New Approaches on Micropropagation of Arracacia xanthorrhiza (“Arracacha”): In Vitro Establishment, Senescence Reduction and Plant Growth Regulators Balance
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. In Vitro Introduction, Basal Medium, and Growing Conditions
2.3. Antibiotic Testing
2.4. Plantlet Senescence Reduction and Silver Nitrate Effects over Plantlet Morphogenesis and Resilience
2.5. Different Exogenous Cytokinins and Determination of Optimal Concentrations in the Culture Medium
2.6. Statistical Analysis
3. Results and Discussion
3.1. Ampicillin Promotes Efficient Explant Asepsis Without Toxicity
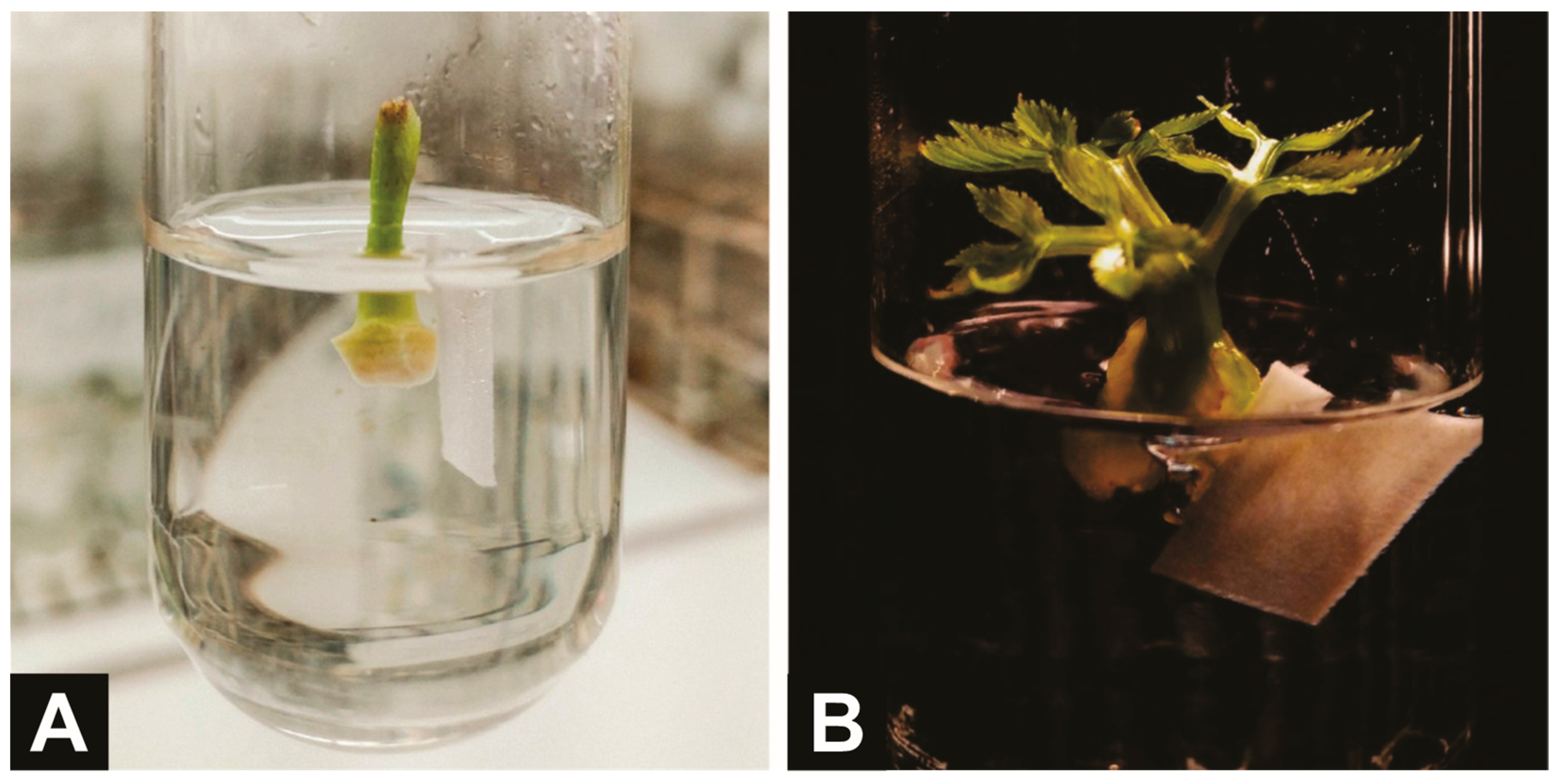
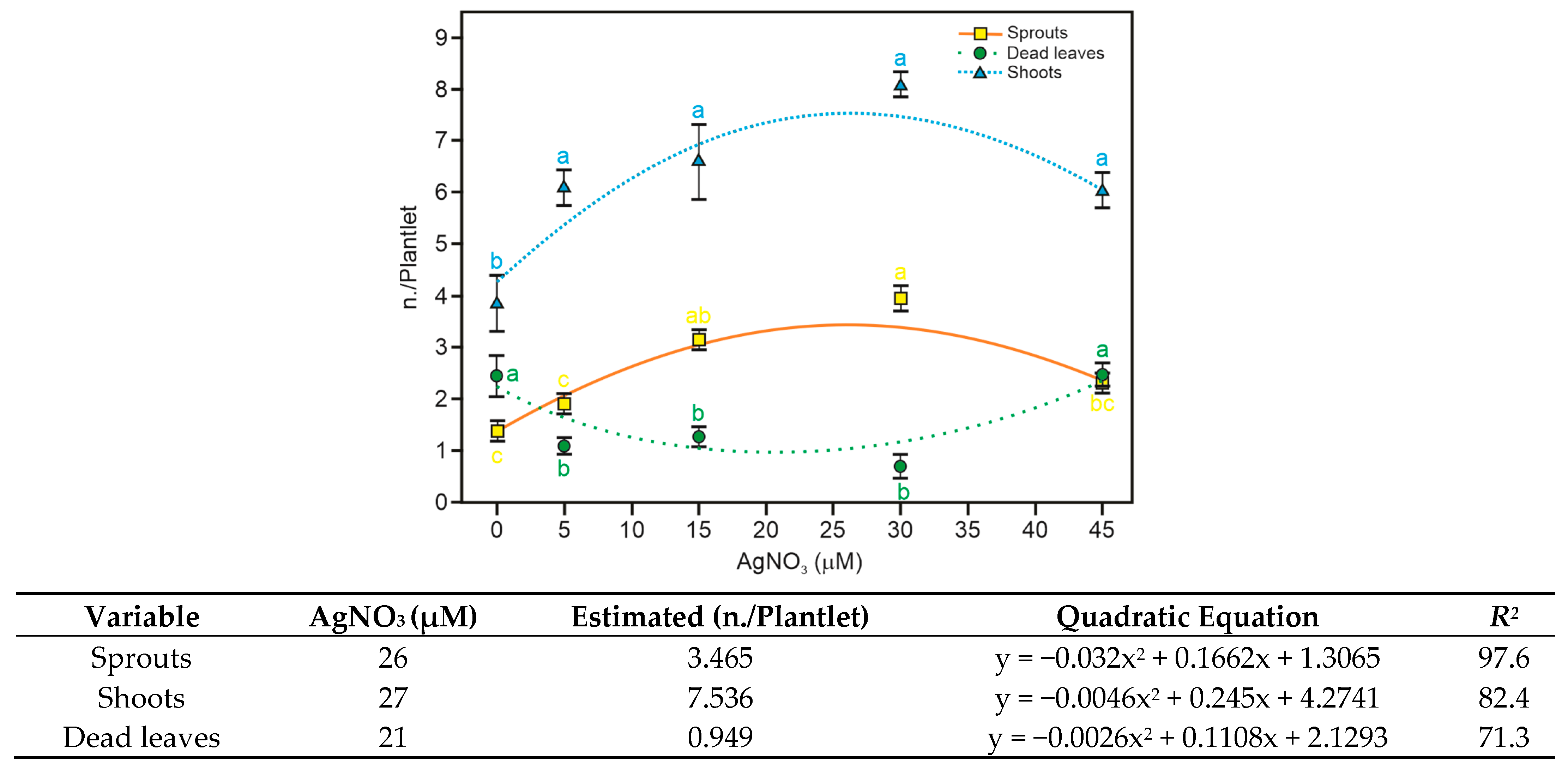
3.2. Silver Nitrate Reduces In Vitro Premature Culture Senescence
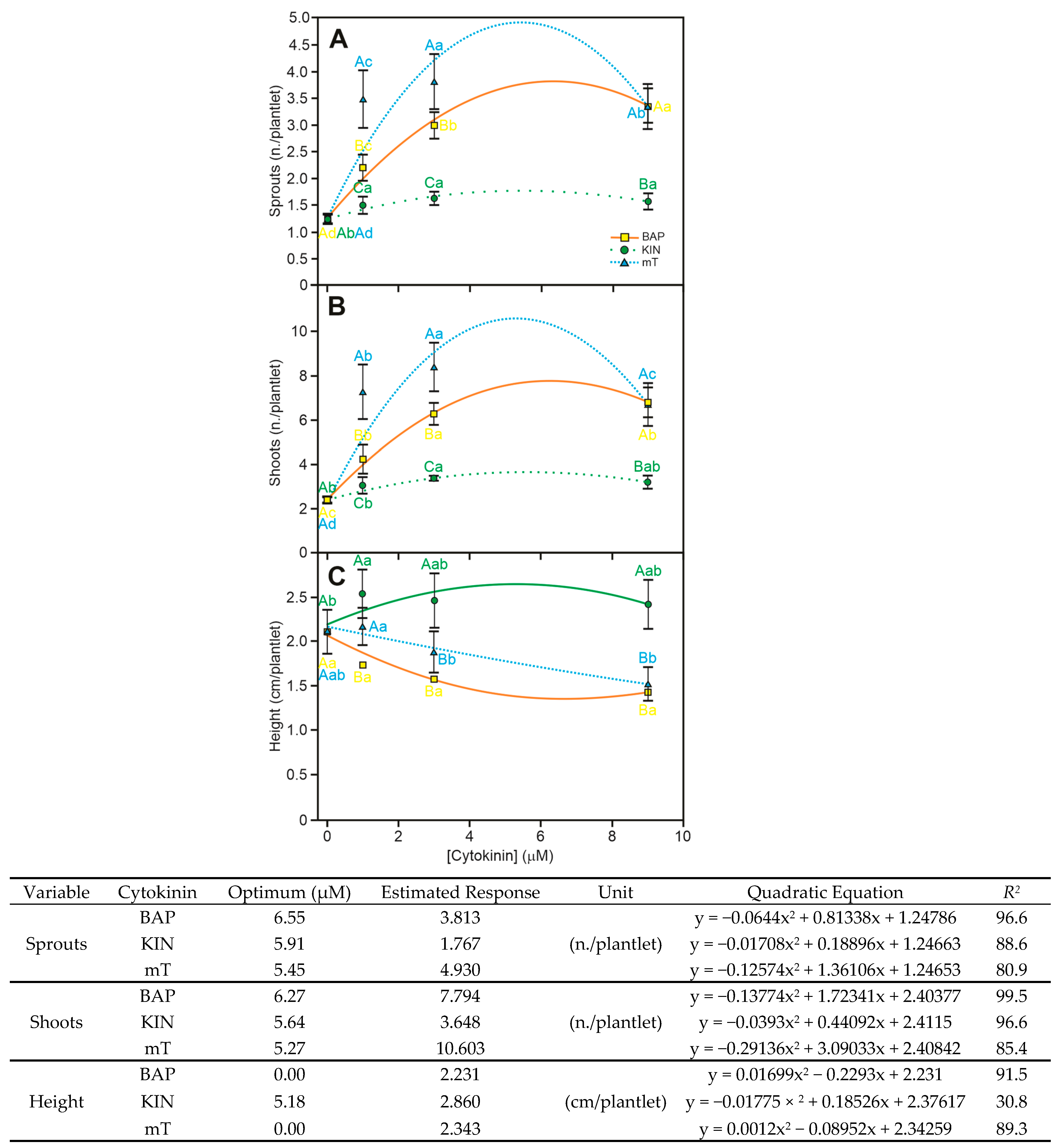
