caruso, G. Yield and nutritional response of greenhouse grown tomato cultivars to sustainable fertilization and irrigation management. Plants 2020, 9, 1053. [Google Scholar] [CrossRef]
- Hamburda, S.B.; Munteanu, N. The Technology of Runner Beans (Phaseolus coccineus) Cultivation; Performantica: Iasi, Romania, 2016. (In Romanian) [Google Scholar]
- Popa, D.L.; Teliban, G.C.; Stoleru, V.; Vlăduţ, N.V. Agrobiological Principles of Runner Bean (Phaseolus coccineus L.) Cultivation; Ion Ionescu de la Brad: Iasi, Romania, 2020. (In Romanian) [Google Scholar]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Cuevas, F.J.; Pradas, I.; Ruiz-Moreno, M.J.; Arroyo, F.T.; Perez-Romero, L.F.; Montenegro, J.C.; Moreno-Rojas, J.M. Effect of organic and conventional management on bio-functional quality of thirteen plum cultivars (Prunus salicina Lindl.). PLoS ONE 2015, 10, e0136596. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Castaño, M.; Mejía Díaz, D.P.; Contreras-Calderón, J.; Gallardo Cabrera, C. Physicochemical properties of bean pod (Phaseolus vulgaris) flour and its potential as a raw material for the food industry. Rev. Fac. Nac. Agron. Medellín 2020, 73, 9179–9187. [Google Scholar] [CrossRef]
- Álvarez-Iglesias, L.; Vales, M.I.; De Ron, A.M.; Rodiño, A.P.; Tejada-Hinojoza, J.L.; Taboada, A.; Revilla, P. Variability of photosynthetic and related traits in maize and other summer crops in a temperate humid area. Plant Physiol. Rep. 2022, 27, 596–602. [Google Scholar] [CrossRef]
- Hou, Y.; Guo, S. Comparative analysis of photosynthetic traits, yield, and fruit quality among different chestnut cultivars. Agronomy 2024, 14, 2635. [Google Scholar] [CrossRef]
- Gong, X.; Liu, C.; Dang, K.; Wang, H.; Du, W.; Qi, H.; Jiang, Y.; Feng, B. Mung bean (Vigna radiata L.) source leaf adaptation to shading stress affects not only photosynthetic physiology metabolism but also control of key gene expression. Front. Plant Sci. 2022, 13, 753264. [Google Scholar] [CrossRef] [PubMed]
- Ukwu, U.N.; Muller, O.; Meier-Grüll, M.; Uguru, M.I. Agrivoltaics shading enhanced the microclimate, photosynthesis, growth and yields of Vigna radiata genotypes in tropical Nigeria. Sci. Rep. 2025, 15, 1190. [Google Scholar] [CrossRef]
- Stefan, M.; Munteanu, N.; Stoleru, V.; Marius, M. Effects of inoculation with plant growth promoting rhizobacteria on photosynthesis, antioxidant status and yield of runner bean. Rom. Biotechnol. Lett. 2013, 18, 8132–8143. [Google Scholar]
- Tantray, A.Y.; Hazzazi, Y.; Ahmad, A. Physiological, agronomical, and proteomic studies reveal crucial players in rice nitrogen use efficiency under low nitrogen supply. Int. J. Mol. Sci. 2022, 23, 6410. [Google Scholar] [CrossRef]
- Jawad Moharam Al-Bayati, H.; Ibraheem, F.F.; Allela, W.; Al-Taey, D. Role of organic and chemical fertilizer on growth and yield of two cultivars of pea (Pisum sativum L.). Plant Arch. 2019, 19, 2581–6063. [Google Scholar]
- Teliban, G.-C.; Burducea, M.; Mihalache, G.; Zheljazkov, V.D.; Dincheva, I.; Badjakov, I.; Popa, L.-D.; Bodale, I.; Vlăduț, N.-V.; Cojocaru, A.; et al. Morphological, physiological and quality performances of basil cultivars under different fertilization types. Agronomy 2022, 12, 3219. [Google Scholar] [CrossRef]
- Nasar, J.; Wang, G.-Y.; Ahmad, S.; Muhammad, I.; Zeeshan, M.; Gitari, H.; Adnan, M.; Fahad, S.; Khalid, M.H.B.; Zhou, X.-B.; et al. Nitrogen fertilization coupled with iron foliar application improves the photosynthetic characteristics, photosynthetic nitrogen use efficiency, and the related enzymes of maize crops under different planting patterns. Front. Plant Sci. 2022, 13, 988055. [Google Scholar] [CrossRef]
- Liu, Y.; Lan, X.; Hou, H.; Ji, J.; Liu, X.; Lv, Z. Multifaceted ability of organic fertilizers to improve crop productivity and abiotic stress tolerance: Review and perspectives. Agronomy 2024, 14, 1141. [Google Scholar] [CrossRef]
- Khan, M.T.; Aleinikovienė, J.; Butkevičienė, L.-M. Innovative organic fertilizers and cover crops: Perspectives for sustainable agriculture in the era of climate change and organic agriculture. Agronomy 2024, 14, 2871. [Google Scholar] [CrossRef]
- Precupeanu, C.; Rădeanu, G.; Teliban, G.-C.; Roșca, M.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Stoleru, V. Effects of mulch and fertilization on the quantity and quality of perennial wall–rocket (Diplotaxis tenuifolia). Plants 2025, 14, 1421. [Google Scholar] [CrossRef] [PubMed]
- Asargew, M.F.; Masutomi, Y.; Kobayashi, K.; Aono, M. Water stress changes the relationship between photosynthesis and stomatal conductance. Sci. Total Environ. 2024, 907, 167886. [Google Scholar] [CrossRef] [PubMed]
- Khonghintaisong, J.; Onkaeo, A.; Songsri, P.; Jongrungklang, N. Water use efficiency characteristics and their contributions to yield in diverse sugarcane genotypes with varying drought resistance levels under different field irrigation conditions. Agriculture 2024, 14, 1952. [Google Scholar] [CrossRef]
- Papathanasiou, F.; Ninou, E.; Mylonas, I.; Baxevanos, D.; Papadopoulou, F.; Avdikos, I.; Sistanis, I.; Koskosidis, A.; Vlachostergios, D.N.; Stefanou, S.; et al. The evaluation of common bean (Phaseolus vulgaris L.) genotypes under water stress based on physiological and agronomic parameters. Plants 2022, 11, 2432. [Google Scholar] [CrossRef]
- Cheng, M.; Wang, H.; Zhang, F.; Wang, X.; Liao, Z.; Zhang, S.; Yang, Q.; Fan, J. Effects of irrigation and fertilization regimes on tuber yield, water-nutrient uptake and productivity of potato under drip fertigation in sandy regions of Northern China. Agric. Water Manag. 2023, 287, 108459. [Google Scholar] [CrossRef]
- Touckia, G.I.; Elian, H.D.B.; Aba-Toumnou, L.; Rekya, F.A.; Orolo, S.N.; Zimaga, D.B.; Yongo, O.D.; Kokou, K. Agro-morphological performances of common bean varieties (Phaseolus vulgaris L.) introduced in the Central African Republic. Int. J. Plant Soil. Sci. 2021, 33, 1–9. [Google Scholar] [CrossRef]
- Geleta, R.J.; Roro, A.G.; Terfa, M.T. Phenotypic and yield responses of common bean (Phaseolus vulgaris L.) varieties to different soil moisture levels. BMC Plant Biol. 2024, 24, 242. [Google Scholar] [CrossRef]
- Arriagada, O.; Schwember, A.R.; Greve, M.J.; Urban, M.O.; Cabeza, R.A.; Carrasco, B. Morphological and molecular characterization of selected Chilean runner bean (Phaseolus coccineus L.) genotypes shows moderate agronomic and genetic variability. Plants 2021, 10, 1688. [Google Scholar] [CrossRef]
- Petrova, R.; Matev, A. Defficit irrigation for green beans—Late field production. Sci. Pap. Ser. A Agron. 2020, LXIII, 475–483. [Google Scholar]
- Negash, T.W.; Tefera, A.T.; Bayisa, G.D.; Ashami, G.; Bizuneh, K.T.; Worku, T.; Gurms, A. Optimal irrigation water allocation for enhanced productivity of Haricot bean (Phaseolus Vulgaris) and economic gain: An experiment conducted in the semi-arid area of Ethiopia. J. Agric. Food Res. 2025, 19, 101668. [Google Scholar] [CrossRef]
- Polania, J.A.; Poschenrieder, C.; Beebe, S.; Rao, I.M. Effective use of water and increased dry matter partitioned to grain contribute to yield of common bean improved for drought resistance. Front. Plant Sci. 2016, 7, 660. [Google Scholar] [CrossRef]