3.3. Meta-Topolin Performs Better than 6-Benzilaminopurine in A. xanthorrhiza In Vitro Organogenesis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrade, N.J.P.; Sevillano, R.H.B.; Sørensen, M. Traditional uses, processes, and markets: The case of arracacha (Arracacia xanthorrhiza Bancroft). In Traditional Starch Food Products: Application and Processing, 4th ed.; Cereda, M.P., Vilpoux, O.F., Eds.; Academic Press: London, UK, 2025; pp. 325–332. [Google Scholar]
- EFSA. EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on the safety of Arracacia xanthorrhiza as a novel food. EFSA J. 2015, 13, 3954. [Google Scholar] [CrossRef]
- Sanchez-Portillo, S.; Salazar-Sánchez, M.D.R.; Campos-Muzquiz, L.G.; Ascacio-Valdés, J.A.; Solanilla-Duque, J.F.; Lopez Badillo, C.M.; Flores-Gallegos, A.C.; Rodríguez-Herrera, R. Proximal characteristics, phenolic compounds profile, and functional properties of Ullucus tuberosus and Arracacia xanthorrhiza. Explor. Foods Foodomics 2024, 2, 672–686. [Google Scholar] [CrossRef]
- Lescano, M.; Vásquez, N.; Vásquez, S.; Yoplac, I.; Velásquez-Barreto, F.K. Development and optimization of biofilms made from potato or arracacha starch. Starch 2021, 73, 2100075. [Google Scholar] [CrossRef]
- Morillo, E.; Sécond, G. Tracing the domestication of the Andean root crop arracacha (Arracacia xanthorrhiza Bancr.): A molecular survey confirms the selection of a wild form apt to asexual reproduction. Plant Genet. Resour. 2017, 15, 380–387. [Google Scholar] [CrossRef]
- Madeira, N.R.; Carvalho, A.D.F.; Silva, G.O.; Brotel, N.; Bortoletto, A.C. Mandioquinha-Salsa Arracacia xanthorrhiza Bancroft; Embrapa: Brasília, DF, Brazil, 2021. [Google Scholar]
- Embrapa. Mandioquinha-Salsa (Arracacia xanthorrhiza). Embrapa Hortaliças, Sistemas de Produção, 4. 2008. Available online: https://sistemasdeproducao.cnptia.embrapa.br/FontesHTML/Mandioquinha/MandioquinhaSalsa/apresentacao.html (accessed on 15 September 2025).
- Mesquita Filho, M.V.; Souza, A.F.; Silva, H.R.; Santos, F.F.; Oliveira, S.A. Adubação nitrogenada e fosfatada para produção comercializável de mandioquinhasalsa em latossolo vermelho amarelo. Hortic. Bras. 1996, 14, 211–215. [Google Scholar]
- Alencar, G. Cientistas Desenvolvem Variedades de Mandioquinha-Salsa Que Produzem até 80% Mais Que a Cultivar Tradicional. Embrapa News. 2018. Available online: https://encurtador.com.br/hkYnL (accessed on 15 September 2025).
- Macedo, A. Mandioquinha-Salsa: Embrapa Multiplica Ações em Novas Áreas de Cultivo no Paraná. Embrapa News. 2019. Available online: https://encurtador.com.br/zwKVC (accessed on 15 September 2025).
- Fletcher, P.J.; Fletcher, J.D. In vitro virus elimination in three Andean root crops: Oca (Oxalis tuberosa), ulluco (Ullucus tuberosus), and arracacha (Arracacia xanthorrhiza). N. Z. J. Crop Hort. Sci. 2001, 29, 23–27. [Google Scholar] [CrossRef]
- Abdalla, N.; El-Ramady, H.; Seliem, M.K.; El-Mahrouk, M.E.; Taha, N.; Bayoumi, Y.; Shalaby, T.A.; Dobránszki, J. An academic and technical overview on plant micropropagation challenges. Horticulturae 2022, 8, 677. [Google Scholar] [CrossRef]
- Slíva, Š.; Viehmannová, I.; Vítámvás, J. Micropropagation and morphogenesis of arracacha (Arracacia xanthorrhiza Bancroft). Agric. Trop. Subtrop. 2010, 43, 206–211. [Google Scholar]
- Madeira, N.R.; Santos, F.F.; Souza, R.J. Desempenho de clones de mandioquinha-salsa (Arracacia xanthorrhiza Bancroft) na região de Lavras-MG. Ciênc. Agrotec. 2002, 26, 711–718. [Google Scholar]
- Manchanda, P.; Gosal, S.S. Effect of activated charcoal, carbon sources and gelling agents on direct somatic embryogenesis and regeneration in sugarcane via leaf roll segments. Sugar Tech. 2012, 14, 168–173. [Google Scholar] [CrossRef]
- Khatoon, N.; Alam, H.; Khan, A.; Raza, K.; Sardar, M. Ampicillin silver nanoformulations against multidrug resistant bacteria. Sci. Rep. 2019, 9, 6848. [Google Scholar] [CrossRef] [PubMed]
- Madeira, N.R. Micropropagação e Indexação de Mandioquinha-Salsa. Ph.D. Thesis, Universidade Federal de Lavras, Lavras, MG, Brazil, 2004. [Google Scholar]
- Matos, E.; Marcano, M.; Azócar, C.J.; Mora, A. Establecimiento y multiplicación in vitro de cinco cultivares de apio (Arracacia xanthorrhiza Bancroft) colectados en Venezuela. Bioagro 2015, 27, 121–130. [Google Scholar]
- Staba, E.J. Plant tissue culture as a technique for the phytochemist. In Recent Advances in Phytochemistry; Seikel, M.K., Runcekles, V.C., Eds.; Appleton-Century-Crofts: New York, NY, USA, 1969; Volume 2, pp. 75–106. [Google Scholar]
- Vrundha, C.P.K.; Aswathi, N.V.; Thomas, T.D. The role of meta-topolin in plant morphogenesis in vitro. In Meta-Topolin: A Growth Regulator for Plant Biotechnology and Agriculture; Ahmad, N., Strnad, M., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2021; pp. 93–118. [Google Scholar]
- Vasconcellos, E.S.; Cruz, C.D.; Regazzi, A.J.; Bhering, L.L.; Rosado, T.B.; Vasconcelos, F.S. Regression models grouping for genotype adaptability and stability analysis. Pesqui. Agropecu. Bras. 2010, 45, 1357–1362. [Google Scholar]
- Lozano-Isla, F.; Benites Alfaro, O.; Santana, D.G.; Ranal, M.A.; Pompelli, M.F. Germination Indexes for Seed Germination Variables for Ecophysiological Studies [R Package GerminaR Version 1.4.2]; R Development Core Team: Kaysville, UT, USA, 2020. [Google Scholar]
- Rusu, A.; Buta, E.L. The development of third-generation tetracycline antibiotics and new perspectives. Pharmaceutics 2021, 13, 2085. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.S.M.; Ullman, J.L.; Teel, A.L.; Watts, R.J. pH and temperature effects on the hydrolysis of three β-lactam antibiotics: Ampicillin, cefalotin and cefoxitin. Sci. Total Environ. 2014, 466–467, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Guerra, W.; Silva-Caldeira, P.P.; Terenzi, H.; Pereira-Maia, E.C. Impact of metal coordination on the antibiotic and non-antibiotic activities of tetracycline-based drugs. Coord. Chem. Rev. 2016, 327–328, 188–199. [Google Scholar] [CrossRef]