- Nabati, J.; Nezami, A.; Yousefi, A.; Oskoueian, E.; Oskoueian, A.; Ahmadi-Lahijani, M.J. Biofertilizers containing plant growth promoting rhizobacteria enhance nutrient uptake and improve the growth and yield of chickpea plants in an arid environment. Sci. Rep. 2025, 15, 8331. [Google Scholar] [CrossRef] [PubMed]
- Khlila, I.; Baidani, A.; Hnizil, O.; Amamou, A. Integrated management of water, nitrogen, and genotype selection for enhanced wheat productivity in Moroccan arid and semi-arid regions. Agronomy 2025, 15, 612. [Google Scholar] [CrossRef]
- Dong, J.; Shen, X.; Zhang, X.; Chen, J.; Li, H.; Li, Q.; He, J.; Liu, H. Optimizing water-nitrogen management enhances productivity for peanut (Arachis hypogaea L.) with Drip-irrigated under mulched in northwest of China. Agric. Water Manag. 2025, 317, 109659. [Google Scholar] [CrossRef]
- Song, Y.; Xu, J.; Zhang, S.; Xing, J.; Wang, L.; Wang, X.; Hu, C.; Li, W.; Tan, Z.; Cheng, Y. Optimizing water–fertilizer coupling across different growth stages of tomato in yellow sand substrate: Toward enhanced yield, quality, and resource use efficiency. Plants 2025, 14, 936. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salazar, N.A.; Sánchez-Chávez, E.; Sánchez, D.E.; Chávez-Martínez, A.; Soto-Caballero, M.C.; Flores-Cordova, M.A. Influence of fertilization schemes on bioactive compounds, antioxidant activity and volatile profile in Pinto Centauro bean (Phaseolus vulgaris L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2025, 53, 14499. [Google Scholar] [CrossRef]
- Nina, N.; Theoduloz, C.; Tapia, G.; Jimenéz-Aspee, F.; Márquez, K.; Schmeda-Hirschmann, G. Changes in polyphenol composition, antioxidant capacity and enzyme inhibition in Phaseolus Vulgaris L. submitted to hydric stress. Sci. Hortic. 2023, 317, 112070. [Google Scholar] [CrossRef]
- Smith, E.N.; van Aalst, M.; Tosens, T.; Niinemets, Ü.; Stich, B.; Morosinotto, T.; Alboresi, A.; Erb, T.J.; Gómez-Coronado, P.A.; Tolleter, D.; et al. Improving photosynthetic efficiency toward food security: Strategies, advances, and perspectives. Mol. Plant 2023, 16, 1547–1563. [Google Scholar] [CrossRef]
- Wang, P.; Liu, W.-C.; Han, C.; Wang, S.; Bai, M.-Y.; Song, C.-P. Reactive oxygen species: Multidimensional regulators of plant adaptation to abiotic stress and development. J. Integr. Plant Biol. 2024, 66, 330–367. [Google Scholar] [CrossRef]
- Reshi, Z.A.; Ahmad, W.; Lukatkin, A.S.; Javed, S.B. From nature to lab: A review of secondary metabolite biosynthetic pathways, environmental influences, and in vitro approaches. Metabolites 2023, 13, 895. [Google Scholar] [CrossRef]
- Topali, C.; Antonopoulou, C.; Chatzissavvidis, C. Effect of waterlogging on growth and productivity of fruit crops. Horticulturae 2024, 10, 623. [Google Scholar] [CrossRef]
- Luo, H.; Liu, S.; Song, Y.; Qin, T.; Xiao, S.; Li, W.; Xu, L.; Zhou, X. Effects of waterlogging stress on root growth and soil nutrient loss of winter wheat at seedling stage. Agronomy 2024, 14, 1247. [Google Scholar] [CrossRef]
- Lin, C. Role of inorganic fertilizers in modern agriculture: Nourishing plants with minerals. Int. J. Manures Fertil. 2023, 11, 1–2. [Google Scholar]
- Liu, J.; Wang, D.; Yan, X.; Jia, L.; Chen, N.; Liu, J.; Zhao, P.; Zhou, L.; Cao, Q. Effect of nitrogen, phosphorus and potassium fertilization management on soil properties and leaf traits and yield of Sapindus mukorossi. Front. Plant Sci. 2024, 15, 1300683. [Google Scholar] [CrossRef] [PubMed]
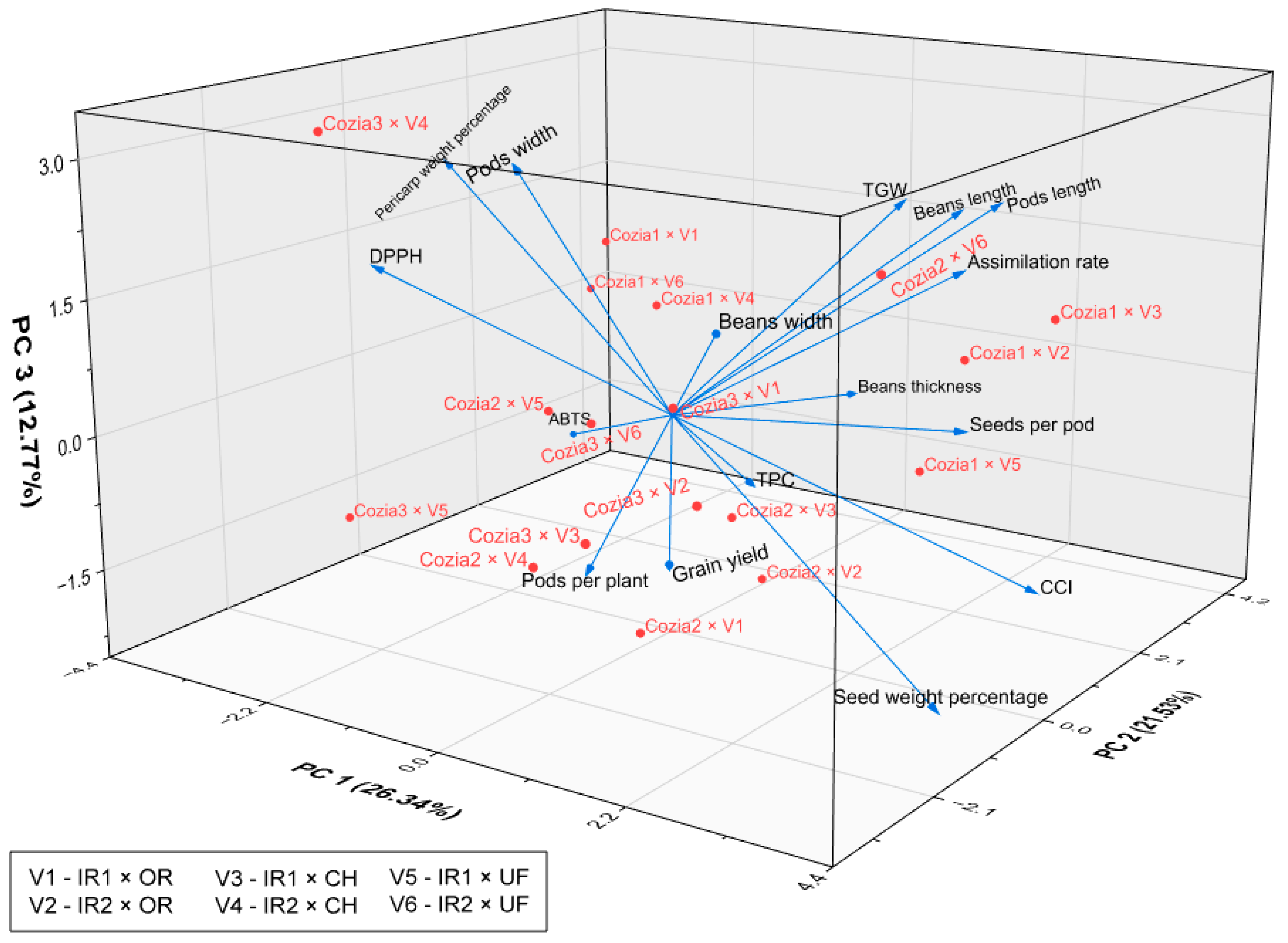
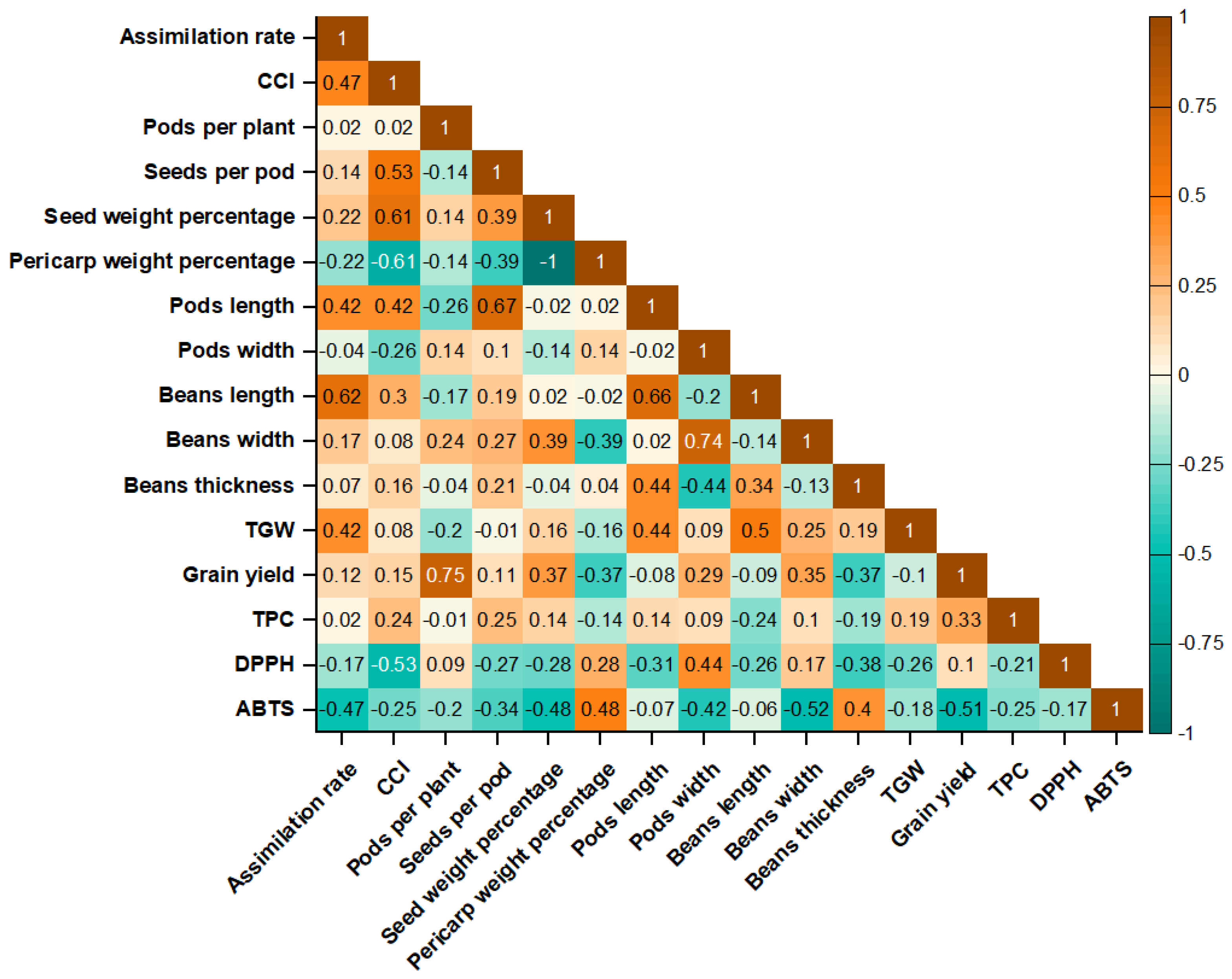