- Romadanova, N.V.; Tolegen, A.B.; Kushnarenko, S.V.; Zholdybayeva, E.V.; Bettoni, J.C. Effect of plant preservative mixtureTM on endophytic bacteria eradication from in vitro-grown apple shoots. Plants 2022, 11, 2624. [Google Scholar] [CrossRef]
- Leone, G.F.; Almeida, C.V.; Abreu-Tarazi, M.F.; Batagin-Piotto, K.D.; Artioli-Coelho, F.A.; Almeida, M.D. Antibiotic therapy in Pineapple (Ananas comosus) microplants. Ciên. Rural 2016, 46, 89–94. [Google Scholar] [CrossRef]
- El-Banna, A.N.; El-Mahrouk, M.E.; Dewir, Y.H.; Farid, M.A.; Abou Elyazid, D.M.; Schumacher, H.M. Endophytic bacteria in banana in vitro cultures: Molecular identification, antibiotic susceptibility, and plant survival. Horticulturae 2021, 7, 526. [Google Scholar] [CrossRef]
- Khan, T.; Abbasi, B.H.; Iqrar, I.; Khan, M.A.; Shinwari, Z.K. Molecular identification and control of endophytic contamination during in vitro plantlet development of Fagonia indica. Acta Physiol. Plant 2018, 40, 150. [Google Scholar] [CrossRef]
- Palú, E.G.; Corrêa, L.S.; Suzuki, A.N.; Boliani, A.C. Uso de antibióticos para o controle de bactérias endógenas visando à micropropagação da figueira (Ficus carica L.). Rev. Bras. Frutic. 2011, 33, 587–592. [Google Scholar] [CrossRef]
- Pollock, K.; Barfield, D.G.; Shields, R. The toxicity of antibiotics to plant cell cultures. Plant Cell Rep. 1983, 2, 36–39. [Google Scholar] [CrossRef]
- Pereira, J.E.S.; Fortes, G.R.L. Antibiotics toxicity on the in vitro potato cultivation in semi-solid and liquid media. Pesqui. Agropecu. Bras. 2003, 38, 1273–1279. [Google Scholar] [CrossRef]
- Salehi, H.; Khosh-Khui, M. A simple procedure for disinfection of ‘Baby Masquerade’ miniature rose explants. Sci. Hort. 1997, 68, 145–148. [Google Scholar] [CrossRef]
- Thomas, P. In vitro decline in plant cultures: Detection of a legion of covert bacteria as the cause for degeneration of long-term micropropagated triploid watermelon cultures. Plant Cell Tiss. Org. Cult. 2004, 77, 173–179. [Google Scholar] [CrossRef]
- Najafi, F.; Mohammadi, S.; Asgharian, P.; Kosari-Nasab, M. The impact of activated charcoal and graphite on growth parameters and production of secondary metabolites of Plantago maritima through in vitro culture. BMC Chem. 2025, 19, 222. [Google Scholar] [CrossRef] [PubMed]
- Sáenz, L.; Herrera-Herrera, G.; Uicab-Ballote, F.; Chan, J.L.; Oropeza, C. Influence of form of activated charcoal on embryogenic callus formation in coconut (Cocos nucifera). Plant Cell Tiss. Organ. Cult. 2010, 100, 301–308. [Google Scholar] [CrossRef]
- Thomas, T.D. The role of activated charcoal in plant tissue culture. Biotechnol. Adv. 2008, 26, 618–631. [Google Scholar] [CrossRef]
- Kumar, V.; Parvatam, G.; Ravishankar, G.A. AgNO3: A potential regulator of ethylene activity and plant growth modulator. Electron. J. Biotechnol. 2009, 12, 1–15. [Google Scholar] [CrossRef]
- Gao, H.; Xia, X.; An, L.; Xin, X.; Liang, Y. Reversion of hyperhydricity in pink (Dianthus chinensis L.) plantlets by AgNO3 and its associated mechanism during in vitro culture. Plant Sci. 2017, 254, 1–11. [Google Scholar] [CrossRef]
- Husak, V.V.; Stambulska, U.Y.; Pitukh, A.M.; Lushchak, V.I. Exposure of Paulownia seedlings to silver nitrate improves growth parameters via stimulation of mild oxidative stress. Agric. Conspec. Sci. 2024, 89, 209–218. [Google Scholar]
- Schaller, G.E.; Binder, B.M. Inhibitors of ethylene biosynthesis and signaling. In Ethylene Signaling. Methods in Molecular Biology; Binder, B., Eric Schaller, G., Eds.; Humana Press: New York, NY, USA, 2017; Volume 1573, pp. 223–235. [Google Scholar]
- Mahendran, D.; Geetha, N.; Venkatachalam, P. Role of silver nitrate and silver nanoparticles on tissue culture medium and enhanced the plant growth and development. In In vitro Plant Breeding Towards Novel Agronomic Traits; Kumar, M., Muthusamy, A., Kumar, V., Bhalla-Sarin, N., Eds.; Springer: Singapore, 2019; pp. 59–74. [Google Scholar]
- Türkoğlu, A.; Haliloğlu, K.; Demirel, F.; Aydin, M.; Çiçek, S.; Yiğider, E.; Demirel, S.; Piekutowska, M.; Szulc, P.; Niedbała, G. Machine learning analysis of the impact of silver nitrate and silver nanoparticles on wheat (Triticum aestivum L.): Callus induction, plant regeneration, and DNA methylation. Plants 2023, 12, 4151. [Google Scholar] [CrossRef] [PubMed]
- Enfeshi, N.; Abdulali, E.; Salama, M.; Geath, Z.; Shaaban, A.; Ben Saad, Z.; Abughnia, E. Effect of silver nitrate (AgNO3) and copper sulphate (CuSO4) on callus formation and plant regeneration from two pepper varieties (Chile Ancho and Misraty) in vitro. Sci. J. Fac. Sci. Sirte Univ. 2023, 3, 150–157. [Google Scholar]
- Zhang, P.; Phansiri, S.; Puonti-Kaerlas, J. Improvement of cassava shoot organogenesis by the use of silver nitrate in vitro. Plant Cell Tiss. Org. Cult. 2001, 67, 47–54. [Google Scholar] [CrossRef]
- Giridhar, P.; Indu, E.P.; Vijaya Ramu, D.; Ravishankar, G.A. Effect of silver nitrate on in vitro shoot growth of Coffee. Trop. Sci. 2003, 43, 144–146. [Google Scholar] [CrossRef]
- Tamimi, S.M. Effects of ethylene inhibitors, silver nitrate (AgNO3), cobalt chloride (CoCl2) and aminooxyacetic acid (AOA), on in vitro shoot induction and rooting of banana (Musa acuminata L.). Afr. J. Biotechnol. 2015, 14, 2511–2516. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Giridhar, P.; Ravishankar, G.A. Induction of in vitro flowering in Capsicum frutescens under the influence of silver nitrate and cobalt chloride and pollen transformation. Electron. J. Biotechnol. 2008, 11, 84–89. [Google Scholar] [CrossRef]
- Lopes, F.S. Morfogênese In Vitro Como Auxílio ao Melhoramento da Mandioquinha-Salsa (Arracacia xanthorrhiza Bancroft). Master’s Thesis, Universidade Federal do Espírito Santo, Vitória, ES, Brazil, 2009. [Google Scholar]
- Senna Neto, N. Micropropagação de Mandioquinha-Salsa (Arracacia xanthorrhiza Bancroft); Universidade Federal de Viçosa: Viçosa, MG, Brazil, 1990. [Google Scholar]
- Landazuri, P. Introduccion In Vitro, Micropropagacion, Conservacion y Aclimatizacion de Zanahoria Blanca (Arracacia xanthorrhiza) y Achira (Canna edulis); Pontificia Universidad Catolica del Ecuador: Quito, Ecuador, 1996. [Google Scholar]
- Vitamvas, J.; Viehmannova, I.; Cepkova, P.H.; Mrhalova, H.; Eliasova, K. Assessment of somaclonal variation in indirect morphogenesis-derived plants of Arracacia xanthorrhiza. Pesqui. Agropecu. Bras. 2019, 54, e00301. [Google Scholar] [CrossRef]
- Karwasra, R.K.; Nazir, R.; Pandey, D.K. Meta-topolin mediated improved micropropagation, phytochemical profiling, and assessment of genetic fidelity in Chlorophytum borivilianum Sant. et Fernand., an endangered medicinal herb. Ind. Crop. Prod. 2024, 214, 118463. [Google Scholar] [CrossRef]
- Luz, J.M.Q. Obtenção In Vitro de Plantas de Mandioquinha-Salsa (Arracacia xanthorrhiza Bancroft) via Cultura de Meristemas. Master’s Thesis, Universidade Federal de Lavras, Lavras, MG, Brazil, 1993. [Google Scholar]