| Treatment | Pods per Plant | Seeds per Pod | Seed Weight Percentage (%) | Pericarp Weight Percentage (%) | Pods Length (cm) | Pods Width (mm) |
|---|---|---|---|---|---|---|
| Cozia1 | 29.72 ± 0.22 b | 2.30 ± 0.02 | 74.04 ± 0.16 b | 25.96 ± 0.16 a | 10.16 ± 0.03 a | 18.19 ± 0.09 b |
| Cozia2 | 33.30 ± 0.07 a | 2.30 ± 0.02 | 75.94 ± 0.20 a | 24.06 ± 0.20 b | 9.70 ± 0.02 b | 18.50 ± 0.03 b |
| Cozia3 | 32.84 ± 0.19 a | 2.30 ± 0.01 | 74.32 ± 0.34 b | 25.68 ± 0.34 a | 9.75 ± 0.09 b | 19.06 ± 0.14 a |
| Signification | * | ns | * | * | * | * |
| IR1 | 30.46 ± 0.47 | 2.28 ± 0.01 | 75.05 ± 0.06 | 24.95 ± 0.06 | 9.80 ± 0.07 | 18.40 ± 0.22 |
| IR2 | 33.45 ± 0.29 | 2.31 ± 0.02 | 74.48 ± 0.08 | 25.52 ± 0.08 | 9.94 ± 0.03 | 18.77 ± 0.09 |
| Signification | * | ns | * | * | ns | ns |
| OR | 32.23 ± 0.22 b | 2.31 ± 0.02 | 75.06 ± 0.27 | 24.94 ± 0.27 | 9.95 ± 0.03 | 18.28 ± 0.10 b |
| CH | 33.85 ± 0.31 a | 2.32 ± 0.01 | 74.70 ± 0.16 | 25.30 ± 0.16 | 9.93 ± 0.06 | 18.88 ± 0.03 a |
| UF | 29.78 ± 0.52 c | 2.26 ± 0.02 | 74.54 ± 0.27 | 25.46 ± 0.27 | 9.72 ± 0.12 | 18.60 ± 0.15 ab |
| Signification | * | ns | ns | ns | ns | * |
| Treatment | Beans Length (mm) | Beans Width (mm) | Beans Thickness (mm) | TGW (g) | Grain Yield (kg·ha−1) |
|---|---|---|---|---|---|
| Cozia1 | 21.01 ± 0.16 a | 13.03 ± 0.02 b | 8.69 ± 0.10 a | 1444.40 ± 10.00 a | 2505.60 ± 39.54 c |
| Cozia2 | 20.05 ± 0.02 b | 13.64 ± 0.02 a | 8.61 ± 0.06 a | 1412.84 ± 14.62 ab | 2674.23 ± 17.26 b |
| Cozia3 | 20.00 ± 0.17 b | 13.45 ± 0.11 a | 7.98 ± 0.02 b | 1388.69 ± 8.01 b | 2951.81 ± 19.94 a |
| Signification | * | * | * | * | * |
| IR1 | 20.34 ± 0.03 | 13.25 ± 0.10 | 8.33 ± 0.04 | 1407.88 ± 3.38 | 2648.38 ± 40.19 |
| IR2 | 20.37 ± 0.02 | 13.49 ± 0.02 | 8.52 ± 0.06 | 1422.74 ± 10.68 | 2772.70 ± 27.52 |
| Signification | ns | ns | ns | ns | ns |
| OR | 20.40 ± 0.05 | 13.29 ± 0.03 | 8.49 ± 0.06 | 1405.71 ± 12.49 | 2807.84 ± 32.11 a |
| CH | 20.28 ± 0.01 | 13.54 ± 0.03 | 8.47 ± 0.06 | 1409.35 ± 20.03 | 2836.43 ± 68.70 a |
| UF | 20.37 ± 0.06 | 13.28 ± 0.12 | 8.32 ± 0.10 | 1430.88 ± 4.96 | 2487.37 ± 57.67 b |
| Signification | ns | ns | ns | ns | * |
| Treatment | Pods per Plant | Seeds per Pod | Seed Weight Percentage (%) | Pericarp Weight Percentage (%) | Pods Length (cm) | Pods Width (mm) |
|---|---|---|---|---|---|---|
| Cozia1 × IR1 | 30.78 ± 1.92 b | 2.29 ± 0.02 | 74.39 ± 0.20 b | 25.61 ± 0.20 a | 10.20 ± 0.05 a | 18.17 ± 0.21 b |
| Cozia1 × IR2 | 28.67 ± 1.50 b | 2.31 ± 0.02 | 73.69 ± 0.23 b | 26.31 ± 0.23 a | 10.12 ± 0.01 ab | 18.21 ± 0.09 b |
| Cozia2 × IR1 | 29.54 ± 0.61 b | 2.29 ± 0.01 | 75.97 ± 0.46 a | 24.03 ± 0.46 b | 9.60 ± 0.03 d | 18.29 ± 0.11 b |
| Cozia2 × IR2 | 37.05 ± 0.58 a | 2.31 ± 0.02 | 75.90 ± 0.23 a | 24.10 ± 0.23 b | 9.80 ± 0.02 cd | 18.70 ± 0.09 b |
| Cozia3 × IR1 | 31.06 ± 0.53 b | 2.26 ± 0.04 | 74.80 ± 0.12 ab | 25.20 ± 0.12 ab | 9.62 ± 0.18 cd | 18.75 ± 0.34 b |
| Cozia3 × IR2 | 34.63 ± 0.17 a | 2.33 ± 0.01 | 73.84 ± 0.70 b | 26.16 ± 0.70 a | 9.88 ± 0.05 bc | 19.38 ± 0.17 a |
| Signification | * | ns | * | * | * | * |
| Cozia1 × OR | 28.93 ± 0.32 c | 2.29 ± 0.02 | 74.00 ± 0.41 cd | 26.00 ± 0.41 ab | 10.22 ± 0.03 ab | 17.91 ± 0.09 d |
| Cozia1 × CH | 29.89 ± 0.38 c | 2.34 ± 0.03 | 73.92 ± 0.34 cd | 26.08 ± 0.34 ab | 10.36 ± 0.05 a | 18.50 ± 0.17 bcd |
| Cozia1 × UF | 30.34 ± 0.38 c | 2.27 ± 0.01 | 74.21 ± 0.39 cd | 25.79 ± 0.39 ab | 9.91 ± 0.10 bc | 18.16 ± 0.11 cd |
| Cozia2 × OR | 34.44 ± 0.26 b | 2.35 ± 0.05 | 76.20 ± 0.55 a | 23.80 ± 0.55 d | 9.81 ± 0.08 c | 18.01 ± 0.27 cd |