- Abdouli, D.; Placková, L.; Dolezal, K.; Bettaieb, T.; Werbrouck, S.P.O. Topolin cytokinins enhanced shoot proliferation, reduced hyperhydricity and altered cytokinin metabolism in Pistacia vera L. seedling explants. Plant Sci. 2022, 322, 111360. [Google Scholar] [CrossRef]
- Ríos-Ríos, A.M.; Silva, J.V.S.; Fernandes, J.V.M.; Batista, D.S.; Silva, T.D.; Chagas, K.; Pinheiro, M.V.M.; Faria, D.V.; Otoni, W.C.; Fernandes, S.A. Micropropagation of Piper crassinervium: An improved protocol for faster growth and augmented production of phenolic compounds. Plant Cell Tiss. Organ. Cult. 2019, 137, 495–509. [Google Scholar] [CrossRef]

| Treatment | Survival (%) ** | Bacteria (B %) ** | Fungi (F %) ** | B + F (%) ** |
|---|---|---|---|---|
| Control | 69.3 ± 5.2 b * | 65.6 ± 5.8 a | 6.0 ± 3.5 a | 8.2 ± 1.0 a |
| Ampicillin | 92.4 ± 7.8 a | 25.2 ± 1.8 b | 4.1 ± 1.9 a | 1.3 ± 0.1 c |
| Tetracycline | 92.8 ± 6.7 a | 61.2 ± 6.5 a | 2.5 ± 1.6 a | 4.6 ± 0.3 b |
| Average (%) | 84.8 ± 13 | 50.7 ± 19.3 | 4.1 ± 2.9 | 4.7 ± 3.0 |
| CV% | 15.3 | 38.1 | 68.9 | 63.7 |
| Treatment | Survival (%) | Sprouts (n./Plantlet) | Shoots (n./Plantlet) |
|---|---|---|---|
| Control | 78.5 ± 3.5 b * | 2.1 ± 0.1 b | 2.7 ± 0.1 b |
| AgNO3 (10 µM) | 97.2 ± 2.4 a | 3.0 ± 0.4 a | 4.6 ± 0.2 a |
| Activated charcoal (1.75 g L−1) | 70.2 ± 5.5 c | 1.2 ± 0.3 c | 1.7 ± 0.3 c |
| General Average (%) | 82.0 ± 12.2 | 2.1 ± 0.8 | 3.0 ± 1.2 |
| CV (%) | 14.9 | 35.6 | 41.4 |
| Report | Saline Formulation | NAA | BAP | mT | GA3 |
|---|---|---|---|---|---|
| Senna Neto (1990) [50] | B5 | 0.11 | 0.44 | - | - |
| Luz (1993) [54] | B5 | 0.57 | 0.88 | - | 0.72 |
| Landázuri (1996) [51] | MS | 0.27 | 24.86 | - | - |
| Madeira (2002) [14] | B5 | 0.54 | 1.33 | - | 0.72 |
| Slíva et al. (2010) [13] | MS | 0.54 | 4.44 | - | - |
| Matos et al. (2015) [18] | B5 | 0.54 | 1.33 | - | - |
| Presented results | B5 | 0.5 | 1 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, P.D.; Ornellas, T.S.; Fritsche, Y.; Mund, I.F.; Caprestano, C.A.; Stefenon, V.M.; Pompelli, M.F.; Guerra, M.P. New Approaches on Micropropagation of Arracacia xanthorrhiza (“Arracacha”): In Vitro Establishment, Senescence Reduction and Plant Growth Regulators Balance. Horticulturae 2025, 11, 1134. https://doi.org/10.3390/horticulturae11091134
Marques PD, Ornellas TS, Fritsche Y, Mund IF, Caprestano CA, Stefenon VM, Pompelli MF, Guerra MP. New Approaches on Micropropagation of Arracacia xanthorrhiza (“Arracacha”): In Vitro Establishment, Senescence Reduction and Plant Growth Regulators Balance. Horticulturae. 2025; 11(9):1134. https://doi.org/10.3390/horticulturae11091134
Chicago/Turabian StyleMarques, Patrick Dias, Thiago Sanches Ornellas, Yohan Fritsche, Ingrilore Flores Mund, Clarissa Alves Caprestano, Valdir Marcos Stefenon, Marcelo F. Pompelli, and Miguel Pedro Guerra. 2025. "New Approaches on Micropropagation of Arracacia xanthorrhiza (“Arracacha”): In Vitro Establishment, Senescence Reduction and Plant Growth Regulators Balance" Horticulturae 11, no. 9: 1134. https://doi.org/10.3390/horticulturae11091134
APA StyleMarques, P. D., Ornellas, T. S., Fritsche, Y., Mund, I. F., Caprestano, C. A., Stefenon, V. M., Pompelli, M. F., & Guerra, M. P. (2025). New Approaches on Micropropagation of Arracacia xanthorrhiza (“Arracacha”): In Vitro Establishment, Senescence Reduction and Plant Growth Regulators Balance. Horticulturae, 11(9), 1134. https://doi.org/10.3390/horticulturae11091134