| Cozia2 × CH | 36.98 ± 0.59 a | 2.31 ± 0.01 | 75.73 ± 0.02 ab | 24.27 ± 0.02 cd | 9.62 ± 0.09 c | 18.49 ± 0.10 bcd |
| Cozia2 × UF | 28.47 ± 0.39 c | 2.24 ± 0.01 | 75.88 ± 0.32 a | 24.12 ± 0.32 d | 9.67 ± 0.06 c | 19.01 ± 0.13 b |
| Cozia3 × OR | 33.33 ± 0.67 b | 2.31 ± 0.01 | 74.99 ± 0.09 abc | 25.01 ± 0.09 bcd | 9.84 ± 0.01 c | 18.92 ± 0.07 b |
| Cozia3 × CH | 34.67 ± 1.19 b | 2.30 ± 0.02 | 74.44 ± 0.83 bcd | 25.56 ± 0.83 abc | 9.82 ± 0.08 c | 19.66 ± 0.17 a |
| Cozia3 × UF | 30.52 ± 1.20 c | 2.28 ± 0.08 | 73.53 ± 0.20 d | 26.47 ± 0.20 a | 9.58 ± 0.30 c | 18.61 ± 0.47 bc |
| Signification | * | ns | * | * | * | * |
| IR1 × OR | 31.18 ± 0.61 c | 2.28 ± 0.03 bc | 74.27 ± 0.24 bc | 25.73 ± 0.24 ab | 9.83 ± 0.04 a | 18.21 ± 0.30 c |
| IR1 × CH | 31.01 ± 0.11 cd | 2.39 ± 0.01 a | 75.98 ± 0.15 a | 24.02 ± 0.15 c | 10.09 ± 0.05 a | 18.73 ± 0.07 abc |
| IR1 × UF | 29.18 ± 1.14 d | 2.17 ± 0.05 d | 74.89 ± 0.11 ab | 25.11 ± 0.11 bc | 9.49 ± 0.23 b | 18.27 ± 0.32 c |
| IR2 × OR | 33.28 ± 0.20 b | 2.35 ± 0.02 ab | 75.85 ± 0.52 a | 24.15 ± 0.52 c | 10.08 ± 0.04 a | 18.34 ± 0.11 bc |
| IR2 × CH | 36.69 ± 0.63 a | 2.24 ± 0.03 cd | 73.41 ± 0.17 c | 26.59 ± 0.17 a | 9.78 ± 0.08 ab | 19.03 ± 0.12 a |
| IR2 × UF | 30.38 ± 0.10 cd | 2.36 ± 0.01 ab | 74.18 ± 0.59 bc | 25.82 ± 0.59 ab | 9.95 ± 0.05 a | 18.92 ± 0.07 ab |
| Signification | * | * | * | * | * | * |
| Treatment | Beans Length (mm) | Beans Width (mm) | Beans Thickness (mm) | TGW (g) | Grain Yield (kg·ha−1) |
|---|---|---|---|---|---|
| Cozia1 × IR1 | 21.22 ± 0.36 a | 13.05 ± 0.09 b | 8.62 ± 0.10 a | 1449.82 ± 29.03 a | 2528.13 ± 108.51 b |
| Cozia1 × IR2 | 20.80 ± 0.06 a | 13.00 ± 0.04 b | 8.77 ± 0.12 a | 1438.99 ± 9.82 ab | 2483.07 ± 29.48 b |
| Cozia2 × IR1 | 19.96 ± 0.10 b | 13.55 ± 0.01 a | 8.49 ± 0.09 a | 1388.15 ± 19.39 b | 2508.62 ± 19.20 b |
| Cozia2 × IR2 | 20.14 ± 0.07 b | 13.73 ± 0.05 a | 8.73 ± 0.07 a | 1437.52 ± 10.89 ab | 2839.83 ± 42.35 a |
| Cozia3 × IR1 | 19.83 ± 0.31 b | 13.16 ± 0.23 b | 7.89 ± 0.10 b | 1385.69 ± 8.21 b | 2908.40 ± 65.91 a |
| Cozia3 × IR2 | 20.16 ± 0.02 b | 13.73 ± 0.03 a | 8.08 ± 0.07 b | 1391.70 ± 15.73 b | 2995.21 ± 32.56 a |
| Signification | * | * | * | * | * |
| Cozia1 × OR | 21.11 ± 0.03 ab | 12.88 ± 0.02 c | 8.69 ± 0.09 a | 1455.05 ± 15.48 ab | 2584.88 ± 1.39 bcd |
| Cozia1 × CH | 20.69 ± 0.14 abc | 13.25 ± 0.06 bc | 8.69 ± 0.07 a | 1453.49 ± 3.42 ab | 2506.75 ± 55.26 cd |
| Cozia1 × UF | 21.23 ± 0.58 a | 12.94 ± 0.02 c | 8.70 ± 0.22 a | 1424.67 ± 11.69 abc | 2425.17 ± 64.82 d |
| Cozia2 × OR | 19.93 ± 0.12 cd | 13.37 ± 0.04 b | 8.71 ± 0.14 a | 1352.81 ± 4.89 c | 2745.76 ± 13.25 bc |
| Cozia2 × CH | 19.92 ± 0.02 cd | 13.59 ± 0.05 ab | 8.78 ± 0.15 a | 1382.65 ± 11.61 bc | 2872.18 ± 65.49 ab |
| Cozia2 × UF | 20.30 ± 0.17 bcd | 13.97 ± 0.03 a | 8.35 ± 0.03 ab | 1503.05 ± 40.52 a | 2404.74 ± 51.88 d |
| Cozia3 × OR | 20.15 ± 0.08 cd | 13.61 ± 0.07 ab | 8.06 ± 0.04 bc | 1409.25 ± 17.18 bc | 3092.85 ± 85.37 a |
| Cozia3 × CH | 20.25 ± 0.16 bcd | 13.78 ± 0.09 a | 7.96 ± 0.11 bc | 1391.92 ± 52.65 bc | 3130.37 ± 197.78 a |
| Cozia3 × UF | 19.58 ± 0.56 d | 12.95 ± 0.36 c | 7.92 ± 0.18 c | 1364.91 ± 19.04 c | 2632.19 ± 153.27 bcd |
| Signification | * | * | * | * | * |
| IR1 × OR | 20.33 ± 0.08 | 13.14 ± 0.07 bc | 8.36 ± 0.05 ab | 1374.10 ± 21.13 b | 2621.90 ± 8.82 bc |
| IR1 × CH | 20.34 ± 0.07 | 13.54 ± 0.02 a | 8.47 ± 0.11 ab | 1429.27 ± 19.03 ab | 2800.98 ± 19.75 ab |
| IR1 × UF | 20.35 ± 0.10 | 13.08 ± 0.25 c | 8.17 ± 0.14 b | 1420.29 ± 8.01 ab | 2522.27 ± 125.06 c |
| IR2 × OR | 20.46 ± 0.03 | 13.43 ± 0.02 ab | 8.62 ± 0.14 a | 1437.31 ± 7.82 a | 2993.76 ± 55.51 a |
| IR2 × CH | 20.24 ± 0.07 | 13.54 ± 0.04 a | 8.48 ± 0.05 ab | 1389.44 ± 22.45 ab | 2871.88 ± 124.45 a |
| IR2 × UF | 20.40 ± 0.02 | 13.49 ± 0.01 a | 8.48 ± 0.08 ab | 1441.47 ± 15.09 a | 2452.47 ± 25.01 c |
| Signification | ns | * | * | * | * |
| Treatment | Pods per Plant | Seeds per Pod | Seed Weight Percentage (%) | Pericarp Weight Percentage (%) | Pods Length (cm) | Pods Width (mm) |
|---|---|---|---|---|---|---|
| Cozia1 × IR1 × OR | 30.27 ± 2.71 c÷g | 2.20 ± 0.03 d÷g | 71.96 ± 0.28 e | 28.04 ± 0.28 a | 10.04 ± 0.06 bc | 17.95 ± 0.13 efg |
| Cozia1 × IR2 × OR | 27.58 ± 2.75 efg | 2.39 ± 0.01 abc | 76.04 ± 0.61 ab | 23.96 ± 0.61 de | 10.40 ± 0.05 ab | 17.86 ± 0.14 fg |
| Cozia1 × IR1 × CH | 30.79 ± 1.43 c÷f | 2.41 ± 0.01 ab | 75.14 ± 0.40 abc | 24.86 ± 0.40 cde | 10.67 ± 0.04 a | 18.57 ± 0.41 b÷g |
| Cozia1 × IR2 × CH | 29.01 ± 0.84 d÷g | 2.28 ± 0.03 b÷e | 72.69 ± 0.34 de | 27.31 ± 0.34 ab | 10.04 ± 0.09 bc | 18.42 ± 0.09 b÷g |
| Cozia1 × IR1 × UF | 31.28 ± 2.14 cde | 2.28 ± 0.01 b÷e | 76.06 ± 0.49 ab | 23.94 ± 0.49 de | 9.89 ± 0.17 bcd | 17.97 ± 0.18 d÷g |
| Cozia1 × IR2 × UF | 29.41 ± 1.37 d÷g | 2.26 ± 0.01 c÷f | 72.36 ± 0.30 de | 27.64 ± 0.30 ab | 9.94 ± 0.07 bc | 18.35 ± 0.14 b÷g |
| Cozia2 × IR1 × OR | 32.85 ± 1.31 bcd | 2.33 ± 0.04 a÷d | 76.77 ± 0.96 a | 23.23 ± 0.96 e | 9.61 ± 0.05 c÷f | 17.80 ± 0.55 g |
| Cozia2 × IR2 × OR | 36.02 ± 1.38 b | 2.36 ± 0.06 abc | 75.62 ± 0.87 ab | 24.38 ± 0.87 de | 10.01 ± 0.11 bc | 18.21 ± 0.02 c÷g |
| Cozia2 × IR1 × CH | 25.83 ± 0.38 g | 2.43 ± 0.02 a | 76.11 ± 0.47 ab | 23.89 ± 0.47 de | 9.84 ± 0.11 cde | 18.46 ± 0.11 b÷g |
| Cozia2 × IR2 × CH | 48.13 ± 0.95 a | 2.18 ± 0.01 efg | 75.35 ± 0.52 abc | 24.65 ± 0.52 cde | 9.39 ± 0.08 def | 18.51 ± 0.14 b÷g |
| Cozia2 × IR1 × UF | 29.94 ± 0.56 c÷g | 2.10 ± 0.02 g | 75.02 ± 0.20 abc | 24.98 ± 0.20 cde | 9.35 ± 0.05 ef | 18.63 ± 0.11 b÷g |
| Cozia2 × IR2 × UF | 27.00 ± 0.99 efg | 2.38 ± 0.02 abc | 76.74 ± 0.69 a | 23.26 ± 0.69 e | 10.00 ± 0.08 bc | 19.38 ± 0.14 ab |
| Cozia3 × IR1 × OR | 30.42 ± 0.55 c÷g | 2.31 ± 0.02 a÷e | 74.09 ± 0.15 bcd | 25.91 ± 0.15 bcd | 9.86 ± 0.03 cd | 18.89 ± 0.30 b÷f |
| Cozia3 × IR2 × OR | 36.24 ± 0.83 b | 2.30 ± 0.01 a÷e | 75.89 ± 0.16 ab | 24.11 ± 0.16 de | 9.83 ± 0.05 cde | 18.95 ± 0.18 b÷e |
| Cozia3 × IR1 × CH | 36.42 ± 1.07 b | 2.34 ± 0.01 abc | 76.71 ± 0.32 a | 23.29 ± 0.32 e | 9.76 ± 0.07 cde | 19.15 ± 0.24 bc |
| Cozia3 × IR2 × CH | 32.92 ± 1.33 bcd | 2.26 ± 0.04 c÷f | 72.18 ± 1.35 de | 27.82 ± 1.35 ab | 9.89 ± 0.08 bcd | 20.17 ± 0.30 a |
| Cozia3 × IR1 × UF | 26.33 ± 1.83 fg | 2.14 ± 0.14 fg | 73.60 ± 0.59 cde | 26.40 ± 0.59 abc | 9.24 ± 0.57 f | 18.20 ± 0.90 c÷g |
| Cozia3 × IR2 × UF | 34.72 ± 0.68 bc | 2.43 ± 0.02 a | 73.46 ± 0.87 cde | 26.54 ± 0.87 abc | 9.92 ± 0.05 bc | 19.02 ± 0.12 bcd |
| Treatment | Beans Length (mm) | Beans Width (mm) | Beans Thickness (mm) | TGW (g) | Grain Yield (kg·ha−1) |
|---|---|---|---|---|---|
| Cozia1 × IR1 × OR | 20.87 ± 0.02 abc | 12.71 ± 0.05 h | 8.74 ± 0.14 abc | 1424.81 ± 34.81 a÷d | 2193.43 ± 70.42 i |
| Cozia1 × IR2 × OR | 21.35 ± 0.04 ab | 13.06 ± 0.08 d÷h | 8.65 ± 0.06 a÷e | 1485.29 ± 4.24 ab | 2976.34 ± 71.44 bcd |
| Cozia1 × IR1 × CH | 21.07 ± 0.15 abc | 13.48 ± 0.13 b÷g | 8.67 ± 0.08 a÷d | 1524.20 ± 21.83 a | 2758.95 ± 118.60 def |
| Cozia1 × IR2 × CH | 20.30 ± 0.14 bcd | 13.01 ± 0.01 e÷h | 8.70 ± 0.08 abc | 1382.78 ± 15.11 bcd | 2254.53 ± 41.62 hi |
| Cozia1 × IR1 × UF | 21.72 ± 1.14 a | 12.96 ± 0.08 fgh | 8.45 ± 0.13 b÷e | 1400.44 ± 36.29 bcd | 2632.00 ± 159.87 d÷h |
| Cozia1 × IR2 × UF | 20.74 ± 0.18 abc | 12.93 ± 0.04 gh | 8.95 ± 0.31 ab | 1448.90 ± 16.93 abc | 2218.33 ± 31.51 i |
| Cozia2 × IR1 × OR | 19.96 ± 0.15 cd | 13.30 ± 0.05 c÷g | 8.27 ± 0.10 c÷g | 1323.20 ± 13.92 d | 2688.80 ± 86.01 d÷g |
| Cozia2 × IR2 × OR | 19.90 ± 0.14 cd | 13.43 ± 0.05 c÷g | 9.14 ± 0.36 a | 1382.43 ± 20.77 bcd | 2802.72 ± 107.69 c÷f |
| Cozia2 × IR1 × CH | 19.83 ± 0.10 cd | 13.61 ± 0.09 bcd | 8.91 ± 0.31 ab | 1360.35 ± 33.29 cd | 2316.76 ± 17.91 ghi |
| Cozia2 × IR2 × CH | 20.01 ± 0.13 cd | 13.56 ± 0.06 b÷e | 8.65 ± 0.04 a÷e | 1404.95 ± 22.37 bcd | 3427.60 ± 121.10 a |
| Cozia2 × IR1 × UF | 20.08 ± 0.20 bcd | 13.73 ± 0.02 abc | 8.30 ± 0.06 c÷g | 1480.92 ± 24.00 ab | 2520.31 ± 42.84 e÷i |
| Cozia2 × IR2 × UF | 20.52 ± 0.22 a÷d | 14.21 ± 0.04 a | 8.40 ± 0.11 b÷f | 1525.19 ± 57.11 a | 2289.18 ± 100.44 ghi |
| Cozia3 × IR1 × OR | 20.16 ± 0.17 bcd | 13.41 ± 0.12 c÷g | 8.05 ± 0.06 efg | 1374.30 ± 33.22 cd | 2983.48 ± 41.05 bcd |
| Cozia3 × IR2 × OR | 20.14 ± 0.01 bcd | 13.81 ± 0.02 abc | 8.07 ± 0.04 efg | 1444.20 ± 7.89 abc | 3202.23 ± 130.76 abc |
| Cozia3 × IR1 × CH | 20.11 ± 0.26 bcd | 13.52 ± 0.11 b÷f | 7.84 ± 0.05 fg | 1403.26 ± 45.89 bcd | 3327.24 ± 136.65 ab |
| Cozia3 × IR2 × CH | 20.04 ± 0.07 bcd | 14.04 ± 0.08 ab | 8.08 ± 0.16 d÷g | 1380.58 ± 59.78 bcd | 2933.51 ± 258.96 bcd |
| Cozia3 × IR1 × UF | 19.24 ± 0.99 d | 12.55 ± 0.68 h | 7.76 ± 0.38 g | 1379.51 ± 31.68 bcd | 2414.49 ± 282.39 f÷i |
| Cozia3 × IR2 × UF | 19.93 ± 0.14 cd | 13.34 ± 0.03 c÷g | 8.09 ± 0.04 d÷g | 1350.32 ± 12.28 cd | 2849.90 ± 37.39 cde |
| Treatment | TPC (mg GAE·100 g−1 d.w.) | DPPH (mmol TE·100 g−1 d.w.) | ABTS (mmol TE·100 g−1 d.w.) |
|---|---|---|---|
| Cozia1 | 0.32 ± 0.00 b | 0.12 ± 0.00 b | 0.28 ± 0.00 |
| Cozia2 | 0.31 ± 0.01 b | 0.12 ± 0.00 ab | 0.27 ± 0.01 |
| Cozia3 | 0.37 ± 0.01 a | 0.13 ± 0.00 a | 0.26 ± 0.00 |
| Signification | * | * | ns |
| IR1 | 0.33 ± 0.00 | 0.13 ± 0.00 | 0.27 ± 0.00 |
| IR2 | 0.34 ± 0.01 | 0.12 ± 0.00 | 0.27 ± 0.00 |
| Signification | ns | ns | ns |
| OR | 0.33 ± 0.01 | 0.12 ± 0.00 b | 0.27 ± 0.01 |
| CH | 0.35 ± 0.01 | 0.14 ± 0.01 a | 0.26 ± 0.00 |
| UF | 0.32 ± 0.01 | 0.12 ± 0.00 b | 0.27 ± 0.01 |
| Signification | ns | * | ns |
| Treatment | TPC (mg GAE·100 g−1 d.w.) | DPPH (mmol TE·100 g−1 d.w.) | ABTS (mmol TE·100 g−1 d.w.) |
|---|---|---|---|
| Cozia1 × IR1 | 0.28 ± 0.00 c | 0.11 ± 0.00 b | 0.27 ± 0.00 ab |
| Cozia1 × IR2 | 0.36 ± 0.01 ab | 0.13 ± 0.00 ab | 0.28 ± 0.00 a |
| Cozia2 × IR1 | 0.33 ± 0.00 bc | 0.13 ± 0.00 a | 0.27 ± 0.00 ab |
| Cozia2 × IR2 | 0.30 ± 0.02 c | 0.12 ± 0.01 b | 0.26 ± 0.01 b |
| Cozia3 × IR1 | 0.39 ± 0.01 a | 0.13 ± 0.00 a | 0.27 ± 0.01 ab |
| Cozia3 × IR2 | 0.35 ± 0.01 ab | 0.13 ± 0.00 a | 0.26 ± 0.00 b |
| Signification | * | * | * |
| Cozia1 × OR | 0.28 ± 0.00 d | 0.13 ± 0.00 bc | 0.27 ± 0.01 |
| Cozia1 × CH | 0.40 ± 0.01 ab | 0.12 ± 0.00 bcd | 0.27 ± 0.01 |
| Cozia1 × UF | 0.28 ± 0.00 d | 0.11 ± 0.00 d | 0.29 ± 0.01 |
| Cozia2 × OR | 0.30 ± 0.04 d | 0.11 ± 0.00 cd | 0.29 ± 0.02 |
| Cozia2 × CH | 0.36 ± 0.01 bc | 0.14 ± 0.01 b | 0.26 ± 0.00 |
| Cozia2 × UF | 0.31 ± 0.01 cd | 0.12 ± 0.01 bcd | 0.26 ± 0.01 |
| Cozia3 × OR | 0.44 ± 0.02 a | 0.12 ± 0.00 cd | 0.26 ± 0.00 |
| Cozia3 × CH | 0.29 ± 0.00 d | 0.16 ± 0.01 a | 0.26 ± 0.01 |
| Cozia3 × UF | 0.38 ± 0.02 b | 0.12 ± 0.00 bcd | 0.27 ± 0.00 |
| Signification | * | * | ns |
| IR1 × OR | 0.30 ± 0.00 c | 0.13 ± 0.00 ab | 0.28 ± 0.01 |
| IR1 × CH | 0.39 ± 0.00 a | 0.13 ± 0.00 ab | 0.26 ± 0.00 |
| IR1 × UF | 0.31 ± 0.01 c | 0.12 ± 0.00 bc | 0.28 ± 0.01 |
| IR2 × OR | 0.37 ± 0.03 ab | 0.11 ± 0.00 c | 0.27 ± 0.01 |
| IR2 × CH | 0.31 ± 0.01 c | 0.15 ± 0.01 a | 0.27 ± 0.01 |
| IR2 × UF | 0.33 ± 0.00 bc | 0.12 ± 0.01 bc | 0.27 ± 0.00 |
| Signification | * | * | ns |
| Treatment | TPC (mg GAE·100 g−1 d.w.) | DPPH (mmol TE·100 g−1 d.w.) | ABTS (mmol TE·100 g−1 d.w.) |
|---|---|---|---|
| Cozia1 × IR1 × OR | 0.17 ± 0.00 i | 0.14 ± 0.01 bcd | 0.29 ± 0.01 abc |
| Cozia1 × IR2 × OR | 0.39 ± 0.01 bcd | 0.12 ± 0.01 c÷g | 0.25 ± 0.01 cd |
| Cozia1 × IR1 × CH | 0.47 ± 0.01 a | 0.11 ± 0.00 fgh | 0.27 ± 0.00 bcd |
| Cozia1 × IR2 × CH | 0.34 ± 0.03 def | 0.14 ± 0.01 bcd | 0.28 ± 0.01 a÷d |
| Cozia1 × IR1 × UF | 0.21 ± 0.00 hi | 0.10 ± 0.00 gh | 0.27 ± 0.01 bcd |
| Cozia1 × IR2 × UF | 0.35 ± 0.00 de | 0.12 ± 0.01 c÷f | 0.31 ± 0.01 a |
| Cozia2 × IR1 × OR | 0.28 ± 0.00 fgh | 0.14 ± 0.01 bc | 0.28 ± 0.02 a÷d |
| Cozia2 × IR2 × OR | 0.28 ± 0.03 fgh | 0.09 ± 0.00 h | 0.30 ± 0.02 ab |
| Cozia2 × IR1 × CH | 0.37 ± 0.00 cd | 0.13 ± 0.01 b÷e | 0.26 ± 0.01 bcd |
| Cozia2 × IR2 × CH | 0.35 ± 0.02 d | 0.14 ± 0.01 bc | 0.25 ± 0.00 cd |
| Cozia2 × IR1 × UF | 0.33 ± 0.00 def | 0.12 ± 0.00 c÷f | 0.28 ± 0.02 a÷d |
| Cozia2 × IR2 × UF | 0.28 ± 0.01 efg | 0.12 ± 0.01 c÷f | 0.24 ± 0.01 d |
| Cozia3 × IR1 × OR | 0.45 ± 0.01 ab | 0.12 ± 0.00 c÷f | 0.26 ± 0.01 bcd |
| Cozia3 × IR2 × OR | 0.43 ± 0.05 abc | 0.12 ± 0.00 d÷g | 0.26 ± 0.01 bcd |
| Cozia3 × IR1 × CH | 0.34 ± 0.01 def | 0.15 ± 0.00 ab | 0.25 ± 0.00 cd |
| Cozia3 × IR2 × CH | 0.24 ± 0.02 gh | 0.17 ± 0.01 a | 0.27 ± 0.02 bcd |
| Cozia3 × IR1 × UF | 0.39 ± 0.05 bcd | 0.13 ± 0.01 cde | 0.28 ± 0.00 a÷d |
| Cozia3 × IR2 × UF | 0.37 ± 0.00 cd | 0.11 ± 0.00 efg | 0.25 ± 0.01 cd |
| PC | Eigenvalue | Percentage of Variance (%) | Cumulative Percentage of Variance (%) |
|---|---|---|---|
| 1 | 4.214 | 26.34 | 26.34 |
| 2 | 3.445 | 21.53 | 47.87 |
| 3 | 2.044 | 12.77 | 60.64 |
| 4 | 1.397 | 8.73 | 69.37 |
| 5 | 1.197 | 7.48 | 76.85 |
| 6 | 1.114 | 6.96 | 83.81 |
| 7 | 0.973 | 6.08 | 89.9 |
| 8 | 0.506 | 3.16 | 93.06 |
| 9 | 0.446 | 2.79 | 95.84 |
| 10 | 0.300 | 1.87 | 97.72 |
| 11 | 0.155 | 0.97 | 98.69 |
| 12 | 0.117 | 0.73 | 99.42 |
| 13 | 0.064 | 0.4 | 99.82 |
| 14 | 0.026 | 0.16 | 99.99 |
| 15 | 0.002 | 0.01 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rădeanu, G.; Precupeanu, C.; Teliban, G.-C.; Roșca, M.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Stoleru, V. Interactive Effects of Genotype, Irrigation, and Fertilization on Physiological, Biometric, and Biochemical Traits of Runner Bean (Phaseolus coccineus L.). Horticulturae 2025, 11, 1135. https://doi.org/10.3390/horticulturae11091135
Rădeanu G, Precupeanu C, Teliban G-C, Roșca M, Ordóñez-Díaz JL, Moreno-Rojas JM, Stoleru V. Interactive Effects of Genotype, Irrigation, and Fertilization on Physiological, Biometric, and Biochemical Traits of Runner Bean (Phaseolus coccineus L.). Horticulturae. 2025; 11(9):1135. https://doi.org/10.3390/horticulturae11091135
Chicago/Turabian StyleRădeanu, Georgiana, Cristina Precupeanu, Gabriel-Ciprian Teliban, Mihaela Roșca, José Luis Ordóñez-Díaz, Jose Manuel Moreno-Rojas, and Vasile Stoleru. 2025. "Interactive Effects of Genotype, Irrigation, and Fertilization on Physiological, Biometric, and Biochemical Traits of Runner Bean (Phaseolus coccineus L.)" Horticulturae 11, no. 9: 1135. https://doi.org/10.3390/horticulturae11091135
APA StyleRădeanu, G., Precupeanu, C., Teliban, G.-C., Roșca, M., Ordóñez-Díaz, J. L., Moreno-Rojas, J. M., & Stoleru, V. (2025). Interactive Effects of Genotype, Irrigation, and Fertilization on Physiological, Biometric, and Biochemical Traits of Runner Bean (Phaseolus coccineus L.). Horticulturae, 11(9), 1135. https://doi.org/10.3390/horticulturae11091135










